Green Chemistry in Organic Synthesis: Recent Update on Green Catalytic Approaches in Synthesis of 1,2,4-Thiadiazoles
Abstract
:1. Introduction
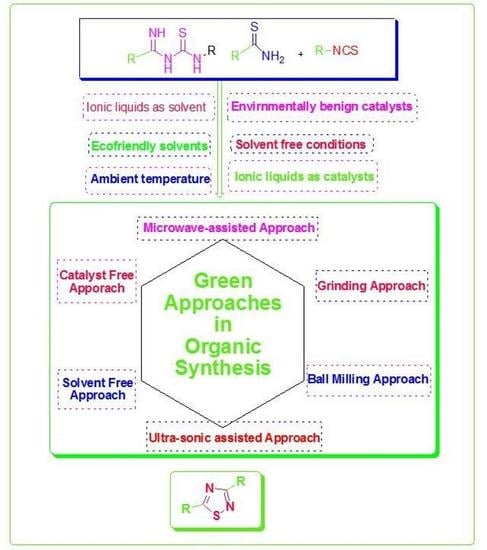
2. Green Approaches in Organic Synthesis
- Solvent-free approach;
- Grinding approach;
- Ball milling approach;
- Solid–wet approach;
- Ultrasonic-assisted approach;
- Microwave-assisted approach;
- MOF green synthesis approach;
- Electrochemical green catalytic synthetic approach.
3. Green Catalysts in Organic Synthetic Approaches
4. Green Solvents in Organic Synthetic Approaches
5. Green Synthetic Approaches for Synthesis of 1,2,4-Thiadiazoles
5.1. PIFA Catalyst for Formation of N–S Bond via Intramolecular Cyclization
5.2. Molecular I2 Catalysis and Oxidative N–S Bond Formation
5.3. Molecular I2 Catalysis for Regio-Specific and Expeditious Synthetic Approach
5.4. Molecular I2 Catalysis for One-Pot Green Protocol and Intramolecular Oxidative Coupling
5.5. Ultrasonic-Assisted Synthesis in Water
5.6. HTACas PTC for Green Synthesis Using Molecular Oxygen as an Oxidant
5.7. Transition-Metal-Free Green Protocol Using Air as Oxidant
5.8. Solid–Solid Oxidative Coupling
5.9. Green Synthesis in Wet-Paste Conditions
5.10. Oxidative Dimerization Using CC–DMSO in PEG-400
5.11. Basic Alumina Catalyst for Synthesis of Substituted 1,2,4-Thiadiazoles via Grinding Approach
5.12. Synthesis of 1,2,4-Thiadiazole-5-Carboxylates by Microwave-Assisted Approach
5.13. Green Synthesis of 1,2,4-Thiadiazole via Ionic Liquids
5.14. Copper Salts as Catalyst for Green One-Pot Synthetic Protocol for N–S Bond Formation
5.15. Oxidative Dimerization of Thioamides by Using Oxone as Safe Oxidant
5.16. Molecular I2 as Catalyst for Synthesis of 1,2,4-Thiadiazoles via Oxidative N–S Bond Formation
5.17. Synthesis of N-Fused Imino-1,2,4-Thiadiazolo Isoquinoline via Montmorillonite K10-Catalyst
5.18. H2O2-Catalyzed Synthesis of 1,2,4-Thiadiazoles
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deligeorgiev, T.; Gadjev, N.; Vasilev, A.; Kaloyanova, S.; Vaquero, J.J.; Alvarez-Builla, J. Green Chemistry in Organic Synthesis. Mini-Rev. Org. Chem. 2010, 7, 44–53. [Google Scholar]
- Wardencki, W.; Curylo, J.; Namiesnic, J. Green chemistry—Current and future. Pol. J. Environ. Stud. 2005, 14, 389–395. [Google Scholar]
- Williams, T.M.; Blacker, J. The Importance of Green Chemistry in Process Research and Development, Pharmaceutical Process Development: Current Chemical and Engineering Challenges; Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- The Full Definition Is: Green Chemistry, Also Known as Sustainable Chemistry, Is the Design of Chemical Products and Processes that Reduce or Eliminate the Use or Generation of Hazardous Substances. Available online: https://www.epa.gov/greenchemistry/basics-green-chemistry (accessed on 8 February 2011).
- Anastas, T.P.; Warner, C.J. Green Chemistry applies across the life cycle of a chemical product, including its design, manufacture and use. In Green Chemistry Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Clarke, H.J. Green chemistry: Challenges and opportunities. Green Chem. 1999, 1, 1–8. [Google Scholar] [CrossRef]
- Sheldon, A.R. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Sheldon, A.R. Green chemistry and resource efficiency: Towards a green economy. Green Chem. 2016, 18, 3180–3183. [Google Scholar] [CrossRef]
- Trost, B. The Atom Economy—A Search for Synthetic Efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Draye, M.; Chatel, G.; Duwald, R. Ultrasound for Drug Synthesis: A Green Approach. Pharmaceuticals 2020, 13, 23. [Google Scholar] [CrossRef] [Green Version]
- Chatel, G.; Leclerc, L.; Narechoux, E.; Bas, C.; Kardos, N.; Goux-Henry, C.; Andrioletti, B.; Draye, M. Ultrasonic Properties of Hydrophobic Bis(trifluoromethylsulfonyl)imide-Based Ionic Liquids. J. Chem. Eng. Data. 2012, 57, 3385–3390. [Google Scholar] [CrossRef]
- Tan, D.A.; Kulkarnia, A.; Torok, B. Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches. Green Chem. 2012, 14, 17–37. [Google Scholar] [CrossRef]
- Ahluwalia, V.K.; Kidwai, M. New Trends in Green Chemistry; Kluwer, Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practise. Chem. Soc. Rev. 2020, 29, 301–312. [Google Scholar] [CrossRef]
- Kurniawan, S.Y.; Priyangga, A.T.P.K.; Krisbiantoro, A.; Imawan, C.A. Green Chemistry Influences in Organic Synthesis: A Review. J. Multidiscip. Appl. Nat. Sci. 2021, 1, 1–12. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V.A. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Varma, R.S. Journey on greener pathways: From the use of alternate energy inputs and benign reaction media to sustainable applications of nano-catalysts in synthesis and environmental remediation. Green Chem. 2014, 16, 2027–2041. [Google Scholar] [CrossRef]
- Gawande, M.J.; Bonifacio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by design: Catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.L.Y.; Smith, R.L.; Poliakoff, M. Principles of green chemistry: PRODUCTIVELY. Green Chem. 2005, 7, 761–762. [Google Scholar] [CrossRef]
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Ryabukhin, S.V.; Panov, D.M.; Plaskon, A.S.; Grygorenko, O.O. Approach to the library of 3-hydroxy-1,5-dihydro-2h-pyrrol-2-ones through a three-component condensation. ACS Comb. Sci. 2012, 14, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Graaff, C.; Ruijter, E.; Orru, R.V.A. Recent developments in asymmetric multicomponent reactions. Chem. Soc. Rev. 2012, 41, 3969–4009. [Google Scholar] [CrossRef]
- Toure, B.B.; Hall, D.G. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 2009, 109, 4439–4486. [Google Scholar] [CrossRef]
- Trost, B.M. On inventing reactions for atom economy. Acc. Chem. Res. 2002, 35, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Morken, J.P. Catalytic bismetallative multicomponent coupling reactions: Scope, applications, and mechanisms. Chem. Soc. Rev. 2014, 43, 4368–4380. [Google Scholar] [CrossRef] [PubMed]
- Duvauchelle, V.; Meffre, P.; Benfodda, Z. Green methodologies for the synthesis of 2-aminothiophene. Environ. Chem. Lett. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hafez, A.A.E.; Al-Mousawi, M.S.; Moustafa, S.M.; Sadek, U.K.; Elnagdi, H.M. Green methodologies in organic synthesis: Recent developments in our laboratories. Green Chem. Lett. Rev. 2013, 6, 89–210. [Google Scholar] [CrossRef] [Green Version]
- Banik, B.K.; Sahoo, B.M.; Kumar, B.V.V.R.; Panda, K.C.; Jena, J.; Mahapatra, M.K.; Borah, P. Green Synthetic Approach: An Efficient Eco-Friendly Tool for Synthesis of Biologically Active Oxadiazole Derivatives. Molecules 2021, 26, 1163. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.T.; Anastas, P.T. Innovations and green chemistry. Chem Rev. 2007, 107, 2169–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horváth, I.T.; Anastas, P.T. Introduction: Green chemistry. Chem Rev. 2007, 107, 2167–2168. [Google Scholar] [CrossRef] [Green Version]
- Das, A.; Banik, K. Dipole moment of medicinally active compounds: A sustainable approach in medicinal research: Green and sustainable approach. In Green Approaches in Medicinal Chemistry for Sustainable Drug Design, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 26, pp. 921–964. [Google Scholar]
- Varma, S.R. Greener and Sustainable Trends in Synthesis of Organics and Nanomaterials, ACS Sustainable Chemistry & Engineering. ACS Sustain. Chem. Eng. 2016, 4, 5866–5878. [Google Scholar] [CrossRef]
- Kim, Y.; Li, C.-J. Perspectives on green synthesis and catalysis. Green Synth. Catal. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Dai, S.; Tissot, A.; Serre, C. Metal-Organic Frameworks: From Ambient Green Synthesis to Applications. Bull. Chem. Soc. Jpn. 2021, 94, 2623–2636. [Google Scholar] [CrossRef]
- Cheng, X.; Lei, A.; Mei, T.-S.; Xu, H.-C.; Xu, K.; Zeng, C. Recent Applications of Homogeneous Catalysis in Electrochemical Organic Synthesis. CCS Chem. 2022, 4, 1120–1152. [Google Scholar] [CrossRef]
- Shrikhande, J.J.; Gawande, M.B.; Jayaram, R.V. Cross-aldol and Knoevenagel condensation reactions in aqueous micellar media. Catal. Commun. 2008, 9, 1010–1016. [Google Scholar] [CrossRef]
