Accelerated Decoloration of Organic Dyes from Wastewater Using Ternary Metal/g-C3N4/ZnO Nanocomposites: An Investigation of Impact of g-C3N4 Concentration and Ni and Mn Doping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of g-C3N4
2.3. Synthesis of Composite Nanostructures
2.4. Characterization
The Structure of the Produced Materials Was Studied by XRD (Bruker AXS, D8-S4, USA) at 40 kV and 30 mA at Room Temperature with Cu K Radiation (k = 1.54056)
2.5. Photocatalytic Activity
3. Results and Discussion
3.1. FTIR Analysis
3.2. XRD Analysis
3.3. Compositional and Morphological Study
3.4. Optical Properties
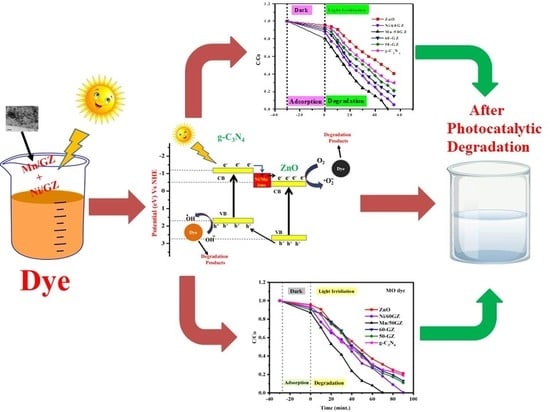
3.5. Photocatalytic Study
3.6. Photoluminescence Spectra
3.7. Recyclability and Constancy of the Mn/50GZ Composite
3.8. Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finke, E.-J.; Beyer, W.; Loderstädt, U.; Frickmann, H. The risk of contracting anthrax from spore-contaminated soil—A military medical perspective. Eur. J. Microbiol. Immunol. 2020, 10, 29–63. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, H.; Jiang, X.; Yuan, G.; Wang, X. Enhanced photocatalytic efficiency in degrading organic dyes by coupling CdS nanowires with ZnFe2O4 nanoparticles. Sol. Energy 2020, 195, 271–277. [Google Scholar] [CrossRef]
- Basaleh, A. Construction of mesoporous ZnFe2O4-g-C3N4 nanocomposites for enhanced photocatalytic degradation of acridine orange dye under visible light illumination adopting soft-and hard-template-assisted routines. J. Mater. Res. Technol. 2021, 11, 1260–1271. [Google Scholar] [CrossRef]
- Dhasmana, A.; Uniyal, S.; Kumar, V.; Gupta, S.; Kesari, K.K.; Haque, S.; Lohani, M.; Pandey, J. Scope of nanoparticles in environmental toxicant remediation. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 31–44. [Google Scholar]
- Sharma, S.; Dutta, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.; Nguyen, V.-H.; VanLe, Q.; Singh, P. An overview of heterojunctioned ZnFe2O4 photocatalyst for enhanced oxidative water purification. J. Environ. Chem. Eng. 2021, 9, 105812. [Google Scholar]
- Alshorifi, F.T.; Alswat, A.A.; Mannaa, M.A.; Alotaibi, M.T.; El-Bahy, S.M.; Salama, R.S. Facile and Green Synthesis of Silver Quantum Dots Immobilized onto a Polymeric CTS–PEO Blend for the Photocatalytic Degradation of p-Nitrophenol. ACS Omega 2021, 6, 30432–30441. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Jilani, A.; Ansari, M.O.; Ur Rehman, G.; Shakoor, M.B.; Hussain, S.Z.; Othman, M.H.D.; Ahmad, S.R.; Dustgeer, M.R.; Alshahrie, A. Phenol removal and hydrogen production from water: Silver nanoparticles decorated on polyaniline wrapped zinc oxide nanorods. J. Ind. Eng. Chem. 2022, 109, 347–358. [Google Scholar] [CrossRef]
- Shekofteh-Gohari, M.; Habibi-Yangjeh, A.; Abitorabi, M.; Rouhi, A. Magnetically separable nanocomposites based on ZnO and their applications in photocatalytic processes: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 806–857. [Google Scholar] [CrossRef]
- Jin, Y.; Long, J.; Ma, X.; Zhou, T.; Zhang, Z.; Lin, H.; Long, J.; Wang, X. Synthesis of caged iodine-modified ZnO nanomaterials and study on their visible light photocatalytic antibacterial properties. Appl. Catal. B Environ. 2019, 256, 117873. [Google Scholar] [CrossRef]
