Synthesis of Polysubstituted 1,2-Dihydro-3H-pyrrolo[1,2-a]indol-3-ones through Domino Palladium-Catalyzed Reactions of Indol-2-ylmethyl Acetates with 1,3-Dicarbonyl Derivatives
Abstract
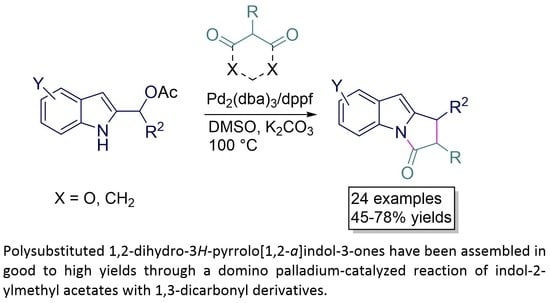
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. General Information
3.2. Synthetic Procedures and Characterization Data
3.2.1. General Procedure for the Preparation of (1H-indol-2-yl)methyl Acetates
3.2.2. Characterization Data of (1H-indol-2-yl)methyl Acetates (1a, c-h; 4a-b)
3.2.3. General Procedure for the Preparation of 5-(aryl-2-ylmethyl)-2,2-dimethyl-1,3-dioxane-4,6-diones (2)
3.2.4. Characterization Data of 5-(aryl-2-ylmethyl)-2,2-dimethyl-1,3-dioxane-4,6-diones (2)
3.2.5. Typical Procedure for the Preparation of 1,2-dihydro-3H-pyrrolo[1,2-a]indol-3-ones (3a-m; 5a-d): Synthesis of 2-methyl-1,2-dihydro-3H-pyrrolo[1,2-a]indol-3-one (3a)
3.2.6. Characterization Data of of 1,2-dihydro-3H-pyrrolo[1,2-a]indol-3-ones (3b-3m; 5a-d)
3.2.7. Typical Procedure for the Preparation of 1,2-dihydro-3H-pyrrolo[1,2-a]indol-3-one (8), ethyl 3-oxo-2,3-dihydro-1H-pyrrolo[1,2-a]indole-2-carboxylate (11) and 2-acetyl-1,2-dihydro-3H-pyrrolo[1,2-a]indol-3-one (13)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dethe, D.H.; Erande, R.D.; Ranjan, A. Biomimetic Total Syntheses of Borreverine and Flinderole Alkaloids. J. Org. Chem. 2013, 78, 10106–10120. [Google Scholar] [CrossRef] [PubMed]
- Vallakati, R.; May, J.A. Biomimetic Synthesis of the Antimalarial Flindersial Alkaloids. J. Am. Chem. Soc. 2012, 134, 6936–6939. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Negri, M.; Heim, R.; Zimmer, C.; Hartmann, R.W. Fine-tuning the selectivity of aldosterone synthase inhibitors: Structure-activity and structure-selectivity insights from studies of heteroaryl substituted 1,2,5,6-tetrahydropyrrolo[3,2,1-ij]quinolin-4-one derivatives. J. Med. Chem. 2011, 54, 2307–2319. [Google Scholar] [CrossRef]
- Dethe, D.H.; Erande, R.D.; Ranjan, A. Biomimetic total syntheses of flinderoles B and C. J. Am. Chem. Soc. 2011, 133, 2864–2867. [Google Scholar] [CrossRef]
- Zeldin, R.M.; Toste, F.D. Synthesis of flinderoles B and C by a gold-catalyzed allene hydroarylation. Chem. Sci. 2011, 2, 1706–1709. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, L.S.; Buchanan, M.S.; Carroll, A.R.; Feng, Y.J.; Quinn, R.J.; Avery, V.M. Flinderoles A-C: Antimalarial bis-indole alkaloids from Flindersia species. Org. Lett. 2009, 11, 329–332. [Google Scholar] [CrossRef]
- Galm, U.; Hager, M.H.; Van Lanen, S.G.; Ju, J.; Thorson, J.S.; Shen, B. Antitumor antibiotics: Bleomycin, enediynes, and mitomycin. Chem. Rev. 2005, 105, 739–758. [Google Scholar]
