Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances
Abstract
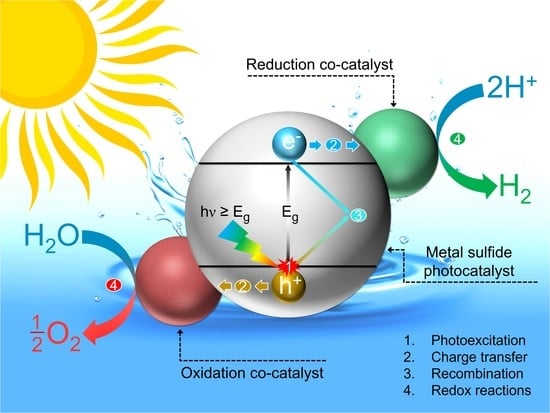
:1. Introduction
Metal Sulfide Photocatalysts: Design and Mechanisms
2. Hydrogen Generation as an Energy Material
2.1. Binary Metal Sulfide Compounds

2.2. Ternary Metal Sulfide Compounds
2.3. Metal Sulfide and Metal Oxide Heterostructures
Metal Sulfide and TiO2-Based Photocatalysts
2.4. Metal Sulfide/g-C3N4 Heterostructures
2.5. Metal Sulfide/MOFs (COFs) Heterojunction Photocatalysts
2.6. Hierarchical Metal Sulfide Photocatalysts
2.7. Plasmonic Co-Catalyst Modified Metal Sulfide Photocatalysts
3. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- United Nations. Sustainable Development Goals. 2021. Available online: https://www.un.org/ (accessed on 27 September 2022).
- Bonde, J.; Moses, P.G.; Jaramillo, T.F.; Nørskov, J.K.; Chorkendorff, I. Hydrogen evolution on nano-particulate transition metal sulfides. Faraday Discuss. 2009, 140, 219–231. [Google Scholar] [CrossRef]
- Mamiyev, Z.Q.; Balayeva, N.O. Preparation and optical studies of PbS nanoparticles. Opt. Mater. 2015, 46, 522–525. [Google Scholar] [CrossRef]
- Ganapathy, M.; Chang, C.T.; Alagan, V. Facile preparation of amorphous SrTiO3-crystalline PbS heterojunction for efficient photocatalytic hydrogen production. Int. J. Hydrogen Energy 2022, 47, 27555–27565. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef] [PubMed]
- Balayeva, N.O.; Mamiyev, Z.Q. Synthesis and characterization of Ag2S/PVA-fullerene (C60) nanocomposites. Mater. Lett. 2016, 175, 231–235. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Dibenedetto, A.; Aresta, M.; Macyk, W. Zinc sulfide functionalized with ruthenium nanoparticles for photocatalytic reduction of CO2. Appl. Catal. B 2015, 178, 170–176. [Google Scholar] [CrossRef]
- Frame, F.A.; Osterloh, F.E. CdSe-MoS2: A Quantum Size-Confined Photocatalyst for Hydrogen Evolution from Water under Visible Light. J. Phys. Chem. C 2010, 114, 10628–10633. [Google Scholar] [CrossRef]
- Keimer, B.; Moore, J. The physics of quantum materials. Nat. Phys. 2017, 13, 1045–1055. [Google Scholar] [CrossRef]
- Sivula, K.; Van De Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Amirav, L.; Alivisatos, A.P. Photocatalytic Hydrogen Production with Tunable Nanorod Heterostructures. J. Phys. Chem. Lett. 2010, 1, 1051–1054. [Google Scholar] [CrossRef]
- Shiga, Y.; Umezawa, N.; Srinivasan, N.; Koyasu, S.; Sakai, E.; Miyauchi, M. A metal sulfide photocatalyst composed of ubiquitous elements for solar hydrogen production. Chem. Commun. 2016, 52, 7470–7473. [Google Scholar] [CrossRef] [Green Version]
- Hou, H.; Yuan, Y.; Cao, S.; Yang, Y.; Ye, X.; Yang, W. CuInS2 nanoparticles embedded in mesoporous TiO2 nanofibers for boosted photocatalytic hydrogen production. J. Mater. Chem. C 2020, 8, 11001–11007. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Kubacka, A.; Berestok, T.; Zhang, T.; Llorca, J.; Arbiol, J.; Cabot, A.; Fernández-García, M. Hydrogen photogeneration using ternary CuGaS2-TiO2-Pt nanocomposites. Int. J. Hydrogen Energy 2020, 45, 1510–1520. [Google Scholar] [CrossRef]
- Singh, J.; Soni, R. Enhanced sunlight driven photocatalytic activity of In2S3 nanosheets functionalized MoS2 nanoflowers heterostructures. Sci. Rep. 2021, 11, 15352. [Google Scholar] [CrossRef] [PubMed]