- Kuang, M.; Zhang, J.; Wang, W.; Chen, J.; Liu, R.; Xie, S.; Wang, J.; Ji, Z. Synthesis of octahedral-like ZnO/ZnFe2O4 heterojunction photocatalysts with superior photocatalytic activity. Solid State Sci. 2019, 96, 105901. [Google Scholar] [CrossRef]
- Umar, K.; Adnan, R. Biomass Mediated Green Synthesis of ZnO/TiO2 Nanocomposite and Enhanced Photocatalytic Activity for the Decolorization of Rhodamine B under Visible Light. In Key Engineering Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2022; pp. 36–42. [Google Scholar]
- Hong, J.; Xia, X.; Wang, Y.; Xu, R. Mesoporous carbon nitride with in situ sulfur doping for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. 2012, 22, 15006–15012. [Google Scholar] [CrossRef]
- Djerdj, I.; Jagličić, Z.; Arčon, D.; Niederberger, M. Co-doped ZnO nanoparticles: Minireview. Nanoscale 2010, 2, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Singh, V.; Kumar, S.; Dhiman, R. Influence of nickel, silver, and sulphur doping on the photocatalytic efficiency of mesoporous ZnO nanoparticles. Arab. J. Sci. Eng. 2020, 45, 249–259. [Google Scholar] [CrossRef]
- Ahmad, I. Comparative study of metal (Al, Mg, Ni, Cu and Ag) doped ZnO/g-C3N4 composites: Efficient photocatalysts for the degradation of organic pollutants. Sep. Purif. Technol. 2020, 251, 117372. [Google Scholar] [CrossRef]
- Zhang, S.; Su, C.; Ren, H.; Li, M.; Zhu, L.; Ge, S.; Wang, M.; Zhang, Z.; Li, L.; Cao, X. In-situ fabrication of g-C3N4/ZnO nanocomposites for photocatalytic degradation of methylene blue: Synthesis procedure does matter. Nanomaterials 2019, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhu, C.; Zhang, G.; Li, M.; Tang, Q.; Cao, J. Palladium modified ZnFe2O4/g-C3N4 nanocomposite as an efficiently magnetic recycling photocatalyst. J. Solid State Chem. 2020, 288, 121389. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Liu, Y.; Alharbi, N.S.; Rabah, S.O.; Wang, S.; Wang, X. Synthesis and fabrication of g-C3N4-based materials and their application in elimination of pollutants. Sci. Total Environ. 2020, 731, 139054. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zong, R.; Zhu, Y. Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods. J. Phys. Chem. C 2013, 117, 9952–9961. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Thomas, A.; Fu, X.; Antonietti, M. Metal-containing carbon nitride compounds: A new functional organic–metal hybrid material. Adv. Mater. 2009, 21, 1609–1612. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Wu, L.; Fu, M.; Wu, Z. Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance. Catal. Sci. Technol. 2012, 2, 1332–1335. [Google Scholar] [CrossRef]
- Shen, B.; Hong, Z.; Chen, Y.; Lin, B.; Gao, B. Template-free synthesis of a novel porous g-C3N4 with 3D hierarchical structure for enhanced photocatalytic H2 evolution. Mater. Lett. 2014, 118, 208–211. [Google Scholar] [CrossRef]
- Chang, F.; Xie, Y.; Li, C.; Chen, J.; Luo, J.; Hu, X.; Shen, J. A facile modification of g-C3N4 with enhanced photocatalytic activity for degradation of methylene blue. Appl. Surf. Sci. 2013, 280, 967–974. [Google Scholar] [CrossRef]