- Butler, R.N.; Coyne, A.G. Water: Nature’s Reaction Enforcer—Comparative Effects for Organic Synthesis “In-Water” and “On-Water”. Chem. Rev. 2010, 110, 6302–6337. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, H.; Khaturia, S. A mini-review on organic synthesis in water. MOJ Biorg. Org. Chem. 2017, 1, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Nasri, S.; Bayat, M.; Miankooshki, F.R.; Samet, N.H. Recent developments in green approaches for sustainable synthesis of indole-derived scaffolds. Mol. Divers. 2022. [Google Scholar] [CrossRef]
- Casti, F.; Basoccu, F.; Mocci, R.; De Luca, L.; Porcheddu, A.; Cuccu, F. Appealing Renewable Materials in Green Chemistry. Molecules 2022, 27, 1988. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.; Cortés, J.F.; Miranda, R. Green Chemistry Metrics, A Review. Processes 2022, 10, 1274. [Google Scholar] [CrossRef]
- Onuegbu, T.U.; Ogbuagu, A.S.; Ekeoma, M.O. The role of catalysts in green synthesis of chemicals for sustainable future. J. Basic Phy. Res. 2011, 2, 86–92. [Google Scholar]
- Kharissova, O.V.; Kharisov, B.I.; Oliva, G.C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastas, T.P.; Kirchhoff, M.M.; Williamson, C.T. Catalysis as a foundational pillar of green chemistry. Appl. Catal. A-Gen. 2001, 221, 3–13. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic versus stoichiometric reagents as a key concept for Green Chemistry. Green Chem. 2016, 18, 590–593. [Google Scholar] [CrossRef]
- Ruslan, A.A.A.N.; Kan, S.-K.; Hamzah, S.A.; Chia, W.P. Natural food additives as green catalysts in organic synthesis: A review. Environ. Chem. Lett. 2021, 19, 3359–3380. [Google Scholar] [CrossRef]
- Matsuo, J.; Tsuchiya, T.; Odashima, K.; Kobayashi, S. Lewis Acid Catalysis in Supercritical Carbon Dioxide. Use of Scandium Tris(heptadecafluorooctanesulfonate) as a Lewis Acid Catalyst in Diels-Alder and Aza Diels-Alder Reactions. Chem. Lett. 2000, 29, 178. [Google Scholar] [CrossRef]
- Kobayashi, S.; Manabe, K. Green Lewis acid catalysis in organic synthesis. Pure Appl. Chem. 2000, 72, 1373–1380. [Google Scholar] [CrossRef]
- Chassaing, S.; Beneteau, V.; Louis, B.; Pale, P. Zeolites as Green Catalysts for Organic Synthesis: The Cases of H-, Cu- & Sc-Zeolites. Curr. Org. Chem. 2017, 21, 779–793. [Google Scholar] [CrossRef]
- Itoha, T.; Hanefeld, U. Enzyme catalysis in organic synthesis. Green Chem. 2017, 19, 331–332. [Google Scholar] [CrossRef]
- Rinaldi, R.; Palkovits, R.; Schüth, F. Depolymerization of cellulose using solid catalysts in ionic liquids. Angew. Chem. Int. Ed. 2010, 47, 8047–8050. [Google Scholar] [CrossRef]
- Akiyama, G.; Matsuda, R.; Sato, H.; Takata, M.; Kitagawaet, S. Cellulose hydrolysis by a new porous coordination polymer decorated with sulfonic acid functional groups. Adv. Mater. 2011, 23, 3294–3297. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fu, Z.; Yin, D.; Xu, Q.; Liu, F.; Lu, C.; Mao, L. Microwave-assisted hydrolysis of crystalline cellulose catalyzed by biomass char sulfonic acids. Green Chem. 2010, 12, 696–700. [Google Scholar] [CrossRef]
- Pang, J.; Wang, A.; Zheng, M.; Zhang, T. Hydrolysis of cellulose into glucose over carbons sulfonated at elevated temperatures. Chem. Commun. 2010, 46, 6935–6937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Deng, X.; Fang, Z.; Zeng, H.; Tian, X.; Kozinski, J. Hydrolysis of microcrystalline cellulose over Zn-Ca-Fe oxide catalyst. Petrochem. Technol. 2011, 40, 43–48. [Google Scholar] [CrossRef]
- Tian, J.; Fang, C.; Cheng, M.; Wang, X. Hydrolysis of cellulose over CsxH3−xPW12O40 (x = 1–3) heteropoly acid catalysts. Chem. Eng. Technol. 2011, 34, 482–486. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.H.; Zhao, S.; Jiang, C.Y.; Zhang, X.; Wang, X.H. Hydrolysis of cellulose by the heteropoly acid H3PW12O40. Cellulose 2010, 17, 587–594. [Google Scholar] [CrossRef]
- Kobayashi, H.; Komanoya, T.; Hara, K.; Fukuoka, A. Water tolermesoporous-carbon-supported ruthenium catalysts for the hydrolysis of cellulose to glucose. ChemSusChem 2010, 3, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.M.; Deng, L.; Guo, Q.X.; Fu, Y. Hydrolysis of biomass by magnetic solid acid. Energy. Environ. Sci. 2011, 4, 3552–3557. [Google Scholar] [CrossRef]
- Komanoya, T.; Kobayashi, H.; Hara, K.; Chun, W.J.; Fukuoka, A. Catalysis and characterization of carbon-supported ruthenium for cellulose hydrolysis. Appl. Catal. A Gen. 2011, 407, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, Y.; Itagaki, S.; Yamaguchi, K.; Mizuno, N. Saccharification of natural lignocellulose biomass and polysaccharides by highly negativecharged heteropolyacids in concentrated aqueous solution. ChemSusChem 2011, 4, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wu, H.; Li, C.; Li, Z.; Li, H.; Zhang, H.; Li, Y.; Su, Y.; Yang, S. Heteropoly Acid-Based Catalysts for Hydrolytic Depolymerization of Cellulosic Biomass. Front. Chem. 2020, 8, 580146. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, R.; Khalilzadeh, A.M.; Zareyee, D. Wood ash biocatalyst as a novel green catalyst and its application for the synthesis of benzochromene derivatives. Sci. Rep. 2022, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Dekamin, M.G.; Ilkhanizadeh, S.; Latifidoost, Z.; Daemi, H.; Karimi, Z.; Barikani, M. Alginic acid: A highly efficient renewable and heterogeneous biopolymeric catalyst for one-pot synthesis of the Hantzsch 1,4-dihydropyridines. RSC Adv. 2014, 4, 56658–56664. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Karimi, Z.; Latifidoost, Z.; Ilkhanizadeh, S.; Daemi, H.; Naimi-Jamal, M.R.; Barikani, M. Alginic acid: A mild and renewable bifunctional heterogeneous biopoly mericorgano catalyst for efficient and facile synthesis of polyhydroquinolines. Int. J. Biol. Macromol. 2018, 108, 1273–1280. [Google Scholar] [CrossRef]
- Hosseinzadeh, Z.; Ramazani, A.; Razzaghi-Asl, N.; Slepokura, K.; Lis, T. Boric acid as an efficient and green catalyst for the synthesis of 2-amino-4,6-diarylnicotinonitrile under microwave irradiation in solvent-freeconditions. Turk. J. Chem. 2019, 43, 464–474. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Maghsoodlou, M.T.; Lashkari, M.; Heydari, R.; Hazeri, N. Green synthesis of polysubstitutedquinolines and xanthene derivatives promoted by tartaric acid as a naturally green catalyst under solvent-free conditions. Chem. J. Mold. 2018, 13, 74–86. [Google Scholar] [CrossRef]
- Singh, A.K.; Dar, B.; Ahad, A.; Pardeshi, R.K. An efficient tartaric acid catalyzed green protocol for the synthesis of 2,3- dihydroquinazolin-4(1H)-ones in aqueous medium. Int. J. Chem. Sci. 2018, 16, 247. [Google Scholar]
- Ahankar, H.; Ramazani, A.; Ślepokura, K.; Lis, T.; Joo, S.W. Synthesis of pyrrolidinone derivatives from aniline, an aldehyde and diethylacetylenedicarboxylate in an ethanolic citric acid solution under ultrasound irradiation. Green Chem. 2016, 18, 3582–3593. [Google Scholar] [CrossRef]
- Shokrollahi, S.; Ramazani, A.; Rezaei, S.J.T.; Malekzadeh, A.M.; Asiabi, P.A.; Joo, S.W. Citric acid as an efficient and green catalyst for the synthesis of hexabenzylhexaazaisowurtzitane (HBIW). Iran. J. Catal. 2016, 6, 65–68. [Google Scholar]
- Shaikh, K.A.; Chaudhar, U.N.; Ningdale, V.B. Citric acid catalyzedsynthesis of amidoalkylnaphthols under solvent-free condition:an eco-friendly protocol. IOSR J. Appl. Chem. 2014, 7, 90–93. [Google Scholar] [CrossRef]
- Kangani, M.; Hazeri, N.; Maghsoodlou, M.T. A mild and environmentally benign synthesis of tetrahydrobenzo[b]pyrans and pyrano[c]chromenes using pectin as a green and biodegradable catalyst. J. Chin. Chem. Soc. 2016, 63, 896–901. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Dharmadhikari, P.P.; Chouthe, S.R.; Fatema, B. Water mediated oxalic acid catalyzed one pot synthesis of 1,8-dioxodecahydroacridines. Arab. J. Chem. 2017, 10, S10–S12. [Google Scholar] [CrossRef] [Green Version]
- Sangshetti, J.N.; Kalam, K.F.A.; Chouthe, R.S.; Zaheer, Z.; Ahmed, R.Z. Water-mediated oxalic acid catalysed one-pot synthesis of 2-(substituted phenyl) phthalazin-1(2 H)-ones. J. Taibah Univ. Sci. 2015, 9, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Sarkate, A.P.; Sangshetti, J.N.; Dharbale, N.B.; Wakte, P.S.; Shinde, D.B. Solvent free oxalic acid catalyzed synthesis of 1,5-benzodiazepines. J. Chil. Chem. Soc. 2013, 58, 2200–2203. [Google Scholar] [CrossRef] [Green Version]
- Mohamadpour, F.; Maghsoodlou, M.T.; Heydari, R.; Lashkari, M. Saccharin: A green, economical and efficient catalyst for the one-pot, multi-component synthesis of 3,4-dihydropyrimidin-2-(1H)-one derivatives and 1H-pyrazolo [1,2-b] phthalazine-5,10-dione derivatives and substituted dihydro-2-oxypyrrole. J. Iran. Chem. Soc. 2016, 13, 1549–1560. [Google Scholar] [CrossRef]
