Magnetron Sputtered Al Co-Doped with Zr-Fe2O3 Photoanode with Fortuitous Al2O3 Passivation Layer to Lower the Onset Potential for Photoelectrochemical Solar Water Splitting
Abstract
:1. Introduction
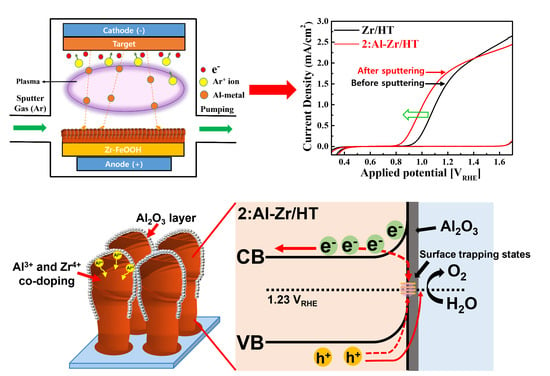
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Zr-Fe2O3 Nanocoral Photoanodes
3.2. Preparation of Al and Zr Co-Doped Fe2O3 via Magnetron Sputtering Method
3.3. Deposition of Cobalt Phosphate (Co-Pi) Cocatalyst on Al-Zr/Fe2O3 Photoanode
3.4. PEC Water Splitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Dai, X.; Bu, Y.; Zhang, H.; Liu, J.; Yuan, W.; Guo, X.; Ao, J.P. Photoelectrochemical Performance Improving Mechanism: Hybridization Appearing at the Energy Band of BiVO4 Photoanode by Doped Quantum Layers Modification. Small 2022, 18, 2200454. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Z.; Kandiel, T.A.; Abdel-Azeim, S.; Jahangir, T.N.; Alhooshani, K. Phosphate ions interfacial drift layer to improve the performance of CoFe−Prussian blue hematite photoanode toward water splitting. Appl. Catal. B 2022, 304, 121014. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Enhanced sunlight-absorption of Fe2O3 covered by PANI for the photodegradation of organic pollutants and antimicrobial inactivation. Adv. Powder Technol. 2022, 33, 103708. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; Hasan, M.; Hussain, F.; Nawaz, M.A.; Iqbal, F. Multi metal oxide NiO-Fe2O3-CdO nanocomposite-synthesis, photocatalytic and antibacterial properties. Appl. Phys. A 2020, 126, 558. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.L.; Song, G.L.; Zheng, D.J. Intrinsic and extrinsic doping to construct hematite nanorod pn homojunctions for highly efficient PEC water splitting. Chem. Eng. J. 2022, 435, 135016. [Google Scholar] [CrossRef]
- Mukhtar, F.; Munawar, T.; Nadeem, M.S.; ur Rehman, M.N.; Khan, S.A.; Koc, M.; Batool, S.; Hasan, M.; Iqbal, F. Dual Z-scheme core-shell PANI-CeO2-Fe2O3-NiO heterostructured nanocomposite for dyes remediation under sunlight and bacterial disinfection. Environ. Res. 2022, 215, 114140. [Google Scholar] [CrossRef]
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Tahir, M.N.; et al. Hematite and magnetite nanostructures for green and sustainable energy harnessing and environmental pollution control: A review. Chem. Res. Toxicol. 2019, 33, 1292–1311. [Google Scholar] [CrossRef]
- Kleiman-Shwarsctein, A.; Huda, M.N.; Walsh, A.; Yan, Y.; Stucky, G.D.; Hu, Y.S.; Al-Jassim, M.M.; McFarland, E.W. Electrodeposited aluminum-doped α-Fe2O3 photoelectrodes: Experiment and theory. Chem. Mater. 2010, 22, 510–517. [Google Scholar] [CrossRef]
- Saremi-Yarahmadi, S.; Wijayantha, K.U.; Tahir, A.A.; Vaidhyanathan, B. Nanostructured α-Fe2O3 electrodes for solar driven water splitting: Effect of doping agents on preparation and performance. J. Phys. Chem. C. 2009, 113, 4768–4778. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Qiu, Y.; Mitsuzak, N.; Chen, Z. The influence of the hydrothermal temperature and time on morphology and photoelectrochemical response of α-Fe2O3 photoanode. J. Alloy. Compd. 2017, 696, 980–987. [Google Scholar] [CrossRef]