- Wojtyła, S.; Baran, T. Photocatalytic H2 production over RuO2@ZnS and RuO2@CuS nanostructures. Int. J. Hydrogen Energy 2019, 44, 14624–14634. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Wang, Z.; Qiu, J. A Li2S-based all-solid-state battery with high energy and superior safety. Sci. Adv. 2022, 8, eabl8390. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Mamiyev, Z. Integrated processes involving adsorption, photolysis, and photocatalysis. In Hybrid and Combined Processes for Air Pollution Control; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–153. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, Y.; Kim, J.; Whitten, A.E.; Wood, K.; Kani, K.; Rowan, A.E.; Henzie, J.; Yamauchi, Y. Mesoporous Metallic Iridium Nanosheets. J. Am. Chem. Soc. 2018, 140, 12434–12441. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Jung, H.S.; Kim, J.M.; Kang, Y.T. Photocatalytic CO2 conversion on highly ordered mesoporous materials: Comparisons of metal oxides and compound semiconductors. Appl. Catal. B 2018, 224, 594–601. [Google Scholar] [CrossRef]
- Tang, J.; Durrant, J.R.; Klug, D.R. Mechanism of Photocatalytic Water Splitting in TiO2. Reaction of Water with Photoholes, Importance of Charge Carrier Dynamics, and Evidence for Four-Hole Chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. [Google Scholar] [CrossRef]
- Meerbach, C.; Klemmed, B.; Spittel, D.; Bauer, C.; Park, Y.J.; Hubner, R.; Jeong, H.Y.; Erb, D.; Shin, H.S.; Lesnyak, V.; et al. General colloidal synthesis of transition-metal disulfide nanomaterials as electrocatalysts for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2020, 12, 13148–13155. [Google Scholar] [CrossRef] [PubMed]
- Mamiyev, Z.Q.; Balayeva, N.O. CuS nanoparticles synthesized by a facile chemical route under different pH conditions. Mendeleev Commun. 2016, 26, 235–237. [Google Scholar] [CrossRef]
- Devi, S.A.; Singh, K.J.; Devi, K.N. Visible light driven photocatalytic activities of metal sulfides synthesized by simple co-precipitation method. Mater. Today Proc. 2022, 65, 2819–2824. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, L.; Wang, C.; Wang, W.; Ling, T.; Yang, J.; Dong, C.; Lin, F.; Du, X.W. Zinc-Blende CdS Nanocubes with Coordinated Facets for Photocatalytic Water Splitting. ACS Catal. 2017, 7, 1470–1477. [Google Scholar] [CrossRef]
- Wu, K.; Rodríguez-Córdoba, W.E.; Liu, Z.; Zhu, H.; Lian, T. Beyond Band Alignment: Hole Localization Driven Formation of Three Spatially Separated Long-Lived Exciton States in CdSe/CdS Nanorods. ACS Nano 2013, 7, 7173–7185. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Y.; Zhou, Y.; Fan, F.; Han, Q.; Xu, Q.; Wang, X.; Xiao, M.; Li, C.; Zou, Z. Construction and Nanoscale Detection of Interfacial Charge Transfer of Elegant Z-Scheme WO3/Au/In2S3 Nanowire Arrays. Nano Lett. 2016, 16, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Shown, I.; Samireddi, S.; Chang, Y.C.; Putikam, R.; Chang, P.H.; Sabbah, A.; Fu, F.Y.; Chen, W.F.; Wu, C.I.; Yu, T.Y.; et al. Carbon-doped SnS2 nanostructure as a high-efficiency solar fuel catalyst under visible light. Nat. Commun 2018, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Guan, B.Y.; Lou, X.W.D. Construction of ZnIn2S4-In2O3 Hierarchical Tubular Heterostructures for Efficient CO2 Photoreduction. J. Am. Chem. Soc. 2018, 140, 5037–5040. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; An, Q.; Watanabe, M.; Cheng, J.; Ho Kim, H.; Akbay, T.; Takagaki, A.; Ishihara, T. Highly correlation of CO2 reduction selectivity and surface electron Accumulation: A case study of Au-MoS2 and Ag-MoS2 catalyst. Appl. Catal. B 2020, 271, 118931. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Hou, C.; Chen, Y.; He, T. Highly efficient visible-light driven solar-fuel production over tetra(4-carboxyphenyl)porphyrin iron(III) chloride using CdS/Bi2S3 heterostructure as photosensitizer. Appl. Catal. B 2018, 238, 656–663. [Google Scholar] [CrossRef]