- Moradi, L.; Aghamohammad, S.M. Sodium saccharin as an effective catalyst for rapid one-pot pseudo-five component synthesis of dihydropyrano[2,3-g]chromenes under microwave irradiation. Acta Chim. Slov. 2017, 64, 506–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häring, M.; Pettignano, A.; Quignard, F.; Tanchoux, N.; Díaz, D.D. Keratin protein-catalyzed nitroaldol (henry) reaction and comparison with other biopolymers. Molecules 2016, 21, 1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhang, Z.; Chi, C.; Wu, G.; Ren, J.; Wang, Z.; Huang, M.; Jiang, Y. Asymmetric hydration of ortho- or para-substituted styrenes catalyzed by biopolymer-metal complex wool-Pd. React. Funct. Polym. 2008, 68, 424–430. [Google Scholar] [CrossRef]
- Wang, X.; Sui, D.; Huang, M.; Jiang, Y. Highly effective hydration of olefins using a wool-palladium complex as a catalyst. Polym. Adv. Technol. 2006, 17, 163–167. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.X.; Mao, S.F.; Huang, M.Y.; Jiang, Y.Y. Hydrogenation of anisol and benzaldehyde catalyzed by chicken feather-palladium complex. Polym. Adv. Technol. 1997, 8, 638–640. [Google Scholar] [CrossRef]
- Jafari, Z.; Seyedi, S.M.; Sadeghian, H. Application of Magnetic Chicken Feather Powder-Cu to the Click Synthesis of 1,2,3-Triazoles. Polycycl. Aromat. Compd. 2020, 40, 245–256. [Google Scholar] [CrossRef]
- Patnam, P.L.; Bhatt, M.; Singh, R.; Saran, S.; Jain, S.L. Magnetically separable chicken feathers: A biopolymer based heterogeneous catalyst for the oxidation of organic substrates. RSC Adv. 2016, 6, 60888–60895. [Google Scholar] [CrossRef]
- Padma Latha, P.; Bhatt, M.; Jain, S.L. Sustainable catalysis using magnetic chicken feathers decorated with Pd(0) for Suzuki-cross coupling reaction. Tetrahedron Lett. 2015, 56, 5718–5722. [Google Scholar] [CrossRef]
- Rizzo, G.; Albano, G.; Lo Presti, M.; Milella, A.; Omenetto, F.G.; Farinola, G.M. Palladium Supported on Silk Fibroin for Suzuki–Miyaura Cross-Coupling Reactions. Eur. J. Org. Chem. 2020, 2020, 6992–6996. [Google Scholar] [CrossRef]
- Rizzo, G.; Albano, G.; Sibillano, T.; Giannini, C.; Musio, R.; Omenetto, F.G.; Farinola, G.M. Silk–Fibroin-Supported Palladium Catalyst for Suzuki-Miyaura and Ullmann Coupling Reactions of Aryl Chlorides. Eur. J. Org. Chem. 2022, 2022, 60–70. [Google Scholar] [CrossRef]
- Clavé, G.; Pelissier, F.; Campidelli, S.; Grison, C. Ecocatalyzed Suzuki cross coupling of heteroaryl compounds. Green Chem. 2017, 19, 4093–4103. [Google Scholar] [CrossRef]
- Grison, C.; Grison, C.; Escande, V.; Petit, E.; Garoux, L. Psychotriadouarrei and Geissois pruinosa, novel resources for the plant-based catalytic chemistry. RSC Adv. 2013, 3, 22340–22345. [Google Scholar] [CrossRef]
- Abu-Dief, M.A.; Abdel-Fatah, M.S. Development and functionalizatituron of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 55–67. [Google Scholar]
- Kim, D.K.; Mikhaylova, M. Anchoring of phosphonate and phosphinate coupling molecules on titania particles. Chem. Mater. 2003, 15, 1617–1627. [Google Scholar] [CrossRef]
- Khojastehnezhad, A.; Rahimizadeh, M.; Eshghi, H.; Moeinpour, F.; Bakavoli, M. Ferric hydrogen sulfate supported on silica-coated nickel ferrite nanoparticles as new and green magnetically separable catalyst for 1,8 dioxodecahydroacridine synthesis. Chin. J. Catal. 2014, 35, 376–382. [Google Scholar] [CrossRef]
- Venkatachalapathy, C.; Pitchumani, K. Fries re-arrangement of esters in montmorillonite clays: Steric control on selectivity. Tetrahedran 1997, 53, 17171–17176. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Kanagaraj, K.; Pitchumani, K. Zn2+-K10-clay (clayzic) as an efficient water-tolerant, solid acid catalyst for the synthesis of benzimidazoles and quinoxalines at room temperature. Tetrahedron Lett. 2011, 52, 69–73. [Google Scholar] [CrossRef]
- Carrado, K.A.; Hayatsu, R.; Botto, R.E.; Winans, R. Reactivity of anisoles on clay and pillared clay surfaces. Clays Clay Miner. 1990, 38, 250–256. [Google Scholar] [CrossRef]
- Guzman, J.; Gates, B.C. Structure and Reactivity of a Mononuclear Gold-Complex Catalyst Supported on Magnesium Oxide. Angew. Chem. Int. Ed. 2003, 42, 690–693. [Google Scholar] [CrossRef]
- Walkey, C.; Das, S.; Seal, S.; Erlichman, J.; Heckman, K.; Ghibelli, L.; Traversa, E.; McGinnis, J.F.; Self, W.T. Catalytic Properties and Biomedical Applications of Cerium Oxide Nanoparticles. Environ. Sci. Nano 2015, 2, 33–53. [Google Scholar] [CrossRef] [Green Version]
- Layek, K.; Kantam, M.L.; Shirai, M.; Nishio-Hamane, D.; Sasaki, T.; Maheswaran, H. Gold Nanoparticles Stabilized on Nanocrystalline Magnesium Oxide as an Active Catalyst for Reduction of Nitroarenes in Aqueous Medium at Room Temperature. Green Chem. 2012, 14, 3164–3174. [Google Scholar] [CrossRef]
- Lopez, N.; Nørskov, J.K. Catalytic CO Oxidation by a Gold Nanoparticle: A Density Functional Study. J. Am. Chem. Soc. 2002, 124, 11262–11263. [Google Scholar] [CrossRef]
- Martínez-Méndez, S.; Henríquez, Y.; Domínguez, O.; D’Ornelas, L.; Krentzien, H. Catalytic Properties of Silica Supported Titanium, Vanadium and Niobium Oxide Nanoparticles towards the Oxidation of Saturated and Unsaturated Hydrocarbons. J. Mol. Catal. A Chem. 2006, 252, 226–234. [Google Scholar] [CrossRef]
- Dupont, J.; Fonseca, G.S.; Umpierre, A.P.; Fichtner, P.F.P.; Teixeira, S.R. Transition-Metal Nanoparticles inImidazolium Ionic Liquids: Recycable Catalysts for Biphasic Hydrogenation Reactions. J. Am. Chem. Soc. 2002, 124, 4228–4229. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, Q.; Kang, X.; Liu, H.; Qian, Q.; Zhang, Z.; Han, B. Molybdenum-Bismuth Bimetallic Chalcogenide Nanosheets for Highly Efficient Electrocatalytic Reduction of Carbon Dioxide to Methanol. Angew. Chem. Int. Ed. 2016, 55, 6771–6775. [Google Scholar] [CrossRef]
- You, D.J.; Kwon, K.; Pak, C.; Chang, H. Platinum-Antimony Tin Oxide Nanoparticle as Cathode Catalyst for Direct Methanol Fuel Cell. Catal. Today 2009, 146, 15–19. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Ghasemzadeh, M.A.; Mehrabi, M. Calcium Oxide Nanoparticles Catalyzed One-Step Multicomponent Synthesis of Highly Substituted Pyridines in Aqueous Ethanol Media. Sci. Iran. 2013, 20, 549–554. [Google Scholar]
- Seabra, A.B.; Durán, N. Nanotoxicology of Metal Oxide Nanoparticles. Metals 2015, 5, 934–975. [Google Scholar] [CrossRef] [Green Version]
- Mazumder, V.; Sun, S. Oleylamine-Mediated Synthesis of Pd Nanoparticles for Catalytic Formic Acid Oxidation. J. Am. Chem. Soc. 2009, 131, 4588–4589. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight Photocatalysis by Carbon-Modified Titanium Dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef]
- Pipelzadeh, E.; Babaluo, A.A.; Haghighi, M.; Tavakoli, A.; Derakhshan, M.V.; Behnami, A.K. Silver Dopingon TiO2 Nanoparticles Using a Sacrificial Acid and Its Photo-catalytic Performance under Medium Pressure Mercury UV Lamp. Chem. Eng. J. 2009, 155, 660–665. [Google Scholar] [CrossRef]
- Baricelli, J.P.; Rodríguez, G.; Rodríguez, A.; Lujano, E.; López-Linares, F. Synthesis, characterization and aqueous-biphase hydrogenation of olefins by the ruthenium complexes Ru(CO)3(TPPMS)2 and RuH2(CO)(TPPMS)3. Appl. Catal. A Gen. 2003, 239, 25–34. [Google Scholar] [CrossRef]
- Baricelli, J.P.; Lzaguirre, L.; López, J.; Lujano, E.; López-Linares, F. Synthesis, characterization and catalytic hydrogenation in aqueous-biphasic system of a new water soluble complex RuH(CO)(NCMe)(TPPMS)3[BF4]. J. Mol. Catal. A Chem. 2004, 208, 67–72. [Google Scholar] [CrossRef]
- Kotzabasakis, V.; Georgopoulou, E.; Pitsikalis, M.; Hadjichristidis, N.; Papadogianakis, G. Catalytic conversions in aqueous media: A novel and efficient hydrogenation of polybutadiene-1,4-block-poly(ethylene oxide) catalyzed by Rh/TPPTS complexes in mixed micellar nanoreactors. J. Mol. Catal. A Chem. 2005, 231, 93–101. [Google Scholar] [CrossRef]
- Zhu, Y.; Carpenter, K.; Ching, C.; Bahnmueller, S.; Chan, P. (R)-Binap-Mediated Asymmetric Hydrogenation with a Rhodacarborane Catalyst in Ionic-Liquid Media. Angew. Chem. Int. Ed. 2003, 42, 3792–3795. [Google Scholar]