- Pu, A.; Deng, J.; Hao, Y.; Sun, X.; Zhong, J. Thickness effect of hematite nanostructures prepared by hydrothermal method for solar water splitting. Appl. Surf. Sci. 2014, 320, 213–217. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Munawar, T.; Mukhtar, F.; ur Rahman, M.N.; Riaz, M.; Hussain, A.; Iqbal, F. Hydrothermally derived co, Ni co-doped ZnO nanorods; structural, optical, and morphological study. Opt. Mater. 2021, 111, 110606. [Google Scholar] [CrossRef]
- Bouhjar, F.; Mollar, M.; Chourou, M.L.; Marí, B.; Bessais, B. Hydrothermal synthesis of nanostructured Cr-doped hematite with enhanced photoelectrochemical activity. Electrochim. Acta. 2018, 260, 838–846. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Anushkkaran, P.; Hwang, J.B.; Chae, W.S.; Kumar, M.; Lee, H.H.; Choi, S.H.; Jang, J.S.; Lee, J.S. Microwave-assisted metal-ion attachment for ex-situ zirconium doping into hematite for enhanced photoelectrochemical water splitting. Renew. Energy 2022, 189, 694–703. [Google Scholar] [CrossRef]
- Kang, K.; Zhang, H.; Kim, J.H.; Byun, W.J.; Lee, J.S. An in situ fluorine and ex-situ titanium two-step co-doping strategy for efficient solar water splitting by hematite photoanodes. Nanoscale Adv. 2022, 4, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yu, C.; Zhang, X. Ti, Zn co-doped hematite photoanode for solar driven photoelectrochemical water oxidation. J. Energy Chem. 2019, 35, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Zandi, O.; Hamann, T.W. Determination of photoelectrochemical water oxidation intermediates on haematite electrode surfaces using operando infrared spectroscopy. Nat. Chem. 2016, 8, 778–783. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Z.; Chen, D.; Yan, W. An efficient hole transfer pathway on hematite integrated by ultrathin Al2O3 interlayer and novel CuCoOx cocatalyst for efficient photoelectrochemical water oxidation. Appl. Catal. B 2020, 277, 119197. [Google Scholar] [CrossRef]
- Hisatomi, T.; Le Formal, F.; Cornuz, M.; Brillet, J.; Tétreault, N.; Sivula, K.; Grätzel, M. Cathodic shift in onset potential of solar oxygen evolution on hematite by 13-group oxide overlayers. Energy Environ. Sci. 2011, 4, 2512–2515. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Fan, F.; Wang, S.; Ding, C.; Zhao, Y.; Li, C. Bridging surface states and current–potential response over hematite-based photoelectrochemical water oxidation. RSC Adv. 2016, 6, 85582–85586. [Google Scholar] [CrossRef]
- Cots, A.; Gómez, R. Ytterbium modification of pristine and molybdenum-modified hematite electrodes as a strategy for efficient water splitting photoanodes. Appl. Catal. B 2017, 219, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.B.; Dhandole, L.K.; Anushkkaran, P.; Chae, W.S.; Choi, S.H.; Lee, H.H.; Jang, J.S. Microwave-assisted surface attachment of aluminium ions on in situ diluted titanium-doped hematite photoanodes for efficient photoelectrochemical water-splitting. Sustain. Energy Fuels 2022, 6, 3056–3067. [Google Scholar] [CrossRef]
- Nakrela, A.; Benramdane, N.; Bouzidi, A.; Kebbab, Z.; Medles, M.; Mathieu, C. Site location of Al-dopant in ZnO lattice by exploiting the structural and optical characterization of ZnO: Al thin films. Results Phys. 2016, 6, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Son, M.K.; Seo, H.; Watanabe, M.; Shiratani, M.; Ishihara, T. Characteristics of crystalline sputtered LaFeO3 thin films as photoelectrochemical water splitting photocathodes. Nanoscale 2020, 12, 9653–9660. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Anushkkaran, P.; Chae, W.S.; Choi, S.H.; Kumar, M.; Cho, M.; Mahadik, M.A.; Lee, H.H.; Jang, J.S. Influence of ZnO Magnetron Sputtering on Controlled Buildout of Zirconium-Doped ZnFe2O4/Fe2O3 Heterojunction Photoanodes for Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2022, 5, 915–929. [Google Scholar] [CrossRef]