- Wolkenberg, S.E.; Boger, D.L. Mechanisms of in Situ Activation for DNA-Targeting Antitumor Agents. Chem. Rev. 2002, 102, 2477–2496. [Google Scholar] [CrossRef]
- Liu, J.-F.; Jiang, Z.-Y.; Wang, R.-R.; Zheng, Y.-T.; Chen, J.-J.; Zhang, X.-M.; Ma, Y.-B. Isatisine A, a Novel Alkaloid with an Unprecedented Skeleton from Leaves of Isatis indigotica. Org. Lett. 2007, 9, 4127–4129. [Google Scholar] [PubMed]
- Dorow, R.L.; Herrington, P.M.; Hohler, R.A.; Maloney, M.T.; Mauragis, M.A.; McGhee, W.E.; Moeslein, J.A.; Strohbach, J.W.; Veley, M.F. Development of an Efficient Synthesis of the Pyrrolquinolone PHA-529311. Org. Process. Res. Dev. 2006, 10, 493–499. [Google Scholar] [CrossRef]
- Elmegeed, G.A.; Baiuomy, A.R.; Abdel-Salam, O.M. Evaluation of the anti-inflammatory and anti-nociceptive activities of novel synthesized melatonin analogues. Eur. J. Med. Chem. 2007, 42, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Protter, A.A.; Chakravarty, S. Compounds and Methods of Treating Hypertension. U.S. Patent WO2012/112961A1, 23 August 2012. [Google Scholar]
- Danishefsky, S.; Taniyama, E. Cyclizations of mercury and palladium substituted acyrylanilides. Tetrahedron Lett. 1983, 24, 15–18. [Google Scholar] [CrossRef]
- Duan, X.-Y.; Tian, Z.; Liu, B.; He, T.; Zhao, L.-L.; Dong, M.; Zhang, P.; Qi, J. Highly Enantioselective Synthesis of Pyrroloindolones and Pyrroloquinolinones via an N-Heterocyclic Carbene-Catalyzed Cascade Reaction. Org. Lett. 2021, 23, 3777–3781. [Google Scholar] [CrossRef]
- Yang, W.-L.; Sun, Z.-T.; Sun, H.; Deng, W.-P. Nickel(II)-Catalyzed Diastereo- and Enantioselective [3 + 2] Cycloaddition of α-Ketoesters with 2-Nitrovinylindoles and 2-Nitrovinylpyrroles. Chin. J. Chem. 2019, 37, 216–220. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Ji, Y.; Qi, L.; Wang, G.; Hui, X.-P. Asymmetric Synthesis of Cyclopenta[3,4]pyrroloindolones via N-Heterocyclic Carbene-Catalyzed Michael/Aldol/Lactamization Cascade Reaction. Org. Lett. 2017, 19, 3271–3274. [Google Scholar] [CrossRef]
- Wang, C.; Wang, A.; Rueping, M. Manganese-Catalyzed C−H Functionalizations: Hydroarylations and Alkenylations Involving an Unexpected Heteroaryl Shift. Angew. Chem. Int. Ed. 2017, 56, 9935–9938. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Bainbridge, D.T.; Stanley, L.M. Enantioselective Model Synthesis and Progress toward the Putative Structure of Yuremamine. J. Org. Chem. 2016, 81, 7945–7951. [Google Scholar] [CrossRef]
- Lu, H.; Lin, J.-B.; Liu, J.-Y.; Xu, P.-F. One-Pot Asymmetric Synthesis of Quaternary Pyrroloindolones through a Multicatalytic N-Allylation/Hydroacylation Sequence. Chem. Eur. J. 2014, 20, 11659–11663. [Google Scholar] [CrossRef]