- Ngo, L.H.; Hu, A.; Lin, W. Catalytic asymmetric hydrogenation of aromatic ketones in room temperature ionic liquids. Tetrahedron Lett. 2005, 46, 595–597. [Google Scholar] [CrossRef]
- Xiong, W.; Lin, Q.; Ma, H.; Zheng, H.; Chen, H.; Li, X. Asymmetric hydrogenation of aromatic ketones in ionic-liquid media catalyzed by Ru-TPPTS–(1S,2S)-DPENDS complexes. Tetrahedron Asymm. 2005, 16, 1959–1962. [Google Scholar] [CrossRef]
- Ackermann, L.; Vicente, R. Catalytic Direct Arylations in Polyethylene Glycol (PEG): Recyclable Palladium(0)Catalyst for C−H Bond Cleavages in the Presence of Air. Org. Lett. 2009, 11, 4922–4925. [Google Scholar] [CrossRef]
- Reddy, C.G.; Balasubramanyam, P.; Salvanna, N.; Das, B. Copper-Mediated C–H Activation of 1,3,4-Oxadiazoles with 1,1-Dibromo-1-alkenes Using PEG-400 as a Solvent Medium: Distinct Approach for the Alkynylation of 1,3,4-Oxadiazoles. Eur. J. Org. Chem. 2011, 2012, 471–474. [Google Scholar] [CrossRef]
- Yang, F.; Koeller, J.; Ackermann, L. Photo-induced Copper-Catalyzed C−H Arylation at Room Temperature. Angew. Chem. Int. Ed. 2016, 55, 4759–4762. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Oliveira, A.C.J.; Shen, Z.; Huang, H.; Ackermann, L. Manganese(II/III/I)-Catalyzed C–H Arylations in Continuous Flow. ACS Catal. 2018, 8, 4402–4407. [Google Scholar] [CrossRef]
- Chen, X.; Souvanhthong, B.; Wang, H.; Zhang, H.; Wang, X.; Huo, M. Polyoxometalate-based ionic liquid as thermoregulated and environmentally friendly catalyst for starch oxidation. Appl. Catal. B Environ. 2013, 161, 138–139. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, W. The oxidation of pyridine and alcohol using the Keggin-type lacunary polytungstophosphate as a temperature-controlled phase transfer catalyst. J. Mol. Catal. A Chem. 2011, 337, 45–51. [Google Scholar] [CrossRef]
- Rafiee, E.; Kahrizi, M. Mechanistic investigation of Heck reaction catalyzed by new catalytic system composed of Fe3O4@OA–Pd and ionic liquids as co-catalyst. J. Mol. Liq. 2016, 218, 625–631. [Google Scholar] [CrossRef]
- Vekariya, L.R. A Review of Ionic Liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2016, 227, 44–60. [Google Scholar] [CrossRef]
- Penín, L.; López, M.; Santos, V.; Parajó, J.C. Evaluation of acidic ionic liquids as catalysts for furfural production from eucalyptus nitens wood. Molecules 2022, 27, 4258. [Google Scholar] [CrossRef]
- Liu, S.; Wang, K.; Yu, H.; Li, B.; Yu, S. Catalytic preparation of levulinic acid from cellobiose via Brønsted-Lewis acidic ionic liquids functional catalysts. Sci. Rep. 2019, 9, 1810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, D.; Chen, G.; Liu, S.; Ji, N.; Ding, H.; Fu, J. Preparation of phosphotungsticacid based poly (ionic liquid) and its application toesterification of palmitic acid. Renew. Energy 2019, 133, 317–324. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Wang, L.; Wang, X.; Li, L.; Xing, Z.; Ji, N.; Liu, S.; Ding, H. Immobilized phosphotungstic acid based ionic liquid: Application for heterogeneous esterification of palmitic acid. Fuel 2018, 216, 364–370. [Google Scholar] [CrossRef]
- Feng, Y.; Li, L.; Wang, X.; Yang, J.; Qiu, T. Stable poly (ionic liquid) with unique crosslinked microsphere structure as efficient catalyst for transesterification of soapberry oil to biodiesel. Energy Convers. Manag. 2017, 153, 649–658. [Google Scholar] [CrossRef]
- Han, M.; Li, Y.; Gu, Z.; Shi, H.; Chen, C.; Wang, Q.; Wan, H.; Guan, G. Immobilization of thiol-functionalized ionic liquids onto the surface of MIL-101 (Cr) frameworks by SCr coordination bond for biodiesel production. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 593–600. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Basic ionic liquid functionalized magnetically responsive Fe3O4@ HKUST-1 composites used for biodiesel production. Fuel 2018, 220, 248–256. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidicvegetable oils. Chem. Eng. J. 2019, 365, 40–50. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Synthesis of heterogenized polyoxometalate-based ionic liquids with brønsted-lewis acid sites: A magnetically recyclable catalyst for biodiesel production from low-quality oils. J. Ind. Eng. Chem. 2020, 87, 162–172. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Li, S.; Zhang, M.; Wang, Y.; Wang, Z.; Peng, Y.; Wang, M.; Li, X.; Pan, H. Recent advances in supported acid/base ionic liquids as catalysts for biodiesel production. Front. Chem. 2022, 10, 999607. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Transition metal nanoparticles in ionic liquids: Synthesis and stabilization. J. Mol. Liq. 2019, 276, 826–849. [Google Scholar] [CrossRef]
- Ma, L.; Haynes, C.J.E.; Grommet, A.B.; Walczak, A.; Parkins, C.C.; Doherty, C.M.; Longley, L.; Tron, A.; Stefankiewicz, A.R.; Bennett, T.D.; et al. Coordination cages as permanently porous ionic liquids. Nat. Chem. 2020, 12, 270–275. [Google Scholar] [CrossRef]
- Bartlewicz, O.; Dabek, I.; Szymańska, A.; Maciejewski, H. Heterogeneous Catalysis with the participation of ionic liquids. Catalysts 2020, 10, 1227. [Google Scholar] [CrossRef]
- Maciejewski, H. Ionic liquids in Catalysis. Catalysts 2021, 11, 367. [Google Scholar] [CrossRef]
- McNeice, P.; Marr, C.P.; Marr, C.A. Basic ionic liquids for catalysis: The road to greater stability. Catal. Sci. Technol. 2021, 11, 726. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Sharma, A. Photocatalytic carbonylation strategies: A recent trend in organic synthesis. J. Org. Chem. 2020, 86, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhang, F.; Pang, H. A review of MOFs and their composites-based photocatalysts: Synthesis and applications. Adv. Funct. Mater. 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Gisbertz, S.; Pieber, B. Heterogeneous photocatalysis in organic synthesis. ChemPhotoChem 2020, 4, 456–475. [Google Scholar] [CrossRef] [Green Version]
- Markushyna, Y.; Savateev, A. Light as a tool in organic photocatalysis: Multi-photon excitation and chromoselective reactions. Eur. J. Org. Chem. 2022, 2022, e202200026. [Google Scholar] [CrossRef]
- Michelin, C.; Hoffmann, N. Photocatalysis applied to organic synthesis—A green chemistry approach. Curr. Opin. Green Sustain. Chem. 2018, 10, 40–45. [Google Scholar] [CrossRef]
- Aghapoor, K.; Mohsenzadeh, F.; Sayahi, H.; Rastgar, S.; Darabi, R.H. Green synthesis of 1,3-dihydrobenzimidazol-2-ones from aromatic diamines by microwave in a tetrabutylammonium bromide–ethanol molten salt paste. Environ. Chem. Lett. 2018, 16, 1109–1116. [Google Scholar] [CrossRef]
- Filippov, A.S.; Amosova, S.V.; Albanov, A.I.; Potapov, V.A. Regioselective synthesis of novel functionalized dihydro-1,4-thiaselenin-2-ylsufanyl derivatives under phase transfer catalysis. Catalysts 2022, 12, 889. [Google Scholar] [CrossRef]
- Banik, B.K.; Banerjee, B.; Kaur, G.; Saroch, S.; Kumar, R. Tetrabutyl ammonium bromide (TBAB) catalyzed synthesis of bioactive heterocycles. Molecules 2020, 25, 5918. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, W. A minireview of phase-transfer catalysis and recent trends. Biomed. J. Sci. Tech. Res. 2022, 45, BJSTR.MS.ID.007237. [Google Scholar] [CrossRef]
- Jaśkowska, J.; Drabczyk, A.K.; Michorczyk, P.; Kułaga, D.; Zaręba, P.; Jodłowski, P.; Majka, Z.; Jakubski, J.; Pindelska, E. Mechanochemical synthesis method for drugs used in the treatment of CNS diseases under PTC conditions. Catalysts 2022, 12, 464. [Google Scholar] [CrossRef]
- Ghosh, A.D. Green solvents for sustainable organic synthesis. IJSR 2015, 6, 2154–2157. [Google Scholar] [CrossRef]
- Shanab, K.; Neudorfer, C.; Schirmer, E.; Spreitzer, H. Green solvents in organic synthesis: An overview. Curr. Org. Chem. 2013, 17, 1179–1187. [Google Scholar] [CrossRef]
- Breeden, S.W.; Clark, J.H.; Macquarrie, D.J.; Sherwood, J.; Zhang, W.; Cue, B.W., Jr. Green Solvents. Green Techniques for Organic Synthesis and Medicinal Chemistry; Wiley: Chichester, UK, 2012; pp. 241–246. [Google Scholar]
- Earle, M.J.; Seddon, K.R. Ionic liquids green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Pena-Pereira, F.; Kloskowski, A.; Namieśnik, J. Perspectives on the replacement of harmful organic solvents in analytical methodologies: A framework toward the implementation of a novel generation of ecofriendly alternatives. Green Chem. 2015, 17, 3687–3705. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for biobased solvents created as petrochemical and fuel products transition towards renewable resources. Int. J. Mol. Sci. 2015, 16, 17101–17159. [Google Scholar] [CrossRef]