- Murthy, N.S. Scattering techniques for structural analysis of biomaterials. In Characterization of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2013; pp. 34–72. [Google Scholar]
- Kumar, D.; Singh, M.; Singh, A.K. Crystallite size effect on lattice strain and crystal structure of Ba1/4Sr3/4MnO3 layered perovskite manganite. AIP Conf. Proc. 2018, 1953, 030185. [Google Scholar]
- Mishra, S.K.; Roy, H.; Lohar, A.K.; Samanta, S.K.; Tiwari, S.; Dutta, K. A comparative assessment of crystallite size and lattice strain in differently cast A356 aluminium alloy. IOP Conf. Ser. Mater. Sci. Eng. 2015, 75, 012001. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Xu, Z.; Yan, S.; Zou, Z. Tuning the ion permeability of an Al2O3 coating layer on Fe2O3 photoanodes for improved photoelectrochemical water oxidation. J. Mater. Chem. 2017, 5, 8402–8407. [Google Scholar] [CrossRef]
- Hwang, J.B.; Kim, S.; Chae, W.S.; Pathan, H.M.; Mahadik, M.A.; Jang, J.S. Engineering of cobalt oxide-integrated nitric acid-functionalized Zr-Fe2O3 nanocoral photoanodes for photoelectrochemical water splitting. Korean J. Chem. Eng. 2021, 38, 1149–1160. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Zhang, X.; Cai, Y.; Wang, W.; Liang, Y. Synergetic effect of surface plasmon resonance and Schottky junction to drastically boost solar-driven photoelectrochemical hydrogen production and photocatalytic performance of CdS/Al nanorod arrays. Energy Convers. Manag. 2022, 268, 115978. [Google Scholar] [CrossRef]
- Cao, X.; Wen, P.; Ma, R.; Liu, Y.; Sun, S.; Ma, Q.; Zhang, P.; Qiu, Y. Ni2P nanocrystals modification on Ta:α-Fe2O3 photoanode for efficient photoelectrochemical water splitting: In situ formation and synergistic catalysis of Ni2P@NiOOH cocatalyst. Chem. Eng. J. 2022, 449, 137792. [Google Scholar] [CrossRef]
- Subramanian, A.; Mahadik, M.A.; Park, J.W.; Jeong, I.K.; Chung, H.S.; Lee, H.H.; Choi, S.H.; Chae, W.S.; Jang, J.S. An effective strategy to promote hematite photoanode at low voltage bias via Zr4+/Al3+ codoping and CoOx OER co-catalyst. Electrochim. Acta 2019, 319, 444–455. [Google Scholar] [CrossRef]
- Tavazohi, A.; Abdizadeh, H.; Golobostanfard, M.R. Hierarchical mesoporous SnO2/BiVO4 photoanode decorated with Ag nanorods for efficient photoelectrochemical water splitting. Int. J. Hydrog. Energy 2022, 47, 18992–19004. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, Y.; Wang, F.; Wang, K.; Liang, Q.; Chen, Z. Design of core–shell-structured ZnO/ZnS hybridized with graphite-like C3N4 for highly efficient photoelectrochemical water splitting. Adv. Mater. Interfaces 2017, 4, 1700681. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, Z.; Spurgeon, J.; Yang, X. Enhanced photoelectrochemical water-splitting performance of semiconductors by surface passivation layers. Energy Environ. Sci. 2014, 7, 2504–2517. [Google Scholar] [CrossRef] [Green Version]