- Huang, L.; Li, B.; Su, B.; Xiong, Z.; Zhang, C.; Hou, Y.; Ding, Z.; Wang, S. Fabrication of hierarchical Co3O4@ CdIn2S4 p–n heterojunction photocatalysts for improved CO2 reduction with visible light. J. Mater. Chem. A 2020, 8, 7177–7183. [Google Scholar] [CrossRef]
- Deng, F.; Lu, X.; Luo, Y.; Wang, J.; Che, W.; Yang, R.; Luo, X.; Luo, S.; Dionysiou, D.D. Novel visible-light-driven direct Z-scheme CdS/CuInS2 nanoplates for excellent photocatalytic degradation performance and highly-efficient Cr(VI) reduction. J. Chem. Eng. 2019, 361, 1451–1461. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Yang, P.; Huang, C.; Wang, X. Boron Carbon Nitride Semiconductors Decorated with CdS Nanoparticles for Photocatalytic Reduction of CO2. ACS Catal. 2018, 8, 4928–4936. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A. Novel Cu2ZnSnS4/Pt/g-C3N4 heterojunction photocatalyst with straddling band configuration for enhanced solar to fuel conversion. Appl. Catal. B 2020, 277, 119239. [Google Scholar] [CrossRef]
- Wu, S.; Pang, H.; Zhou, W.; Yang, B.; Meng, X.; Qiu, X.; Chen, G.; Zhang, L.; Wang, S.; Liu, X.; et al. Stabilizing CuGaS2 by crystalline CdS through an interfacial Z-scheme charge transfer for enhanced photocatalytic CO2 reduction under visible light. Nanoscale 2020, 12, 8693–8700. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Xu, J.; Shao, Y.; Wu, J.; Xu, X.; Pan, Y.; Ju, H.; Zhu, J.; Xie, Y. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 2019, 4, 690–699. [Google Scholar] [CrossRef]
- Kočí, K.; Matějová, L.; Kozák, O.; Čapek, L.; Valeš, V.; Reli, M.; Praus, P.; Šafářová, K.; Kotarba, A.; Obalová, L. ZnS/MMT nanocomposites: The effect of ZnS loading in MMT on the photocatalytic reduction of carbon dioxide. Appl. Catal. B 2014, 158–159, 410–417. [Google Scholar] [CrossRef]
- Sharma, N.; Das, T.; Kumar, S.; Bhosale, R.; Kabir, M.; Ogale, S. Photocatalytic Activation and Reduction of CO2 to CH4 over Single Phase Nano Cu3SnS4: A Combined Experimental and Theoretical Study. ACS Appl. Energy Mater. 2019, 2, 5677–5685. [Google Scholar] [CrossRef]
- Guo, Y.; Ao, Y.; Wang, P.; Wang, C. Mediator-free direct dual-Z-scheme Bi2S3/BiVO4/MgIn2S4 composite photocatalysts with enhanced visible-light-driven performance towards carbamazepine degradation. Appl. Catal. B 2019, 254, 479–490. [Google Scholar] [CrossRef]
- Zeng, R.; Lian, K.; Su, B.; Lu, L.; Lin, J.; Tang, D.; Lin, S.; Wang, X. Versatile synthesis of hollow metal sulfides via reverse cation exchange reactions for photocatalytic CO2 reduction. Angew. Chem. Int. Ed. 2021, 60, 25055–25062. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Chen, B. Uniformly Dispersed Metal Sulfide Nanodots on g-C3N4 as Bifunctional Catalysts for High-Efficiency Photocatalytic H2 and H2O2 Production under Visible-Light Irradiation. Energy Fuels 2021, 35, 10746–10755. [Google Scholar] [CrossRef]