- Abou-Shehada, S.; Clark, J.H.; Paggiola, G.; Sherwood, J. Tunable solvents: Shades of green. Chem. Eng. Process 2016, 99, 88–96. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Dunn, P. Water as a green solvent for pharmaceutical applications. In Handbook of Green Chemistry; Anastas, P.T., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Breslow, R. The Principles of and Reasons for Using Water as a Solvent for Green Chemistry. In Handbook of Green Chemistry; Anastas, P.T., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Blackmond, D.G.; Armstrong, A.; Coombe, V.; Wells, A. Wasser in organokatalytischen Prozessen: Ein Mythos wird entschleiert. Angew. Chem. Int. Ed. 2007, 119, 3872–3874. [Google Scholar] [CrossRef]
- Burk, J.M.; Feng, S.; Gross, F.M.; Tumas, W. Asymmetric catalytic hydrogenation reactions in supercritical carbon dioxide. J. Am. Chem. Soc. 1995, 117, 8277–8278. [Google Scholar] [CrossRef]
- Morita, K.D.; Pesiri, R.D.; David, A.S.; Glaze, H.W.; Tumas, W. Palladium-catalyzed cross-coupling reactions in supercritical carbon dioxide. Chem. Commun. 1998, 13, 1397–1398. [Google Scholar] [CrossRef]
- Horvàth, I.T. Fluorous Biphase Chemistry. Acc. Chem. Res. 1998, 31, 641–650. [Google Scholar] [CrossRef]
- Horváth, I.T. Facile catalyst separation without water: Fluorous biphase hydroformylation of olefins. Science 1994, 266, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Schäffner, B.; Schäffner, F.; Verevkin, S.P.; Borner, A. Organic carbonates as solvents in synthesis and catalysis. Chem. Rev. 2010, 110, 4554–4581. [Google Scholar] [CrossRef]
- Ross, S.D.; Finkelstein, M.; Petersen, R.C. Solvent effects in the reactions of N-bromosuccinimide with toluene, fluorene and acenaphthene; Evidence for a polar mechanism in propylene carbonate. J. Am. Chem. Soc. 1958, 80, 4327–4330. [Google Scholar] [CrossRef]
- Kronick, P.L.; Fuoss, R.M. Quaternization kinetics. II. Pyridine and 4-picoline in propylene carbonate. J. Am. Chem. Soc. 1955, 77, 6114. [Google Scholar] [CrossRef]
- Morcillo, M.; North, M.; Villuendas, P. Amino acid catalysed aldol reactions in cyclic carbonate solvents. Synthesis 2012, 12, 918–1925. [Google Scholar]
- Beattie, C.; North, M.; Villuendas, P. Proline-catalysed amination reactions in cyclic carbonate solvents. Molecules 2011, 16, 3420–3432. [Google Scholar] [CrossRef] [Green Version]
- Miao, X.; Fischmeister, C.; Bruneau, C.; Dixneuf, P.H. Dimethyl carbonate: An eco-friendly solvent in ruthenium-catalyzed olefin metathesis transformations. ChemSusChem 2008, 1, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Arockiam, P.; Poirier, V.; Fischmeister, C.; Bruneau, C.; Dixneuf, P.H. Diethyl carbonate as a solvent for ruthenium catalysed C–H bond functionalization. Green Chem. 2009, 11, 1871–1875. [Google Scholar] [CrossRef]
- Torborg, C.; Huang, J.; Schulz, T.; Schäffner, B.; Zapf, A.; Spannenberg, A.; Borner, A.; Beller, M. Improved palladium-catalyzed Sonogashira coupling reactions of aryl chlorides. Chem. Eur. J. 2009, 15, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Roger, J.; Verrier, C.; Le Goff, R.; Hoarau, C.; Doucet, H. Carbonates: Eco-friendly solvents for palladium-catalyzed direct 2-arylation of oxazole derivatives. ChemSusChem 2009, 2, 951–956. [Google Scholar] [CrossRef]
- Schäffner, B.; Holz, J.; Verevkin, S.P.; Börner, A. Rhodium-catalyzed asymmetric hydrogenation with self-assembling catalysts in propylene carbonate. Tetrahedron Lett. 2008, 49, 768–771. [Google Scholar] [CrossRef]
- Schäffner, B.; Holz, J.; Verevkin, S.P.; Börner, A. Organic carbonates as alternative solvents for palladium-catalyzed substitution reactions. ChemSusChem 2008, 1, 249–253. [Google Scholar] [CrossRef]
- Biliel, H.; Hamdi, N.; Zagrouba, F.; Fischmesiter, C.; Bruneau, C. Cross-metathesis transformations of terpenoids in dialkyl carbonate solvents. Green Chem. 2011, 13, 1448–1452. [Google Scholar] [CrossRef]
- Earle, M.J.; Noè, M.; Perosa-Seddon, A.K.R. Improved synthesis of tadalafil using dimethyl carbonate and ionic liquids. RSC Adv. 2014, 14, 1204–1211. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, C.-H.; Lee, E.Y. Biosynthesis of glycerol carbonate from glycerol by lipase in dimethyl carbonate as the solvent. Bioprocess Biosyst. Eng. 2010, 33, 1059–1065. [Google Scholar] [CrossRef]
- Wan, J.P.; Cao, S.; Jing, Y. Copper-catalyzed homo- and cross-coupling reactions of terminal alkynes in ethyl lactate. Appl. Organomet. Chem. 2014, 28, 631–634. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Wang, C.; Wan, J.-P. Bio-based green solvent mediated disulfide synthesis via thiol couplings free of catalyst and additive. RSC Adv. 2013, 3, 21369–21372. [Google Scholar] [CrossRef]
- Wan, J.-P.; Zhong, S.; Xie, L.; Cao, X.; Liu, Y.; Wei, L. KIO3-catalyzed aerobic cross-coupling reactions of enaminones and thiophenols: Synthesis of polyfunctionalized alkenes by metal-free C-H sulfenylation. Org. Lett. 2016, 18, 584–587. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Bennett, J.S.; Charles, K.L.; Miner, M.R.; Heuberger, C.F.; Spina, E.J.; Bartels, M.F.; Foreman, T. Ethyl lactate as a tunable solvent for the synthesis of aryl aldimines. Green Chem. 2009, 11, 166–168. [Google Scholar] [CrossRef]
- Ghosh, P.P.; Paul, S.; Das, A.R. Light induced synthesis of symmetrical and unsymmetrical dihydropyridines in ethyl lactate–water under tunable conditions. Tetrahedron Lett. 2013, 54, 138–142. [Google Scholar] [CrossRef]
- Yang, J.; Tana, J.-N.; Gu, Y. Lactic acid as an invaluable bio-based solvent for organic reactions. Green Chem. 2012, 14, 3304–3317. [Google Scholar] [CrossRef]
- Cascone, R. Biobutanol—A replacement for bioethanol? Chem. Eng. Prog. 2008, 104, S4–S9. [Google Scholar]
- Fleckenstein, C.A.; Plenio, H. Efficient Suzuki−Miyaura coupling of (hetero)aryl chlorides with thiophene- and furanboronic acids in aqueous n-butanol. J. Org. Chem. 2008, 73, 3236–3244. [Google Scholar] [CrossRef]
- Fleckenstein, C.A.; Plenio, H. Highly efficient Suzuki–Miyaura coupling of heterocyclic substrates through rational reaction design. Chem. Eur. J. 2008, 14, 4267–4279. [Google Scholar] [CrossRef]
- Chemat, S.; Tomao, V.; Chemat, F. Limonene as green solvent for extraction of natural products. In Green Solvents I: Properties and Applications in Chemistry; Mohammad, A., Inamuddin, Eds.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Virot, M.; Tomao, V.; Ginies, C.; Visinoni, F.; Chemat, F. Green procedure with a green solvent for fats and oils’ determination: Microwave-integrated Soxhlet using limonene followed by microwave Clevenger distillation. J. Chromatogr. A 2008, 1196–1197, 147–152. [Google Scholar] [CrossRef]
- Rasina, D.; Kahler-Quesada, A.; Ziarelli, S.; Warratz, S.; Cao, H.; Santoro, S.; Ackermann, L.; Vaccaro, L. A biomass-derived safe medium to replace toxic dipolar solvents and access cleaner Heck coupling reactions. Green Chem. 2016, 18, 5025–5030. [Google Scholar] [CrossRef] [Green Version]
- Pongrácz, P.; Kollárb, L.; Mika, L.T. A step towards hydroformylation under sustainable conditions:platinum-catalysed enantioselective hydroformylation of styrene in gamma-valerolactone. Green Chem. 2016, 18, 842–847. [Google Scholar] [CrossRef]
- Song, J.; Zhou, B.; Liu, H.; Xie, C.; Meng, Q.; Zhang, Z.; Han, B. Biomass-derived γ-valerolactone as an efficient solvent and catalyst for the transformation of CO2 to formamides. Green Chem. 2016, 18, 3956–3961. [Google Scholar] [CrossRef]
- Durand, M.; Zhu, Y.; Molinier, V.; Feron, T.; Aubry, J.-M. Solubilizing and hydrotropic properties of isosorbide monoalkyl- and dimethyl-ethers. J. Surfact. Deterg. 2009, 12, 371–378. [Google Scholar] [CrossRef]
- Mesnager, J.; Quettier, C.; Lambin, A.; Rataboul, F.; Pinel, C. Telomerization of butadiene with starch under mild conditions. ChemSusChem 2009, 2, 1125–1129. [Google Scholar] [CrossRef]
- Sambiagio, C.; Munday, R.H.; Blacker, A.J.; Marsden, S.P.; McGowan, P.C. Green alternative solventsfor the copper-catalysed arylation of phenols and amides. RSC Adv. 2016, 6, 70025–70032. [Google Scholar] [CrossRef]
- Mouret, A.; Leclercq, L.; Mühlbauer, A.; Nardello-Rataj, V. Eco-friendly solvents and amphiphilic catalytic polyoxometalate nanoparticles: A winning combination for olefin epoxidation. Green Chem. 2014, 16, 269–278. [Google Scholar] [CrossRef]