- Cherepy, N.J.; Liston, D.B.; Lovejoy, J.A.; Deng, H.; Zhang, J.Z. Ultrafast studies of photoexcited electron dynamics in γ-and α-Fe2O3 semiconductor nanoparticles. J. Phys. Chem. B 1998, 102, 770–776. [Google Scholar] [CrossRef]
- Barroso, M.; Mesa, C.A.; Pendlebury, S.R.; Cowan, A.J.; Hisatomi, T.; Sivula, K.; Grätzel, M.; Klug, D.R.; Durrant, J.R. Dynamics of photogenerated holes in surface modified α-Fe2O3 photoanodes for solar water splitting. Proc. Natl. Acad. Sci. USA 2012, 109, 15640–15645. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Mahadik, M.A.; Anushkkaran, P.; Chae, W.S.; Choi, S.H.; Jang, J.S. A systematic study of post-activation temperature dependence on photoelectrochemical water splitting of one-step synthesized FeOOH CF photoanodes with erratically loaded ZrO2. Sustain. Energy Fuels 2021, 5, 3414–3427. [Google Scholar] [CrossRef]
- Shen, S.; Guo, P.; Wheeler, D.A.; Jiang, J.; Lindley, S.A.; Kronawitter, C.X.; Zhang, J.Z.; Guo, L.; Mao, S.S. Physical and photoelectrochemical properties of Zr-doped hematite nanorod arrays. Nanoscale 2013, 5, 9867–9874. [Google Scholar] [CrossRef]
- Ma, H.; Hwang, J.B.; Chae, W.S.; Chung, H.S.; Choi, S.H.; Mahadik, M.A.; Lee, H.H.; Jang, J.S. Magnetron sputtering strategy for Zr-Fe2O3 nanorod photoanode fabricated from ZrOx/β-FeOOH nanorods for photoelectrochemical water splitting. Appl. Surf. Sci. 2021, 549, 149233. [Google Scholar] [CrossRef]
- Ding, B.; Huang, S.Y.; Chu, Q.Q.; Li, Y.; Li, C.X.; Li, C.J.; Yang, G.J. Low-temperature SnO2-modified TiO2 yields record efficiency for normal planar perovskite solar modules. J. Mater. Chem. A 2018, 6, 10233–10242. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Liu, C.; Liang, P.; Mitsuzaki, N.; Chen, Z. The effect of fast and slow surface states on photoelectrochemical performance of hematite photoanodes fabricated by electrodeposition and hydrothermal methods. J. Mater. Sci. 2019, 54, 659–670. [Google Scholar] [CrossRef]
- Zhang, H.; Ebaid, M.; Min, J.W.; Ng, T.K.; Ooi, B.S. Enhanced photoelectrochemical performance of InGaN-based nanowire photoanodes by optimizing the ionized dopant concentration. J. Appl. Phys. 2018, 124, 083105. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Koh, T.S.; Anushkkaran, P.; Chung, H.S.; Chae, W.S.; Lee, H.H.; Choi, S.H.; Cho, M.; Jang, J.S. Enhanced charge transfer with tuning surface state in hematite photoanode integrated by niobium and zirconium co-doping for efficient photoelectrochemical water splitting. Appl. Catal. B 2022, 315, 121538. [Google Scholar] [CrossRef]
- Saeidi, M.; Yourdkhani, A.; Ebrahimi, S.A.S.; Poursalehi, R. Candle flame-treatment as an effective strategy to enhance the photoelectrochemical properties of Ti-doped hematite thin films. J. Mater. Chem. C. 2020, 8, 11950–11961. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, Y.K.; Jeong, H.Y.; Lee, J.S. A few atomic FeNbO4 overlayers on hematite nanorods: Microwave-induced high temperature phase for efficient photoelectrochemical water splitting. ACS Catal. 2018, 9, 1289–1297. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, Z.; Guo, Z.; Ruan, M.; Xin, Y. Accelerating the charge separation of ZnFe2O4 nanorods by Cu-Sn ions gradient doping for efficient photoelectrochemical water splitting. J. Colloid Interface Sci. 2019, 552, 111–121. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, C.; Li, S.; Chen, Y.; Zhang, G.; Zhang, D.; Feng, K.; Zhong, J. FeFx and Fe2ZrO5 Co-modified hematite for highly efficient solar water splitting. J. Energy Chem. 2022, 69, 414–420. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Gao, L.; Long, X.; Chai, H.; Li, F.; Wang, Q.; Jin, J. Achieving surface-sealing of hematite nanoarray photoanode with controllable metal–organic frameworks shell for enhanced photoelectrochemical water oxidation. J. Catal. 2022, 413, 398–406. [Google Scholar] [CrossRef]
- Wang, T.; Gao, L.; Wang, P.; Long, X.; Chai, H.; Li, F.; Jin, J. Dual-doping in the bulk and the surface to ameliorate the hematite anode for photoelectrochemical water oxidation. J. Colloid Interface Sci. 2022, 624, 60–69. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Anushkkaran, P.; Chae, W.S.; Chung, H.S.; Lee, H.H.; Choi, S.H.; Cho, M.; Jang, J.S. Efficient charge transfers in hematite photoanode integrated by fluorine and zirconia co-doping for photoelectrochemical water splitting. Chem. Eng. J. 2022, 446, 136957. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, G.; Li, J.; Mo, J.; He, X.; Wang, X.; Yu, Z. Preparation of (Ti, Zr) co-doped hematite photoanode for enhanced photoelectrochemical water splitting. Chem. Phys. Lett. 2020, 754, 137736. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z. Dua-axial gradient doping (Zr and Sn) on hematite for promoting charge separation in photoelectrochemical water splitting. ChemSusChem 2018, 11, 3438–3448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.M.; Borse, P.; Rohatgi, V.K.; Bhoraskar, S.V.; Singh, P.; Sastry, M. Synthesis and structural characterization of nanocrystalline aluminium oxide. Mater. Chem. Phys. 1994, 36, 354–358. [Google Scholar] [CrossRef]
- Fang, R.C.; Sun, Q.Q.; Zhou, P.; Yang, W.; Wang, P.F.; Zhang, D.W. High-performance bilayer flexible resistive random access memory based on low-temperature thermal atomic layer deposition. Nanoscale Res. Lett. 2013, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cushing, S.K.; Zheng, P.; Meng, F.; Chu, D.; Wu, N. Plasmon-induced photonic and energy-transfer enhancement of solar water splitting by a hematite nanorod array. Nat. Commun. 2013, 4, 2651. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, T.S.; Anushkkaran, P.; Hwang, J.B.; Choi, S.H.; Chae, W.-S.; Lee, H.H.; Jang, J.S. Magnetron Sputtered Al Co-Doped with Zr-Fe2O3 Photoanode with Fortuitous Al2O3 Passivation Layer to Lower the Onset Potential for Photoelectrochemical Solar Water Splitting. Catalysts 2022, 12, 1467. https://doi.org/10.3390/catal12111467
Koh TS, Anushkkaran P, Hwang JB, Choi SH, Chae W-S, Lee HH, Jang JS. Magnetron Sputtered Al Co-Doped with Zr-Fe2O3 Photoanode with Fortuitous Al2O3 Passivation Layer to Lower the Onset Potential for Photoelectrochemical Solar Water Splitting. Catalysts. 2022; 12(11):1467. https://doi.org/10.3390/catal12111467
Chicago/Turabian StyleKoh, Tae Sik, Periyasamy Anushkkaran, Jun Beom Hwang, Sun Hee Choi, Weon-Sik Chae, Hyun Hwi Lee, and Jum Suk Jang. 2022. "Magnetron Sputtered Al Co-Doped with Zr-Fe2O3 Photoanode with Fortuitous Al2O3 Passivation Layer to Lower the Onset Potential for Photoelectrochemical Solar Water Splitting" Catalysts 12, no. 11: 1467. https://doi.org/10.3390/catal12111467