- Liu, H.; Tan, P.; Liu, Y.; Zhai, H.; Du, W.; Liu, X.; Pan, J. Ultrafast interfacial charge evolution of the Type-II cadmium Sulfide/Molybdenum disulfide heterostructure for photocatalytic hydrogen production. J. Colloid Interface Sci. 2022, 619, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, D.; Su, P.; Jiang, Z.; Jin, Z. S-scheme W18O49/Mn0.2Cd0.8S Heterojunction for Improved Photocatalytic Hydrogen Evolution. ChemCatChem 2021, 13, 2179–2190. [Google Scholar] [CrossRef]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All-solid-state Z-scheme in CdS–Au–TiO2 three-component nanojunction system. Nat. Mater. 2006, 5, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Balayeva, N.O.; Mamiyev, Z.Q. Synthesis and studies of CdS and ZnS-PE/NBR modified thermoplastic elastomeric copolymer nanocomposite films. Mater. Lett. 2016, 162, 121–125. [Google Scholar] [CrossRef]
- Yendrapati, T.P.; Soumya, J.; Bojja, S.; Pal, U. Robust Co9S8@CdIn2S4 cage for efficient photocatalytic H2 evolution. J. Phys. Chem. C 2021, 125, 5099–5109. [Google Scholar] [CrossRef]
- Gong, H.; Hao, X.; Li, H.; Jin, Z. A novel materials manganese cadmium sulfide/cobalt nitride for efficiently photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 585, 217–228. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Chen, C. Microwave synthesis of Zn, Cd binary metal sulfides with superior photocatalytic H2 evolution performance. Inorg. Chem Commun. 2021, 134, 108993. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.Z.; Li, W.; Zhang, Z.H. Visible-Light-Mediated Oxidative Amidation of Aldehydes by Using Magnetic CdS Quantum Dots as a Photocatalyst. Chem. Eur. J. 2021, 27, 5483–5491. [Google Scholar] [CrossRef]
- Mamiyev, Z.Q.; Balayeva, N.O. Synthesis and characterization of CdS nanocrystals and maleic anhydride octene-1 copolymer nanocomposite materials by the chemical in-situ technique. Compos. B. Eng. 2015, 68, 431–435. [Google Scholar] [CrossRef]
- Long, H.; Wang, P.; Wang, X.; Chen, F.; Yu, H. Optimizing hydrogen adsorption of NixB cocatalyst by integrating P atom for enhanced photocatalytic H2-production activity of CdS. Appl. Surf. Sci. 2022, 604, 154457. [Google Scholar] [CrossRef]
- Li, K.; Chen, X.; Zhao, J.; She, H.; Huang, J.; Wang, L.; Wang, Q. Photodeposition Synthesis of CdS@Ni2P Composites for Efficacious Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2022, 5, 10207–10215. [Google Scholar] [CrossRef]
- Yin, X.L.; Li, L.L.; Gao, G.M.; Lu, Y.; Shang, Q.Q.; Zhao, H.T.; Li, D.C.; Dou, J.M. Direct Z-Scheme NiWO4/CdS nanosheets-on-nanorods nanoheterostructure for efficient visible-light-driven H2 generation. Int. J. Hydrogen Energy 2022, 47, 9895–9904. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Guo, W.; Xu, Q.; Min, Y. A high efficiency water hydrogen production method based on CdS/WN composite photocatalytic. J. Colloid Interface Sci. 2022, 613, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhong, W.; Gao, D.; Wang, X.; Wang, P.; Yu, H. Phosphorus-enriched platinum diphosphide nanodots as a highly efficient cocatalyst for photocatalytic H2 evolution of CdS. J. Chem. Eng. 2022, 439, 135758. [Google Scholar] [CrossRef]
- Lin, Q.; Liang, S.; Wang, J.; Zhang, R.; Wang, X. Cadmium Sulfide 3D Photonic Crystal with Hierarchically Ordered Macropores for Highly Efficient Photocatalytic Hydrogen Generation. Inorg. Chem. 2022, 61, 2920–2928. [Google Scholar] [CrossRef]
- Luan, X.; Dai, H.; Li, Q.; Xu, F.; Mai, Y. A Hybrid Photocatalyst Composed of CdS Nanoparticles and Graphene Nanoribbons for Visible-Light-Driven Hydrogen Production. ACS Appl. Energy Mater. 2022, 5, 8621–8628. [Google Scholar] [CrossRef]