- Ikemoto, H.; Yoshino, T.; Sakata, K.; Matsunaga, S.; Kanai, M. Pyrroloindolone Synthesis via a Cp*CoIII-Catalyzed Redox-Neutral Directed C–H Alkenylation/Annulation Sequence. J. Am. Chem. Soc. 2014, 136, 5424–5431. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Zhang, H.; Grossmann, A.; Loh, C.C.J.; Merkens, C.; Enders, D. Asymmetric Synthesis of Pyrroloindolones by N-Heterocyclic Carbene Catalyzed [2+3] Annulation of α-Chloroaldehydes with Nitrovinylindoles. Angew. Chem. Int. Ed. 2013, 52, 13562–13566. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.; Xu, P.-F. Asymmetric Catalytic Cascade Reactions for Constructing Diverse Scaffolds and Complex Molecules. Acc. Chem. Res. 2015, 48, 1832–1844. [Google Scholar] [CrossRef]
- Arcadi, A.; Cacchi, S.; Fabrizi, G.; Ghirga, F.; Goggiamani, A.; Iazzetti, A.; Marinelli, F. Synthesis of indolo[1,2-c]quinazolines from 2-alkynylaniline derivatives through Pd-catalyzed indole formation/cyclization with N,N-dimethylformamide dimethyl acetal. Beilstein J. Org. Chem. 2018, 14, 2411–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcadi, A.; Blesi, F.; Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Marinelli, F. Multisubstituted benzo[b]furans through a copper- and/or palladium-catalyzed assembly and functionalization process. Tetrahedron 2013, 69, 1857–1871. [Google Scholar] [CrossRef]
- Arcadi, A.; Blesi, F.; Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Marinelli, F. Palladium-Catalyzed Cascade Reactions of 1-(3-Arylprop-2-ynyloxy)-2-bromo Benzene Derivatives with Organoboron Compounds. J. Org. Chem. 2013, 78, 4490–4498. [Google Scholar] [CrossRef] [PubMed]
- Cera, G.; Piscitelli, S.; Chiarucci, M.; Fabrizi, G.; Goggiamani, A.; Ramón, R.S.; Nolan, S.P.; Bandini, M. One-Pot Gold-Catalyzed Synthesis of Azepino[1,2-a]indoles. Angew. Chem. Int. Ed. 2012, 51, 9891–9895. [Google Scholar] [CrossRef]
- Caruana, L.; Mondatori, M.; Corti, V.; Morales, S.; Mazzanti, A.; Fochi, M.; Bernardi, L. Catalytic Asymmetric Addition of Meldrum’s Acid, Malononitrile, and 1,3-Dicarbonyls to ortho-Quinone Methides Generated In Situ Under Basic Conditions. Chem. Eur. J. 2015, 21, 6037–6041. [Google Scholar] [CrossRef] [PubMed]
- Arcadi, A.; Calcaterra, A.; Fabrizi, G.; Fochetti, A.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; Mazzoccanti, G.; Serraiocco, A. One-pot synthesis of dihydroquinolones by sequential reactions of o-aminobenzyl alcohol derivatives with Meldrum’s acids. Org. Biomol. Chem. 2022, 20, 3160–3173. [Google Scholar] [CrossRef]