- Kuriki, R.; Sekizawa, K.; Ishitani, O.; Maeda, K. Visible-light-driven CO2 reduction with carbon nitride: Enhancing the activity of ruthenium catalysts. Angew. Chem. Int. Ed. 2015, 54, 2406–2409. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Sun, X.; Chen, Q.; Liu, Y.; Lou, X.; Zhang, L.; Zhang, X.; Li, Y.; Guan, J. Photocatalytic Applications of g-C3N4 Based on Bibliometric Analysis. Catalysts 2022, 12, 1017. [Google Scholar] [CrossRef]
- Suzuki, V.Y.; Amorin, L.H.; Fabris, G.S.; Dey, S.; Sambrano, J.R.; Cohen, H.; Oron, D.; La Porta, F.A. Enhanced Photocatalytic and Photoluminescence Properties Resulting from Type-I Band Alignment in the Zn2GeO4/g-C3N4 Nanocomposites. Catalysts 2022, 12, 692. [Google Scholar] [CrossRef]
- Machín, A.; Fontánez, K.; Duconge, J.; Cotto, M.C.; Petrescu, F.I.; Morant, C.; Márquez, F. Photocatalytic degradation of fluoroquinolone antibiotics in solution by Au@ ZnO-rGO-gC3N4 composites. Catalysts 2022, 12, 166. [Google Scholar] [CrossRef]
- Yaqoob, A.; Mohd Noor, N.H.B.; Umar, K.; Adnan, R.; Ibrahim, M.N.M.; Rashid, M. Graphene oxide-ZnO nanocomposite: An efficient visible light photocatalyst for degradation of rhodamine B. Appl. Nanosci. 2021, 11, 1291–1302. [Google Scholar] [CrossRef]
- Li, L.; Sun, S.-Q.; Wang, Y.-X.; Wang, C.-Y. Facile synthesis of ZnO/g-C3N4 composites with honeycomb-like structure by H2 bubble templates and their enhanced visible light photocatalytic performance. J. Photochem. Photobiol. A Chem. 2018, 355, 16–24. [Google Scholar] [CrossRef]
- Wang, J.-C.; Cui, C.-X.; Li, Y.; Liu, L.; Zhang, Y.-P.; Shi, W. Porous Mn doped g-C3N4 photocatalysts for enhanced synergetic degradation under visible-light illumination. J. Hazard. Mater. 2017, 339, 43–53. [Google Scholar] [CrossRef]
- Wang, M.; Guo, P.; Zhang, Y.; Lv, C.; Liu, T.; Chai, T.; Xie, Y.; Wang, Y.; Zhu, T. Synthesis of hollow lantern-like Eu (III)-doped g-C3N4 with enhanced visible light photocatalytic perfomance for organic degradation. J. Hazard. Mater. 2018, 349, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Tonda, S.; Kumar, S.; Kandula, S.; Shanker, V. Fe-doped and-mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A 2014, 2, 6772–6780. [Google Scholar] [CrossRef]
- Wu, L.; Liu, X.; Lv, G.; Zhu, R.; Tian, L.; Liu, M.; Li, Y.; Rao, W.; Liu, T.; Liao, L. Study on the adsorption properties of methyl orange by natural one-dimensional nano-mineral materials with different structures. Sci. Rep. 2021, 11, 10640. [Google Scholar] [CrossRef] [PubMed]
- Boudouaia, N.; Bengharez, Z.; Jellali, S. Preparation and characterization of chitosan extracted from shrimp shells waste and chitosan film: Application for Eriochrome black T removal from aqueous solutions. Appl. Water Sci. 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Liang, L.; Ma, J.; Wang, F.; Sun, J. Remarkably enhanced photocatalytic activity of ordered mesoporous carbon/g-C3N4 composite photocatalysts under visible light. Dalton Trans. 2014, 43, 7236–7244. [Google Scholar] [CrossRef]
- Sher, M.; Shahid, S.; Javed, M. Synthesis of a novel ternary (g-C3N4 nanosheets loaded with Mo doped ZnOnanoparticles) nanocomposite for superior photocatalytic and antibacterial applications. J. Photochem. Photobiol. B Biol. 2021, 219, 112202. [Google Scholar] [CrossRef]
- Slobodan, D.; Nada, T.; Zhiyu, P. Absorption spectroscopy of EBT model GAFCHROMIC™ film. Med. Phys. 2007, 34, 2207. [Google Scholar]