- Lei, Y.; Ng, K.H.; Zhang, Y.; Li, Z.; Xu, S.; Huang, J.; Lai, Y. One-pot loading of cadmium sulfide onto tungsten carbide for efficient photocatalytic H2 evolution under visible light irradiation. J. Chem. Eng. 2022, 434, 134689. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, L.; Guo, C.; Chen, T.; Feng, C.; Liu, Z.; Qi, Y.; Wang, W.; Wang, J. Ni-doped CdS porous cubes prepared from prussian blue nanoarchitectonics with enhanced photocatalytic hydrogen evolution performance. Int. J. Hydrogen Energy 2022, 47, 3752–3761. [Google Scholar] [CrossRef]
- Gao, D.; Xu, J.; Wang, L.; Zhu, B.; Yu, H.; Yu, J. Optimizing Atomic Hydrogen Desorption of Sulfur-Rich NiS1+x Cocatalyst for Boosting Photocatalytic H2 Evolution. Adv. Mater. 2022, 34, 2108475. [Google Scholar] [CrossRef]
- Balayeva, O.O.; Azizov, A.A.; Muradov, M.B.; Maharramov, A.M.; Eyvazova, G.M.; Alosmanov, R.M.; Mamiyev, Z.Q.; Aghamaliyev, Z.A. β-NiS and Ni3S4 nanostructures: Fabrication and characterization. Mater. Res. Bull. 2016, 75, 155–161. [Google Scholar] [CrossRef]
- Muniyappa, M.; Kalegowda, S.N.; Shetty, M.; Sriramoju, J.B.; Shastri, M.; Rao, S.V.N.; De, D.; Shankar, M.V.; Rangappa, D. Cocatalyst free nickel sulphide nanostructure for enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 5307–5318. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Jiang, X.; Wang, J.; Xu, L.; Qiu, J.; Lu, W.; Chen, D.; Li, Z. Activate Fe3S4 nanorods by Ni doping for efficient dye-sensitized photocatalytic hydrogen production. ACS Appl. Mater. 2021, 13, 14198–14206. [Google Scholar] [CrossRef]
- Sarilmaz, A.; Yanalak, G.; Aslan, E.; Ozel, F.; Patir, I.H.; Ersoz, M. Shape-controlled synthesis of copper based multinary sulfide catalysts for enhanced photocatalytic hydrogen evolution. Renew. Energy 2021, 164, 254–259. [Google Scholar] [CrossRef]
- Praveen Kumar, D.; Rangappa, A.P.; Kim, S.; Kim, E.; Reddy, K.A.J.; Gopannagari, M.; Bhavani, P.; Reddy, D.A.; Kim, T.K. Boosting charge transfers in cadmium sulfide nanorods with a few layered Ni-doped MoS2 nanosheets for enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Tao, C.L.; Xie, Z.; Xu, D.; Li, X.; Ge, F.; Chen, F.; Jiang, Z.; Gu, W.; Cheng, F.; Wu, X.J. Templated Synthesis of Ultrathin Indium-Based Ternary Metal Sulfide (MIn2S4, M = Zn, Cd, and Ni) Nanoplates for Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2022, 5, 4877–4884. [Google Scholar] [CrossRef]
- Pan, R.; Hu, M.; Liu, J.; Li, D.; Wan, X.; Wang, H.; Li, Y.; Zhang, X.; Wang, X.; Jiang, J.; et al. Two-dimensional all-in-one sulfide monolayers driving photocatalytic overall water splitting. Nano Lett. 2021, 21, 6228–6236. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, Y.; Li, Z.; Xu, S.; Huang, J.; Ng, K.H.; Lai, Y. Molybdenum sulfide cocatalyst activation upon photodeposition of cobalt for improved photocatalytic hydrogen production activity of ZnCdS. J. Chem. Eng. 2021, 425, 131478. [Google Scholar] [CrossRef]
- Zhong, W.; Gao, D.; Yu, H.; Fan, J.; Yu, J. Novel amorphous NiCuSx H2-evolution cocatalyst: Optimizing surface hydrogen desorption for efficient photocatalytic activity. J. Chem. Eng. 2021, 419, 129652. [Google Scholar] [CrossRef]
- Xu, W.; Xie, Z.; Han, W.; Zhang, K.; Guo, D.; Chang, K. Rational design of interfacial energy level matching for CuGaS2 based photocatalysts over hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 11853–11862. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.; Xu, Y.; Zhao, R.; Han, J.; Wang, L. Facile fabrication of CdSe/CuInS2 microflowers with efficient photocatalytic hydrogen production activity. Int. J. Hydrogen Energy 2022, 47, 8294–8302. [Google Scholar] [CrossRef]