- Ouyang, J.; Maji, R.; Leutzsch, M.; Mitschke, B.; List, B. Design of an Organocatalytic Asymmetric (4 + 3) Cycloaddition of 2-Indolylalcohols with Dienolsilanes. J. Am. Chem. Soc. 2022, 144, 8460–8466. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Shi, F. A breakthrough in 2-indolylmethanol-involved organocatalytic asymmetric reactions. Chem. Synth. 2022, 2, 11. [Google Scholar] [CrossRef]
- Bera, K.; Schneider, C. Brønsted Acid Catalyzed [3 + 2]-Cycloaddition of Cyclic Enamides with in Situ Generated 2-Methide-2H-indoles: Enantioselective Synthesis of Indolo[1,2-a]indoles. Org. Lett. 2016, 18, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Schneider, C. Brønsted Acid Catalyzed [3 + 2]-Cycloaddition of 2-Vinylindoles with In Situ Generated 2-Methide-2H-indoles: Highly Enantioselective Synthesis of Pyrrolo[1,2-a]indoles. Chem. Eur. J. 2016, 22, 7074–7078. [Google Scholar] [CrossRef]
- Arcadi, A.; Berden, G.; Ciogli, A.; Corinti, D.; Crestoni, M.E.; De Angelis, M.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; et al. Reactivity of Indolylmethylacetates with N, O, and S Soft Nucleophiles: Evidence of 2-Alkylideneindolenines and 3-Alkylideneindoleninium Generation by ESI-MS and IRMPD Spectroscopy. Eur. J. Org. Chem. 2022, 2022, e202201166. [Google Scholar] [CrossRef]
- Arcadi, A.; Fabrizi, G.; Fochetti, A.; Ghirga, F.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; Mazzoccanti, G.; Serraiocco, A. Palladium-catalyzed Tsuji–Trost-type reaction of benzofuran-2-ylmethyl acetates with nucleophiles. Rsc. Adv. 2021, 11, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Arcadi, A.; Calcaterra, A.; Chiarini, M.; Fabrizi, G.; Fochetti, A.; Goggiamani, A.; Iazzetti, A.; Marrone, F.; Marsicano, V.; Serraiocco, A. Synthesis of Indole/Benzofuran-Containing Diarylmethanes through Palladium-Catalyzed Reaction of Indolylmethyl or Benzofuranylmethyl Acetates with Boronic Acids. Synthesis 2022, 54, 741–753. [Google Scholar]
- Kuwano, R.; Kondo, Y.; Shirahama, T. Transformation of Carbonates into Sulfones at the Benzylic Position via Palladium-Catalyzed Benzylic Substitution. Org. Lett. 2005, 7, 2973–2975. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, R.; Kondo, Y. Palladium-catalyzed benzylation of active methine compounds without additional base: Remarkable effect of 1,5-cyclooctadiene. Org. Lett. 2004, 6, 3545–3547. [Google Scholar] [CrossRef]
- Kuwano, R.; Kondo, Y.; Matsuyama, Y. Palladium-Catalyzed Nucleophilic Benzylic Substitutions of Benzylic Esters. J. Am. Chem. Soc. 2003, 125, 12104–12105. [Google Scholar] [CrossRef] [PubMed]
- Calculated by HF, 3-21G* in Titan 1.0.1 2000; Wavefunction Inc.: Irvine, CA, USA, 2000.