- Jethave, G.; Fegade, U.; Attarde, S.; Ingle, S. Decontamination study of Eriochrome Black-T from waste water by using AlTiPbO nanoparticles (ATPO-NPs) for sustainable clean environment. J. Water Environ. Nanotechnol. 2019, 4, 263–274. [Google Scholar]
- Jamshidi, E.; Manteghi, F. Methyl Orange Adsorption by Fe2O3@ Co-Al-Layered Double Hydroxide. Multidiscip. Digit. Publ. Inst. Proc. 2020, 41, 64. [Google Scholar]
- Qamar, M.A.; Shahid, S.; Javed, M. Synthesis of dynamic g-C3N4/Fe@ ZnO nanocomposites for environmental remediation applications. Ceram. Int. 2020, 46, 22171–22180. [Google Scholar] [CrossRef]
- Palanivel, B.; Maiyalagan, T.; Jayarman, V.; Ayyappan, C.; Alagiri, M. Rational design of ZnFe2O4/g-C3N4 nanocomposite for enhanced photo-Fenton reaction and supercapacitor performance. Appl. Surf. Sci. 2019, 498, 143807. [Google Scholar] [CrossRef]
- Saeed, H.; Nadeem, N.; Zahid, M.; Yaseen, M.; Noreen, S.; Jilani, A.; Shahid, I. Mixed metal ferrite (Mn0. 6Zn0. 4Fe2O4) intercalated g-C3N4 nanocomposite: Efficient sunlight driven photocatalyst for methylene blue degradation. Nanotechnology 2021, 32, 505714. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liming, W.; Yong, S.; Lihui, X.; Mingrui, X. Preparation and photocatalytic properties of magnetic g-C3N4/MnZnFe2O4 composite. J. Phys. Conf. Ser. 2021, 1790, 012075. [Google Scholar] [CrossRef]
- Shanmugam, N.; Suthakaran, S.; Kannadasan, N.; Sathishkumar, K. Synthesis and characterization of Te doped ZnO nanosheets for photocatalytic application. JO Heterocycl. 2015, 105, 15–20. [Google Scholar] [CrossRef]
- Ahmad, A.; Wei, Y.; Syed, F.; Tahir, K.; Rehman, A.U.; Khan, A.; Ullah, S.; Yuan, Q. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb. Pathog. 2017, 102, 133–142. [Google Scholar] [CrossRef]
- Kotresh, M.; Patil, M.; Inamdar, S. Reaction temperature based synthesis of ZnO nanoparticles using co-precipitation method: Detailed structural and optical characterization. Optik 2021, 243, 167506. [Google Scholar] [CrossRef]
- Sher, M.; Khan, S.A.; Shahid, S.; Javed, M.; Qamar, M.A.; Chinnathambi, A.; Almoallim, H.S. Synthesis of novel ternary hybrid g-C3N4@ Ag-ZnO nanocomposite with Z-scheme enhanced solar light-driven methylene blue degradation and antibacterial activities. J. Environ. Chem. Eng. 2021, 9, 105366. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Facile one-pot synthesis of cerium oxide/sulfur-doped graphitic carbon nitride (g-C3N4) as efficient nanophotocatalysts under visible light irradiation. J. Colloid Interface Sci. 2017, 507, 59–73. [Google Scholar] [CrossRef]
- Kalisamy, P.; Lallimathi, M.; Suryamathi, M.; Palanivel, B.; Venkatachalam, M. ZnO-embedded S-doped g-C3N4 heterojunction: Mediator-free Z-scheme mechanism for enhanced charge separation and photocatalytic degradation. RSC Adv. 2020, 10, 28365–28375. [Google Scholar] [CrossRef]
- Norouzzadeh, P.; Golzan, M.; Mabhouti, K.; Naderali, R. Effect of Mn-substitution on impedance spectroscopy and magnetic properties of Al-doped ZnO nanoparticles. Nanotechnology 2020, 31, 325704. [Google Scholar] [CrossRef]
- Rana, A.K.; Kumar, Y.; Rajput, P.; Jha, S.N.; Bhattacharyya, D.; Shirage, P.M. Search for origin of room temperature ferromagnetism properties in Ni-doped ZnO nanostructure. ACS Appl. Mater. Interfaces 2017, 9, 7691–7700. [Google Scholar] [CrossRef] [Green Version]