- Zia, H.; Ma, J.K.H.; O’Donnell, J.P.; Luzzi, L.A. Cosolvency of dimethyl isosorbide for steroid solubility. Pharm. Res. 1991, 8, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Moity, L.; Molinier, V.; Benazzouz, A.; Joosen, B.; Gerbaud, V.; Aubrey, J.-M. A “top-down” in silico approach for designing ad hoc bio-based solvents: Application to glycerol-derived solvents of nitrocellulose. Green Chem. 2016, 18, 3239–3249. [Google Scholar] [CrossRef] [Green Version]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef] [Green Version]
- Mottu, F.; Laurent, A.; Rufenacht, D.A.; Doelker, E. Organic solvents for pharmaceutical parenterals and embolic liquids: A review of toxicity data. PDA J. Pharm. Sci. Technol. 2000, 54, 456–469. [Google Scholar]
- Taygerly, J.P.; Miller, L.M.; Yee, A.; Peterson, E.A. A convenient guide to help select replacement solvents for dichloromethane in chromatography. Green Chem. 2012, 14, 3020–3025. [Google Scholar] [CrossRef]
- Sherwood, J.; Parker, H.L.; Moonen, K.; Farmer, T.J.; Hunt, A.J. N-Butylpyrrolidinone as a dipolar aprotic solvent for organic synthesis. Green Chem. 2016, 18, 3990–3996. [Google Scholar] [CrossRef]
- Sherwood, J.; De Bruyn, M.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef] [PubMed]
- Menges, N.; Şahin, E. Metal- and base-free combinatorial reaction for C-acylation of 1,3-diketo compounds in vegetable oil: The effect of natural oil. ACS Sustain. Chem. Eng. 2014, 2, 226–230. [Google Scholar] [CrossRef]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef] [Green Version]
- McCombs, N.L.; Smirnova, T.; Ghiladi, R.A. Oxidation of pyrrole by dehaloperoxidase-hemoglobin: Chemoenzymatic synthesis of pyrrolin-2-ones. Catal. Sci. Technol. 2017, 7, 3104–3118. [Google Scholar] [CrossRef]
- Gu, Y.; Barrault, J.; Jerome, F. Glycerol as an efficient promoting medium for organic reactions. Adv. Synth. Catal. 2008, 350, 2007–2012. [Google Scholar] [CrossRef]
- Shaik, B.B.; Seboletswe, P.; Mohite, B.S.; Katari, K.N.D.; Bala, D.M.P.; Karpoormath, R.P.; Singh, P.P. Lemon juice: A versatile biocatalyst and green solvent in organic transformations. ChemistrySelect 2022, 7, e202103701. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol, an All-purpose product. Catalysts 2022, 12, 48. [Google Scholar] [CrossRef]
- Jordan, A.; Hall, J.G.C.; Thorp, R.L.; Sneddon, F.H. Replacement of less-preferred dipolar aprotic and ethereal solvents in synthetic organic chemistry with more sustainable alternatives. Chem. Rev. 2022, 122, 6749–6794. [Google Scholar] [CrossRef]
- Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; Dominguez de Maria, P.; Alcantara, A.R. 2-Methyltetrahydrofuran(2-MeTHF): A biomass-derived solvent with broad application in organic chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Bora, U. Eco-friendly Suzuki-Miyaura coupling of arylboronic acids to aromatic ketones catalyzed by the oxime-palladacycle in biosolvent 2-MeTHF. New J. Chem. 2016, 40, 3119–3123. [Google Scholar] [CrossRef]
- Ripin, D.; Vetelino, M. 2-Methyltetrahydrofuran as an alternative to dichloromethane in 2-phase reactions. Synlett 2003, 15, 2353. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Du, W.Y.; Duan, Z.Q.; Yang, R.L.; Bi, Y.H.; Yuan, X.T.; Mao, Y.Y.; Zhao, Y.P.; Wu, J.; Jia, J.B. Efficient regioselective synthesis of the crotonyl polydatin prodrug by Thermomyces lanuginosus lipase: A kinetics study in eco-friendly 2-methyltetrahydrofuran. Appl. Biochem. Biotechnol. 2016, 179, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamagiwa, N.; Torisawa, Y. Cyclopentyl methyl ether as a new and alternative process solvent. Org. Process Res. Dev. 2007, 11, 251–258. [Google Scholar] [CrossRef]
- Watanabe, K. The toxicological assessment of cyclopentyl methyl ether (CPME) as a green solvent. Molecules 2013, 18, 3183–3194. [Google Scholar] [CrossRef] [Green Version]
- Antonucci, V.; Coleman, J.; Ferry, J.B.; Johnson, N.; Mathe, M.; Scott, J.P.; Xu, J. Toxicological assessment of 2-methyltetrahydrofuran and cyclopentyl methyl ether in support of their use in pharmaceutical chemical process development. Org. Process Res. Dev. 2011, 15, 939–941. [Google Scholar] [CrossRef]
- Montanino, M.; Moreno, M.; Alessandrini, F.; Appetecchi, G.B.; Passerini, S.; Zhou, Q.; Henderson, W.A. Physical and electrochemical properties of binary ionic liquid mixtures: (1 − x) PYR14TFSI–(x) PYR14IM14. Electrochim. Acta 2012, 60, 163–169. [Google Scholar] [CrossRef]
- Domańska, U. Physico-chemical properties and phase behavior of pyrrolidinium-based ionic liquids. Int. J. Mol. Sci. 2010, 11, 1825–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonhôte, P.; Dias, P.A.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Walkoli1, T.A.; Sonawane, D.P.D. Ionic liquids: Eco-friendly solvent. RRBB 2022, 9, 9–13. [Google Scholar] [CrossRef]
- Dupont, J.; De Souza, R.F.; Suarez, P.A.Z. Ionic liquid (molten salt) phase organometallic catalysis. Chem. Rev. 2002, 102, 3667–3692. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.H., Jr. Task-specific ionic liquids. Chem. Lett. 2004, 33, 1072–1077. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.P.; Geldbach, J.T. Interface-Ionic Liquids. In The Electrochemical Society Interface; Spring: Chicago, IL, USA, 2007. [Google Scholar]
- Ivaništšev, V.; Fedorov, M.V. Interfaces between Charged Surfaces and Ionic Liquids: Insights from Molecular Simulations. Electrochem. Soc. Interface 2014, 23, 65. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Rawal, A.; Aldous, L. Aprotic vs. Protic Ionic Liquids for Lignocellulosic Biomass Pretreatment: Anion Effects, Enzymatic Hydrolysis, Solid-State NMR, Distillation, and Recycle. ACS Sustain. Chem. Eng. 2019, 7, 11928–11936. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Zubeir, L.F.; Van den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Su, E. Hydrophobic deep eutectic solvents: The new generation of green solvents for diversified and colorful applications in green chemistry. J. Clean. Prod. 2021, 314, 127965. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Meng, Z.; Qian, H.; Zhang, S.; Lu, R.; Gao, H.; Zhou, W. Extraction of benzoylurea pesticides from tea and fruit juices using deep eutectic solvents. J. Chromatogr. B 2020, 1140, 121995. [Google Scholar] [CrossRef]
- Werner, J. Novel deep eutectic solvent-based ultrasounds-assisted dispersive liquid-liquid microextraction with solidification of the aqueous phase for hplc-uv determination of aromatic amines in environmental samples. Microchem. J. 2020, 153, 104405. [Google Scholar] [CrossRef]
- Riveiro, E.; Gonzalez, B.; Domínguez, Á. Extraction of adipic, levulinic and succinic acids from water using topo-based deep eutectic solvents. Separ. Purif. Technol. 2020, 241, 116692. [Google Scholar] [CrossRef]
- Tahghighi, A.; Babalouei, F. Thiadiazoles: The appropriate pharmacological scaffolds with leishmanicidal and antimalarial activities: A review. Iran. J. Basic Med. Sci. 2017, 20, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Iizawa, Y.; Okonogi, K.; Hayashi, R.; Iwahi, T.; Yamazaki, T.; Imada, A. Therapeutic Effect of Cefozopran (SCE-2787), a New Parenteral Cephalosporin, against Experimental Infections in Mice. Antimicrob. Agents Chemother. 1993, 37, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frija, T.M.L.; Pombeiro, L.J.A.; Kopylovich, N.M. Building 1,2,4-Thiadiazole: Ten Years of Progress. Eur. J. Org. Chem. 2017, 2017, 2670–2682. [Google Scholar] [CrossRef]
- Irfan, A.; Batool, F.; Ahmad, S.; Ullah, R.; Sultan, A.; Sattar, R.; Nisar, B.; Rubab, L. Recent trends in the synthesis of 1,2,3-thiadiazoles. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 1098–1115. [Google Scholar] [CrossRef]
- Irfan, A.; Ullah, S.; Anum, A.; Jabeen, N.; Zahoor, A.F.; Kanwal, H.; Kotwica-Mojzych, K.; Mojzych, M. Synthetic Transformations and Medicinal Significance of 1,2,3-Thiadiazoles Derivatives: An Update. Appl. Sci. 2021, 11, 5742. [Google Scholar] [CrossRef]
- Decking, U.K.; Hartmann, M.; Rose, H.R.; Meil, J.B.; Schrader, J. Cardioprotective actions of KC 12291. I. Inhibition of voltage-gated Na+ channels in ischemia delays myocardial Na+ overload. Naunyn Schmiedeberg’s Arch. Pharmacol. 1998, 358, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Proshin, A.N.; Serkov, I.V.; Petrova, L.N.; Bachurin, O.S. 5-Amino-3-(2-aminopropyl)-1,2,4-thiadiazoles as the basis of hybrid multifunctional compounds. Russ. Chem. Bull. 2014, 63, 1148–1152. [Google Scholar] [CrossRef]