- Rossi, E.; Arcadi, A.; Abbiati, G.; Attanasi, O.A.; De Crescentini, L. Sequential base-promoted annulation/ palladium-catalyzed domino 1,5-enyne arylation and vinylation of alpha-propargylaminohydrazones. Angew. Chem. Int. Ed. 2002, 41, 1400–1402. [Google Scholar] [CrossRef]
- Yu, J.-S.; Espinosa, M.; Noda, H.; Shibasaki, M. Traceless Electrophilic Amination for the Synthesis of Unprotected Cyclic β-Amino Acids. J. Am. Chem. Soc. 2019, 141, 10530–10537. [Google Scholar] [CrossRef] [PubMed]
- Chande, M.S.; Khanwelkar, R.R. Michael addition approach for the synthesis of novel spiro compounds and 2-substituted malonic acid derivatives from Meldrum’s acid. Tetrahedron Lett. 2005, 46, 7787–7792. [Google Scholar] [CrossRef]
- Chen, J.-P.; Xu, M.-H. Chiral diene-promoted room temperature conjugate arylation: Highly enantioselective synthesis of substituted chiral phenylalanine derivatives and α,α-di(arylmethyl)acetates. Org. Biomol. Chem. 2020, 18, 4569–4574. [Google Scholar] [CrossRef]
- Saget, T.; König, B. Photocatalytic Synthesis of Polycyclic Indolones. Chem. Eur. J. 2020, 26, 7004–7007. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, S.; Anselmi, E.; Cherry, K.; Kizirian, J.-C.; Thibonnet, J. Synthesis and Reactivity of Oxazinoindolones via Regioselective 6-exo-dig Iodolactonization. Eur. J. Org. Chem. 2018, 45, 6314–6327. [Google Scholar] [CrossRef]
- Goriya, Y.; Ramana, C.V. 2-Aroylindoles from o-bromochalcones via Cu (i)-catalyzed SN Ar with an azide and intramolecular nitrene C–H insertion. Chem. Comm. 2014, 50, 7790–7792. [Google Scholar] [CrossRef]
- Collot, V.; Schmitt, M.; Marwah, P. Regiospecific functionalization of indole-2-carboxylates and diastereoselective preparation of the corresponding indolines. Heterocycles 1999, 51, 2823–2847. [Google Scholar] [CrossRef]
- Akunuri, R.; Veerareddy, V.; Kaul, G.; Akhir, A.; Unnissa, T.; Parupalli, R.; Nanduri, S. Synthesis and antibacterial evaluation of (E)-1-(1H-indol-3-yl) ethanone O-benzyl oxime derivatives against MRSA and VRSA strains. Bioorg. Chem. 2021, 116, 105288. [Google Scholar] [CrossRef]







| Entry | 1 | Catalyst | Base | Solvent | T (°C) | t (h) | Yield 3a (%) b |
|---|---|---|---|---|---|---|---|
| 1 | 1a | / | K2CO3 | DMSO | 100 | 7 | 55 |
| 2 | 1b | / | NaH | DMSO | 100 | 72 | 42(17) c |
| 3 | 1b | Pd2(dba)3/PPh3 | K2CO3 | MeCN | 70 | 5.5 | 68 |
| 4 | 1b | Pd2(dba)3/PPh3 | K2CO3 | MeCN | 80 | 7 | 68 |
| 5 | 1b | Pd2(dba)3/PPh3 | K2CO3 | DMSO | 80 | 1.5 | 75 |
| 6 | 1b | Pd2(dba)3/P(2-furyl)3 | K2CO3 | DMSO | 80 | 1 | 78 |
| 7 | 1b | Pd2(dba)3/dppf | K2CO3 | DMSO | 80 | 1.5 | 85 |
| 8 | 1b | Pd2(dba)3/dppf | / | DMSO | 80 | 24 | (30) c |
| 9 | 1a | Pd2(dba)3/PPh3 | K2CO3 | MeCN | 100 | 40 | 67 |
| 10 | 1a | Pd2(dba)3/PPh3 | K2CO3 | DMSO | 100 | 5.5 | 75 |