- Feng, K.; Wang, C.; Hu, X.; Fan, J.; Liu, E. Highly efficient photocatalytic H2 evolution over Ni-doped Mn0.5Cd0.5S nanorods under visible light. Int. J. Energy Res. 2022, 46, 20828–20837. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Pan, X.; Wang, T.; Li, Y. Ultrathin Nickel-doped ZnIn2S4 Nanosheets with Sulfur Vacancies for Efficient Photocatalytic Hydrogen Evolution. ChemCatChem 2021, 13, 5148–5155. [Google Scholar] [CrossRef]
- Hou, T.; Liang, J.; Wang, L.; Zheng, Z.; Wang, J.; Xing, X.; Tang, H.; Zeng, C.; Wang, B. Cd1-xZnxS biomineralized by engineered bacterium for efficient photocatalytic hydrogen production. Mater. Today Energy 2021, 22, 100869. [Google Scholar] [CrossRef]
- Li, F.; Jiang, J.; Li, N.; Gao, Y.; Ge, L. Design and fabrication of hollow structured Cu2MoS4/ZnIn2S4 nanocubes with significant enhanced photocatalytic hydrogen evolution performance. Int. J. Hydrogen Energy 2021, 46, 37847–37859. [Google Scholar] [CrossRef]
- Raja, A.; Son, N.; Swaminathan, M.; Kang, M. Facile synthesis of sphere-like structured ZnIn2S4-rGO-CuInS2 ternary heterojunction catalyst for efficient visible-active photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 602, 669–679. [Google Scholar] [CrossRef]
- Chong, W.K.; Ng, B.J.; Er, C.C.; Tan, L.L.; Chai, S.P. Insights from density functional theory calculations on heteroatom P-doped ZnIn2S4 bilayer nanosheets with atomic-level charge steering for photocatalytic water splitting. Sci. Rep. 2022, 12, 1927. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Chen, H.; Zhou, Y.; Luo, X.; Tian, D.; Yan, X.; Duan, R.; Di, J.; Kang, L.; Zhou, A.; et al. 2D/2D atomic double-layer WS2/Nb2O5 shell/core nanosheets with ultrafast interfacial charge transfer for boosting photocatalytic H2 evolution. Chin. Chem. Lett. 2021, 32, 3128–3132. [Google Scholar] [CrossRef]
- Ren, X.; Shi, J.; Duan, R.; Di, J.; Xue, C.; Luo, X.; Liu, Q.; Xia, M.; Lin, B.; Tang, W. Construction of high-efficiency CoS@Nb2O5 heterojunctions accelerating charge transfer for boosting photocatalytic hydrogen evolution. Chin. Chem. Lett. 2022, 33, 4700–4704. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Li, Y.; Guo, X.; Jin, Z. Lotus-leaf-like Bi2O2CO3 nanosheet combined with MO2S3 for higher photocatalytic hydrogen evolution. Sep. Purif. Technol. 2022, 288, 120588. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Z.; Shi, Q.; Yang, J.; Xie, M.; Han, W. Toward photocatalytic hydrogen generation over BiVO4 by controlling particle size. Chin. Chem. Lett. 2021, 32, 2419–2422. [Google Scholar] [CrossRef]
- Gogoi, D.; Shah, A.K.; Rambabu, P.; Qureshi, M.; Golder, A.K.; Peela, N.R. Step-Scheme Heterojunction between CdS Nanowires and Facet-Selective Assembly of MnOx-BiVO4 for an Efficient Visible-Light-Driven Overall Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 45475–45487. [Google Scholar] [CrossRef]
- Iwase, A.; Yoshino, S.; Takayama, T.; Ng, Y.H.; Amal, R.; Kudo, A. Water Splitting and CO2 Reduction under Visible Light Irradiation Using Z-Scheme Systems Consisting of Metal Sulfides, CoOx-Loaded BiVO4, and a Reduced Graphene Oxide Electron Mediator. J. Am. Chem. Soc. 2016, 138, 10260–10264. [Google Scholar] [CrossRef]