- Fawzi, A.B.; Macdonald, D.; Benbow, L.L.; Smith-Torhan, A.; Zhang, H.T.; Weig, B.C.; Ho, G.; Tulshian, D.; Linder, M.E.; Graziano, M.P. SCH-202676: An allosteric modulator of both agonist and antagonist binding to G protein-coupled receptors. Mol. Pharmacol. 2001, 59, 30. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Decking, U.K.M.; Schrader, J. Cardioprotective actions of KC 12291 II. Delaying Na+ overload in ischemia improves cardiac function and energy status in reperfusion. Naunyn Schmiedeberg’s Arch. Pharmacol. 1998, 358, 554. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Alonso, M.; Castro, A.; Pérez, C.; Moreno, F.J. First Non-ATP Competitive Glycogen Synthase Kinase 3 â (GSK-3â) Inhibitors: Thiadiazolidinones (TDZD) as Potential Drugs for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2002, 45, 1292–1299. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, N.-M.; Chang, K.-H.; Shah, K. Synthesis and anticancer activity of 5-(3-indolyl)-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 4664–4668. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Baraldi, G.P.; Carrion, D.M.; Cruz-Lopez, O.; Preti, D.; Tabrizi, A.M.; Fruttarolo, F.; Heilmann, F.; Bermejo, J.; Estevez, F. Hybrid molecules containing benzo[4,5]imidazo-[1,2-d][1,2,4]thiadiazole and a-bromoacryloyl moieties as potent apoptosis inducers on human myeloid leukaemia cells. Bioorg. Med. Chem. Lett. 2007, 17, 2844. [Google Scholar] [CrossRef] [PubMed]
- Camoutsis, c.; Geronikaki, A.; Ciric, A.; Soković, M.; Zoumpoulakis, P.; Zervou, M. Sulfonamide-1,2,4-thiadiazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Chem. Pharm. Bull. 2010, 58, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.T.; Connor, D.T.; Sercel, A.D.; Sorenson, R.J.; Doubleday, R.; Unangst, P.C.; Roth, B.D.; Beylin, V.G.; Gilbertsen, R.B.; Chan, K.; et al. Synthesis, Structure–Activity Relationships, and in Vivo Evaluations of Substituted Di-tert-butylphenols as a Novel Class of Potent, Selective, and Orally Active Cyclooxygenase-2 Inhibitors. 2. 1,3,4- and 1,2,4-Thiadiazole Series. J. Med. Chem. 1999, 42, 1161. [Google Scholar] [CrossRef] [PubMed]
- Leung-Toung, R.; Tam, F.T.; Zhao, Y.; Simpson, D.C.; Li, W.; Desilets, D.; Karimian, K. Synthesis of 3-substituted bicyclic imidazo[1,2-d][1,2,4]thiadiazoles and tricyclic benzo[4,5]imidazo[1,2-d][1,2,4]thiadiazoles. J. Org. Chem. 2005, 70, 6230–6241. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, P.; Pandeya, S.N.; Kashaw, S.K.; Kashaw, V.; Stables, J.P. Synthesis and anticonvulsant activity of some substituted 1,2,4-thiadiazoles. Eur. J. Med. Chem. 2009, 44, 1100–1105. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, Y.; Wang, A.; Sieber-McMaster, E.; Chen, X.; Pelton, P.; Xu, J.Z.; Yang, M.; Zhu, P.; Zhou, L.; et al. Synthesis and Identification of [1,2,4]Thiadiazole Derivatives as a New Series of Potent and Orally Active Dual Agonists of Peroxisome Proliferator-Activated Receptors α and δ. J. Med. Chem. 2007, 50, 3954. [Google Scholar] [CrossRef]
- Nieuwendijk, D.V.; Pietra, D.; Heitman, L.; Göblyös, A.; Ijzerman, A.P. Synthesis and Biological Evaluation of 2,3,5-Substituted [1,2,4]Thiadiazoles as Allosteric Modulators of Adenosine Receptors. J. Med. Chem. 2004, 47, 663. [Google Scholar] [CrossRef]
- Unangst, P.C.; Shrum, G.P.; Connor, D.T.; Dyer, R.D.; Schrier, D.J. Novel 1,2,4-oxadiazoles and 1,2,4-thiadiazoles as dual 5-lipoxygenase and cyclooxygenase inhibitors. J. Med. Chem. 1992, 35, 3691. [Google Scholar] [CrossRef]
- Tam, T.F.; Leung-Toung, R.; Li, W.; Spino, M.; Karimian, K. Medicinal chemistry and properties of 1,2,4-thiadiazoles. Mini Rev. Med. Chem. 2005, 5, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. Thiadiazole-a Promising Structure in Medicinal Chemistry. ChemMedChem 2013, 8, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, A.; Rajaguru, K.; Noufal, M.C.; Muthusubramanian, S.; Bhuvanesh, N. Hypervalent Iodine(III) Mediated Synthesis of 3-Substituted 5-Amino-1,2,4-thiadiazoles through Intramolecular Oxidative S–N Bond Formation. J. Org. Chem. 2016, 81, 6573. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Meng, Y.; Zhou, Y.; Ren, L.; Wu, J.; Yu, W.; Chang, J. ynthesis of 5-Amino and 3,5-Diamino Substituted 1,2,4-Thiadiazoles by I2-Mediated Oxidative N–S Bond Formation. J. Org. Chem. 2017, 82, 5898. [Google Scholar] [CrossRef]
- Tumula, N.; Jatangi, N.; Palakodety, K.R.; Balasubramanian, S.; Nakka, M. I2-Catalyzed Oxidative N–S Bond Formation: Metal-Free Regiospecific Synthesis of N-Fused and 3,4-Disubstituted 5-Imino-1,2,4-thiadiazoles. J. Org. Chem. 2017, 82, 5310. [Google Scholar] [CrossRef]
- Chai, L.; Xu, Y.; Ding, T.; Fang, S.; Zhang, W.; Wang, Y.; Lu, M.; Xu, H.; Yang, X. One-pot synthesis of 3,5-disubstituted 1,2,4-thiadiazoles from nitriles and thioamides via I2-mediated oxidative formation of an N–S bond. Org. Biomol. Chem. 2017, 15, 8410. [Google Scholar] [CrossRef]
- Chauhan, S.; Verma, P.; Mishra1, A.; Srivastava, V. An Expeditious Ultrasound-Initiated Green Synthesis of 1,2,4-Thiadiazoles in Water. Chem. Heterocycl. Comp. 2020, 56, 123. [Google Scholar] [CrossRef]
- Zhao, J.-W.; Xu, J.-X.; Guo, X.-Z. Green synthesis of 1,2,4-thiadizoles from thioamides in water using molecular oxygen as an oxidant. Chin. Chem. Lett. 2014, 25, 1499. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, T.; Liu, S.; Li, A.; Liu, K.; Yang, T.; Zhou, C. Transition-metal-free S–N bond formation: Synthesis of 5-amino-1,2,4-thiadiazoles from isothiocyanates and amidines. New J. Chem. 2019, 43, 6465. [Google Scholar] [CrossRef]
- Boeini, Z.H. Green Protocol for Synthesis of the 3,5-disubstituted 1,2,4-thiadiazoles Using N-benzyl-DABCO-tribromide in Aqueous Media. J. Iran. Chem. Soc. 2009, 6, 547. [Google Scholar] [CrossRef]
- Boeini, Z.H. Highly Efficient Synthesis of 3,5-Diaryl-1,2,4-thiadiazoles in Water–Wet Paste Conditions. Synth. Commun. 2011, 41, 2932. [Google Scholar] [CrossRef]
- Khosropour, R.A.; Noei, J. Monatsh. A convenient strategy for the synthesis of 3,5-diaryl-1,2,4-thiadiazoles: Oxidative dimerization of arylthioamides using CC–DMSO in PEG-400. Monatshefte für Chemie 2010, 141, 649. [Google Scholar] [CrossRef]
- Xua, Y.; Chena, J.; Gao, W.; Jina, H.; Dinga, J.; Wua, H. Solvent-free synthesis of 3,5-di(hetero)aryl-1,2,4-thiadiazoles by grinding of thioamides under oxidative conditions. J. Chem. Res. 2010, 34, 151–153. [Google Scholar] [CrossRef]
- Fordyce, A.F.E.; Morrison, A.J.; Sharp, R.D.; Paton, M.R. Microwave-induced generation and reactions of nitrile sulfides: An improved method for the synthesis of isothiazoles and 1,2,4-thiadiazole. Tetrahedron 2010, 66, 7192–7197. [Google Scholar] [CrossRef]
- Zali-Boeinia, H.; Shokrolahib, A.; Zalib, A.; Ghanib, K. Highly efficient synthesis of 3,5-disubstituted 1,2,4-thiadiazoles using pentylpyridinium tribromide as a solvent/reagent ionic liquid. J. Sulphur Chem. 2012, 33, 165–170. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kwak, H.S.; Lee, G.-H.; Gong, Y.-D. Copper-catalyzed synthesis of 3-substituted-5-amino-1,2,4-thiadiazoles via intramolecular NeS bond formation. Tetrahedron 2014, 70, 8737–8743. [Google Scholar] [CrossRef]
- Yoshimura, A.; Todora, D.A.; Kastern, J.B.; Koski, R.S.; Zhdankin, V.V. Synthesis of 1,2,4-Thiadiazoles by Oxidative Dimerization of Carbothioamides by Using Oxone. Eur. J. Org. Chem. 2014, 2014, 5149–5152. [Google Scholar] [CrossRef]
- Jatangi, N.; Tumula, N.; Palakodety, K.R.; Nakka, M. I2-Mediated oxidative C-N and N-S bond formation in water:A metal-free synthesis of 4,5-disubstituted/N-fused 3-amino-1,2,4-triazoles and 3-substituted 5-amino-1,2,4-thiadiazoles. J. Org. Chem. 2018, 83, 5715–5723. [Google Scholar] [CrossRef]
- Chacko, P.; Shivashankar, K. Montmorillonite K10-catalyzed synthesis of N-fused imino-1,2,4-thiadiazolo isoquinoline derivatives. Synth. Commun. 2018, 48, 1363–1376. [Google Scholar] [CrossRef]
- Cao, X.-T.; Zheng, Z.-L.; Liu, J.; Hu, Y.-H.; Yu, H.-Y.; Cai, S.; Wang, G. H2O2-Mediated Synthesis of 1,2,4-Thiadiazole Derivatives in Ethanol at Room Temperature. Adv. Synth. Catal. 2022, 364, 689–694. [Google Scholar] [CrossRef]






















| Green Catalyst Type | Examples |