- Simmons, E. Reflectance spectroscopy: Application of the Kubelka-Munk theory to the rates of photoprocesses of powders. Appl. Opt. 1976, 15, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Luu Thi, L.A.; Neto, M.M.; Van, T.P.; Nguyen Ngoc, T.; Nguyen Thi, T.M.; Nguyen, X.S.; Nguyen, C.T. In Situ g-C3N4@ Zno Nanocomposite: One-Pot Hydrothermal Synthesis and Photocatalytic Performance under Visible Light Irradiation. Adv. Mater. Sci. Eng. 2021, 2021, 6651633. [Google Scholar] [CrossRef]
- Rosli, N.I.M.; Lam, S.-M.; Sin, J.-C.; Satoshi, I.; Mohamed, A.R. Photocatalytic performance of ZnO/g-C3N4 for removal of phenol under simulated sunlight irradiation. J. Environ. Eng. 2018, 144, 04017091. [Google Scholar] [CrossRef]
- Mohamed, R.; Shawky, A. CNT supported Mn-doped ZnO nanoparticles: Simple synthesis and improved photocatalytic activity for degradation of malachite green dye under visible light. Appl. Nanosci. 2018, 8, 1179–1188. [Google Scholar] [CrossRef]
- Labhane, P.; Patle, L.; Sonawane, G.; Sonawane, S. Fabrication of ternary Mn doped ZnO nanoparticles grafted on reduced graphene oxide (RGO) sheet as an efficient solar light driven photocatalyst. Chem. Phys. Lett. 2018, 710, 70–77. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Panchal, P.; Malik, R.; Sharma, A.; Tomer, V.K.; Nehra, S. Silver doped graphitic carbon nitride for the enhanced photocatalytic activity towards organic dyes. J. Nanosci. Nanotechnol. 2019, 19, 5241–5248. [Google Scholar] [CrossRef]
- Sun, J.-X.; Yuan, Y.-P.; Qiu, L.-G.; Jiang, X.; Xie, A.-J.; Shen, Y.-H.; Zhu, J.-F. Fabrication of composite photocatalyst g-C3N4–ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756–6763. [Google Scholar] [CrossRef]
- Farouq, R. Coupling Adsorption-Photocatalytic Degradation of Methylene Blue and Maxilon Red. J. Fluoresc. 2022, 32, 1381–1388. [Google Scholar] [CrossRef]
- Iqbala, S.; Iqbala, M.; Sibtaina, A.; Iqbalb, A.; Farooqic, Z.H.; Ahmadd, S.; Mustafaa, K.; Musaddiqa, S. Solar driven photocatalytic degradation of organic pollutants via Bi. Desalin. Water Treat. 2021, 216, 140–150. [Google Scholar] [CrossRef]
- Sobahi, T.R.; Amin, M. Synthesis of ZnO/ZnFe2O4/Pt nanoparticles heterojunction photocatalysts with superior photocatalytic activity. Ceram. Int. 2020, 46, 3558–3564. [Google Scholar] [CrossRef]
- Falak, P.; Hassanzadeh-Tabrizi, S.; Saffar-Teluri, A. Synthesis, characterization, and magnetic properties of ZnO-ZnFe2O4 nanoparticles with high photocatalytic activity. J. Magn. Magn. Mater. 2017, 441, 98–104. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Lam, S.-M.; Sin, J.-C.; Zeng, H.; Mohamed, A.R. Magnetically recoverable Pd-loaded BiFeO3 microcomposite with enhanced visible light photocatalytic performance for pollutant, bacterial and fungal elimination. Sep. Purif. Technol. 2020, 236, 116195. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Liu, X.; Yang, X.; Yang, Y. A ternary magnetic recyclable ZnO/Fe3O4/g-C3N4 composite photocatalyst for efficient photodegradation of monoazo dye. Nanoscale Res. Lett. 2019, 14, 147. [Google Scholar] [CrossRef]