| 11 | 1a | Pd2(dba)3/dppf | K2CO3 | DMSO | 100 | 2 | 88 |
| 12 | 1a | Pd2(dba)3/dppf | K2CO3 | DMSO | 100 | 2 | /(/) c,d |

| Entry | 1 | R1 | R2 | 2 | R3 | t (h) | Yield 3 (%) b |
|---|---|---|---|---|---|---|---|
| 1 | 1a | H | H | 2b | -CH2(4-OMe-C6H4) | 1 | 3b (78) |
| 2 | 1a | H | H | 2c | -CH2(furyl) | 4 | 3c (63) |
| 3 | 1a | H | H | 2d | -Ph | 24 | (/) |
| 4 | 1a | H | H | 2e | -CH2CH2CO2Me | 3 | 3d (74) |
| 5 | 1c | 5-Me | H | 2a | -Me | 3 | 3e (70) |
| 6 | 1d | 5-Br | H | 2a | -Me | 5 | 3f (50) |
| 7 | 1e | 5-(4-Me-C6H4) | H | 2a | -Me | 4.5 | 3g (70) |
| 8 | 1f | 5-(4-F,3-Me-C6H3) | H | 2a | -Me | 5 | 3h (70) |
| 9 | 1g | H | Ph | 2a | -Me | 3 | 3i (58) |
| 10 | 1g | H | -Ph | 2b | -CH2(4-OMe-C6H4) | 2 | 3j (64) |
| 11 | 1g | H | -Ph | 2c | -CH2(2-furyl) | 2 | 3k (54) |
| 12 | 1g | H | -Ph | 2e | -CH2CH2CO2Me | 2.5 | 3l (66) |
| 13 | 1h | H | 4-CF3-C6H4 | 2a | -Me | 1 | 3m (71) |
| 14 | H | H | 2a | -Me | 3 | 3a (72) c |

| Entry | 4 | R1 | 2 | R2 | t (h) | Ratio 5/5′ b | Yield 5 + 5′ (%) c |
|---|---|---|---|---|---|---|---|
| 1 | 4a | -Ph | 2a | -Me | 2 | 84/16 | 5a + 5′a (74) |
| 2 | 4a | -Ph | 2b | -CH2(4-OMe-C6H4) | 3 | 94/6 | 5b + 5′b (50) |
| 3 | 4a | -Ph | 2c | -CH2(furyl) | 2 | 74/26 | 5c + 5′c (52) |
| 4 | 4b | -Me | 2a | -Me | 24 | 84/16 | 5d + 5′d (76) |

| Entry | 14 | R1 | R2 | t (h) | Yield 3 (%) | Yield 15 (%) b |
|---|---|---|---|---|---|---|
| 1 | 14a | -Me | -Me | 1 | 3a (45) | 15a (18) c |
| 2 | 14a | -Me | -Me | 3 | 3a (46) | traces |
| 3 | 14b | -OEt | -Me | 24 | / | 15b (60) |
| 4 | 14c | -Me | -CH2CH = CH | 4 | 3n (71) | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iazzetti, A.; Arcadi, A.; Dessalvi, S.; Fabrizi, G.; Goggiamani, A.; Marrone, F.; Serraiocco, A.; Sferrazza, A.; Ullah, K. Synthesis of Polysubstituted 1,2-Dihydro-3H-pyrrolo[1,2-a]indol-3-ones through Domino Palladium-Catalyzed Reactions of Indol-2-ylmethyl Acetates with 1,3-Dicarbonyl Derivatives. Catalysts 2022, 12, 1516. https://doi.org/10.3390/catal12121516
Iazzetti A, Arcadi A, Dessalvi S, Fabrizi G, Goggiamani A, Marrone F, Serraiocco A, Sferrazza A, Ullah K. Synthesis of Polysubstituted 1,2-Dihydro-3H-pyrrolo[1,2-a]indol-3-ones through Domino Palladium-Catalyzed Reactions of Indol-2-ylmethyl Acetates with 1,3-Dicarbonyl Derivatives. Catalysts. 2022; 12(12):1516. https://doi.org/10.3390/catal12121516
Chicago/Turabian StyleIazzetti, Antonia, Antonio Arcadi, Stefano Dessalvi, Giancarlo Fabrizi, Antonella Goggiamani, Federico Marrone, Andrea Serraiocco, Alessio Sferrazza, and Karim Ullah. 2022. "Synthesis of Polysubstituted 1,2-Dihydro-3H-pyrrolo[1,2-a]indol-3-ones through Domino Palladium-Catalyzed Reactions of Indol-2-ylmethyl Acetates with 1,3-Dicarbonyl Derivatives" Catalysts 12, no. 12: 1516. https://doi.org/10.3390/catal12121516