|---|---|
| Lewis acids catalysts in water | Scandium tris(heptadecafluorooctanesulfonate) (Sc(O3 SC8 F17)3) in supercritical carbon dioxide (scCO2), cationic surfactant, cetyltrimethylammonium bromide (CTAB), Sc(OTf)3–SDS and rare earth metal triflates can be used in carbon–carbon bond-forming reactions in aqueous media [47,48]. |
| Zeolites as green catalysts | H-, Cu- and Sc-zeolites as green Lewis catalysts for the carbonylation, glycosylation, aldolization, click reactions, multicomponent reactions, halogenation, cycloadditions, coupling reactions and cyclization [49]. |
| Enzyme catalysis | Enzymatic redox catalyst, lipases, aldolases, transaminases, hydroxynitrile lyases and hydrolases [50]. |
| Heteropoly acid-based (HPAs) catalysis | HPAs can be designed in homogeneous and heterogeneous systems such as Amberlyst-15, PCPs–SO3H, BC–SO3H, CMK-3-SO3H, Zn–Ca–Fe, CsH2PW12O40, Ru/CMK-3, Fe3O4-SBA–SO3H, CaFe2O4, H3PW12O40, H5BW12O40, H5AlW12O40, H5GaW12O40 and H6CoW12O40 [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. |
| Natural materials and foods as catalysts | Wood ash biocatalyst [63], alginic acid [64,65], boric acid [66], tartaric acid [67,68], citric acid [69,70,71], pectin [72], oxalic acid [73,74,75], saccharin [46,76,77], wool and keratin deriving from wool fibers [78,79,80], feathers [81,82,83,84], silk [85,86], plant derivatives, lemon juice [87,88]. |
| Nano particles (NPs)/materials as catalysts | Magnetic nano catalysts (magnetic Fe3O4, magnetic zinc ferrite ZnFe2O4, CuFe2O4, CoFe2O4, NiFe2O4, NiFe2O4@Cu) and oxides; ferrites with a shell; metallic with a shell [89,90,91,92], K10 clay, K10 montmorillonite and clayfen [93,94], magnesium oxide NPs, cerium oxide NPs, gold NPs, silica titanium oxide NPs, silica vanadium oxide NPs, iridium oxide NPs, molybdenum–bismuth bimetallic chalcogenide NPs, platinum–antimony tin oxide NPs, calcium oxide NPs, palladium NPs, tin oxide NPs [95,96,97,98,99,100,101,102,103,104,105,106,107] |
| Transition metals as green catalysts | Ru(CO)3(TPPMS)2 (TPPMS = (C6H5)2P(m-C6H4SO3Na)), RuH2(CO)(TPPMS)3, [RuH(CO)(NCMe)(TPPMS)3][BF4], Rh/TPPTS complexes, Ru/(R)-BINAP,Ru/(R)-13-[(S,S)-DPEN]Cl2, Ru/(S)-BINAP, Pd-DPPP, Pd(OAc)2, CuI,MnBr(CO)5 [108,109,110,111,112,113,114,115,116,117] |
| Ionic liquids as catalysts | POM-based ILs (POM-ILs) such as (4-sulfonic acid) butyltributyl amine (TBABS) such as cations and H5PMo10V2O40 (Mo10V2) such as anion (3-sulfonic acid) propylpyridine (PyPS), (4-sulfonic acid) butylpyridine (PyBS), palladium deposited oleic acid coated-Fe3O4 NPs (Fe3O4@OA–Pd) and (4-sulfonic acid) butyltrimethyl amine (TMABS) [118,119,120,121]. Acidic ionic liquids such as [HO3S-(CH2)3-mim] Cl-FeCl3 and Brønsted Lewis acidic ILs, (1-butyl-3-methylimidazolium hydrogen sulfate or 1-(3-sulfopropyl)-3-methylimidazolium hydrogen sulfate) ILs [122,123]. Following different ionic liquids also used as catalysts for the productions of biodiesel, which are: SBA- IL-3, PIL-3, P(VB-VS)HSO4, MIL-101(Cr)@ MBIAILs, Fe3O4@HKUST-1, AILs/HPW/UiO-66-2COOH and CoFe2O4/MIL-88B(Fe)-NH2/(Py-Ps)PMo [124,125,126,127,128,129,130,131]. Ionic liquids are classified into three groups: solid catalyst with ionic liquid layers (SCILL), porous ionic liquids and supported ionic liquid phase catalyst (SILPC). Binary alkoxide ionic liquids catalyzed organic reaction and examples of such ILs are ([Pyrr1,4][NTf2]x[OiPr] 1,3-butylmethylimidazolium hydroxide([BMIM][OH]) and [C2DABCO][NTf2] [132,133,134,135,136]. |
| Photocatalyst (PC) | Carbonylation approaches in organic synthesis were mediated by photocatalysts, such as [Ir(4-Fppy)2(bpy)]+, Ru(bpy)32+, fac-Ir(ppy)3, 4-CzIPN, fluorescein, Ir[(dF(CF3)(ppy)]2(dtbbpy)+, eosin and various MOFs composite-based photocatalyst were afforded for their applications in different synthetic approaches such as PCN-250-Fe3, Uio-68-TZDC, MIL-88A(Fe), ZIF-8, Ni-MOF and Ru(bpy)3@NKMOF-7. Following, different heterogeneous photocatalysis have been used in organic synthesis such as TiO2, TiO2 P25, dye-sensitized TiO2, metal doped TiO2, bismuth (III) oxide-based PCs, cadmium sulfide and cadmium-selenide-based PCs, lead halide perovskites and graphitic carbon nitrides (g-CN) PCs, [137,138,139,140,141]. |
| Phase transfer catalyst (PTC) | Tetrabutylammonium bromide (TBAB), triethylbenzylammonium chloride (TEBAC) and tetrabutylammonium iodide (TBAI) are famous phase-transfer green catalysts used in organic synthetic transformations. The types of PTC are the following: onium salt phase-transfer catalysts, crown ether and polyether phase-transfer catalyst and supported phase-transfer catalysts [142,143,144,145,146]. |
| Type of Green Solvent | Examples |
|---|---|
| Aqueous and super critical carbon dioxide | H2O, scCO2, scCO2 + H2O [121,122,123,124,125,155,156,157,158,159]. |
| Fluorous solvents | 1,1,1-trifluoroethanol,perfluoromethyl cyclohexane/toluene—[160,161]. |
| Organic carbonates | Butylene carbonate, propylene carbonate, diethyl Carbonate and dimethyl carbonate (CH3OCOOCH3) [162,163,164,165,166,167,168,169,170,171,172,173,174,175]. |
| Lactates and general solvents | Lactic acid, ethyl lactate, lactate dehydrogenase, transaminase and n-butanol [176,177,178,179,180,181,182,183,184,185]. |
| Natural and biosolvents | Limonene and P-cymene as solvent, γ-valerolactone (GVL), sugar-derived dimethylisosorbide (DMI), glycerol and glycerol derivatives as solvents such as glycerol carbonate, glycerol-derived acetals and ketals, 2,3-propanediol, 1,3-propanediol, monoacylglycerol MAGs, diacylglycerols DAGs, triacylglycerols TAGs, glycidyl monoalkyl ethers, glycidyl dialkyl ethers, glycidyl trialkyl ethers and dihydrolevoglucosenone (cyrene), etc. [186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201]. Corn oil, glycerol, oxidoreductases, transferases, hydrolases, lyases, isomerases, ligases, dehaloperoxidase (DHP), lemon juice as solvent and eucalyptol used as solvent for the synthesis of N, O and S heterocycles, cygnet a family of green solvents and dimethyl isosorbide (DMI) solvent derived from cellulose [202,203,204,205,206,207,208,209]. |
| Archetypal green solvents | 2-methyl tetrahydrofuran (2-MeTHF) and cyclopentyl methyl ether (CPME), etc. [210,211,212,213,214,215,216]. |
| Ionic liquids as solvents | 1-allyl-3-methylimidazolium chloride, 1-butyl-3-methylimidazolium chloride ([BMIM]Cl), sodium dicyanamide, sodium thiocyanate, silver nitrate, sodium nitrate, chloroauric acid, 1-ethyl-3-methyl-(EMIM), 1-butyl-3-methyl-(BMIM), 1-octyl-3 methyl (OMIM), 1-decyl-3-methyl-(DMIM), 1-dodecyl-3-methyl- docecylMIM), 1-ethyl-3-methyl imidazolium salts, etc. [217,218,219,220,221,222,223,224,225,226,227]. |
| Deep eutectic solvents (DESs) | The classification and general examples of DES are as follows: Class I Cat + X-zMClx, M = Zn, Sn, Fe, Al, Ga. Class II Cat + X-zMClx• yH2O, M = Cr, Co, Cu, Ni, Fe. Class III Cat + X-zRZ Z = CONH2, COOH, OH. Class IV MClx + RZ = MClx-1 + ⋅RZ + MClx+ -1 M = Al, Zn and Z = CONH2, OH. New type Class V RZ + RP Z = CONH2, COOH, OH and P––C6H4OH, CO, NH2. Hydrophobic deep eutectic solvents (HDESs) play a role in green chemistry. The first HDESs (cecanoic acid (DecA)) were synthesized by Osch et al. The following solvents (tetradecyl) phosphonium tetrafluoroborate (P14,666Cl), trioctylphosphine oxide (TOPO) and N-didodecylammonium chloride (DDDACl) have been developed as green HDESs [228,229,230,231]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubab, L.; Anum, A.; Al-Hussain, S.A.; Irfan, A.; Ahmad, S.; Ullah, S.; Al-Mutairi, A.A.; Zaki, M.E.A. Green Chemistry in Organic Synthesis: Recent Update on Green Catalytic Approaches in Synthesis of 1,2,4-Thiadiazoles. Catalysts 2022, 12, 1329. https://doi.org/10.3390/catal12111329
Rubab L, Anum A, Al-Hussain SA, Irfan A, Ahmad S, Ullah S, Al-Mutairi AA, Zaki MEA. Green Chemistry in Organic Synthesis: Recent Update on Green Catalytic Approaches in Synthesis of 1,2,4-Thiadiazoles. Catalysts. 2022; 12(11):1329. https://doi.org/10.3390/catal12111329
Chicago/Turabian StyleRubab, Laila, Ayesha Anum, Sami A. Al-Hussain, Ali Irfan, Sajjad Ahmad, Sami Ullah, Aamal A. Al-Mutairi, and Magdi E. A. Zaki. 2022. "Green Chemistry in Organic Synthesis: Recent Update on Green Catalytic Approaches in Synthesis of 1,2,4-Thiadiazoles" Catalysts 12, no. 11: 1329. https://doi.org/10.3390/catal12111329