- Kulkarni, S.D.; Kumbar, S.; Menon, S.G.; Choudhari, K.; Santhosh, C. Magnetically separable core–shell ZnFe2O4@ ZnO nanoparticles for visible light photodegradation of methyl orange. Mater. Res. Bull. 2016, 77, 70–77. [Google Scholar] [CrossRef]
- Kumaresan, N.; Sinthiya, M.M.A.; Kumar, M.P.; Ravichandran, S.; Babu, R.R.; Sethurman, K.; Ramamurthi, K. Investigation on the g-C3N4 encapsulated ZnO nanorods heterojunction coupled with GO for effective photocatalytic activity under visible light irradiation. Arab. J. Chem. 2020, 13, 2826–2843. [Google Scholar] [CrossRef]
- Anitha, S.; Muthukumaran, S. Microstructure, crystallographic and photoluminescence examination of Ni doped ZnO nanoparticles co-doped with Co by sol–gel method. J. Mater. Sci. Mater. Electron. 2017, 28, 12995–13005. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Amin, S.; Guo, F.; Shi, W.; Li, Y. Engineering of 2D/3D architectures type II heterojunction with high-crystalline g-C3N4 nanosheets on yolk-shell ZnFe2O4 for enhanced photocatalytic tetracycline degradation. Mater. Res. Bull. 2022, 150, 111789. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Di, A.; Yang, X.; Chen, G. Enhanced photocatalytic performance of hierarchical ZnFe2O4/g-C3N4 heterojunction composite microspheres. Catal. Lett. 2018, 148, 2179–2189. [Google Scholar] [CrossRef]













| Sr. No | Photocatalyst | Contaminant | Light Source | Dye (mg/L) | Catalyst (mg/L) | Radiation Time (min.) | Decoloration% | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Mn-ZnO/CSAC | BG | Sunlight | 30 | 200 | 175 | 96 | [62] |
| 2 | ZnO/Fe2O4/Pt | Ciprofloxacin | Xe lamp | 10 | 150 | 75 | 100 | [63] |
| 3 | Mn-ZnO/RGO | RhB | Visible | 20 | 20 | 140 | 99 | [58] |
| 4 | ZnO/ZnFe2O4 | MB | Visible | 10 | 50 | 100 | 98 | [64] |
| 5 | Pt-BiFeO3 | MG | Visible | 10 | 100 | 240 | 96 | [65] |
| 6 | ZnO/Fe3O4/g-C3N4 | MO | Visible | 30 | 10 | 150 | 97.87 | [66] |
| 7 | Mn-ZnO/CNT | MG | Visible | 50 | 100 | 150 | >95 | [57] |
| 8 | ZnFe2O4@ZnO | MO | Visible | 56 | 660 | 240 | 99 | [67] |
| 9 | Mn/50% g-C3N4/ZnO | EBT | Sunlight | 10 | 200 | 50 | 100 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qamar, M.A.; Shahid, S.; Javed, M.; Shariq, M.; Fadhali, M.M.; Madkhali, O.; Ali, S.K.; Syed, I.S.; Awaji, M.Y.; Shakir Khan, M.; et al. Accelerated Decoloration of Organic Dyes from Wastewater Using Ternary Metal/g-C3N4/ZnO Nanocomposites: An Investigation of Impact of g-C3N4 Concentration and Ni and Mn Doping. Catalysts 2022, 12, 1388. https://doi.org/10.3390/catal12111388
Qamar MA, Shahid S, Javed M, Shariq M, Fadhali MM, Madkhali O, Ali SK, Syed IS, Awaji MY, Shakir Khan M, et al. Accelerated Decoloration of Organic Dyes from Wastewater Using Ternary Metal/g-C3N4/ZnO Nanocomposites: An Investigation of Impact of g-C3N4 Concentration and Ni and Mn Doping. Catalysts. 2022; 12(11):1388. https://doi.org/10.3390/catal12111388
Chicago/Turabian StyleQamar, Muhammad Azam, Sammia Shahid, Mohsin Javed, Mohammad Shariq, Mohammed M. Fadhali, Osama Madkhali, Syed Kashif Ali, Imam Saheb Syed, Majed Yusef Awaji, Mohd. Shakir Khan, and et al. 2022. "Accelerated Decoloration of Organic Dyes from Wastewater Using Ternary Metal/g-C3N4/ZnO Nanocomposites: An Investigation of Impact of g-C3N4 Concentration and Ni and Mn Doping" Catalysts 12, no. 11: 1388. https://doi.org/10.3390/catal12111388