- Ghouri, M.; Ahmed, E.; Ali, A.; Ramzan, M.; Irfan, M. Improved photocatalytic H2 evolution over composites based on niobium pentoxide, metal sulfides and graphene. Mater. Sci. Semicond. Process. 2021, 122, 105492. [Google Scholar] [CrossRef]
- Liu, M.; Li, P.; Wang, S.; Liu, Y.; Zhang, J.; Chen, L.; Wang, J.; Liu, Y.; Shen, Q.; Qu, P.; et al. Hierarchically porous hydrangea-like In2S3/In2O3 heterostructures for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 587, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, Q.; Guo, E.; Wei, M.; Pang, Y. Hierarchical Co9S8/ZnIn2S4 Nanoflower Enables Enhanced Hydrogen Evolution Photocatalysis. Energy Fuels 2022, 36, 4541–4548. [Google Scholar] [CrossRef]
- Xiang, D.; Hao, X.; Jin, Z. Cu/CdS/MnOx Nanostructure-Based Photocatalyst for Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 13848–13860. [Google Scholar] [CrossRef]
- Khanal, V.; Balayeva, N.O.; Günnemann, C.; Mamiyev, Z.; Dillert, R.; Bahnemann, D.W.; Subramanian, V.R. Photocatalytic NOx removal using tantalum oxide nanoparticles: A benign pathway. Appl. Catal. B 2021, 291, 119974. [Google Scholar] [CrossRef]
- Kohlsdorf, A.; Taffa, D.H.; Wark, M. Microwave assisted synthesis of Ta2O5 nanostructures for photocatalytic hydrogen production. J. Photochem. Photobiol. A Chem. 2018, 366, 41–47. [Google Scholar] [CrossRef]
- Ravi, P.; Navakoteswara Rao, V.; Shankar, M.; Sathish, M. Heterojunction engineering at ternary Cu2S/Ta2O5/CdS nanocomposite for enhanced visible light-driven photocatalytic hydrogen evolution. Mater. Today Energy 2021, 21, 100779. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z.; Tsubaki, N. Activating and optimizing the MoS2@MoO3 S-scheme heterojunction catalyst through interface engineering to form a sulfur-rich surface for photocatalyst hydrogen evolution. J. Chem. Eng. 2022, 438, 135238. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Fleisch, M.; Bahnemann, D.W. Surface-grafted WO3/TiO2 photocatalysts: Enhanced visible-light activity towards indoor air purification. Catal. Today 2018, 313, 63–71. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Visible-light-mediated photocatalytic aerobic dehydrogenation of N-heterocycles by surface-grafted TiO2 and 4-amino-TEMPO. ACS Catal. 2019, 9, 10694–10704. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Mamiyev, Z.; Dillert, R.; Zheng, N.; Bahnemann, D.W. Rh/TiO2-Photocatalyzed Acceptorless Dehydrogenation of N-Heterocycles upon Visible-Light Illumination. ACS Catal. 2020, 10, 5542–5553. [Google Scholar] [CrossRef]
- Lin, B.; Ma, B.; Chen, J.; Zhou, Y.; Zhou, J.; Yan, X.; Xue, C.; Luo, X.; Liu, Q.; Wang, J.; et al. Sea-urchin-like ReS2 nanosheets with charge edge-collection effect as a novel cocatalyst for high-efficiency photocatalytic H2 evolution. Chin. Chem. Lett. 2022, 33, 943–947. [Google Scholar] [CrossRef]
- Rao, V.N.; Ravi, P.; Sathish, M.; Sakar, M.; Yang, B.L.; Yang, J.M.; Kumari, M.M.; Shankar, M. Titanate quantum dots-sensitized Cu2S nanocomposites for superficial H2 production via photocatalytic water splitting. Int. J. Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Chen, F.; Feng, H.F.; Luo, W.; Wang, P.; Yu, H.G.; Fan, J.J. Simultaneous realization of direct photodeposition and high H2-production activity of amorphous cobalt sulfide nanodot-modified rGO/TiO2 photocatalyst. Rare Met. 2021, 40, 3125–3134. [Google Scholar] [CrossRef]
- Yu, B.; Meng, F.; Zhou, T.; Fan, A.; Khan, M.W.; Wu, H.; Liu, X. Construction of hollow TiO2/CuS nanoboxes for boosting full-spectrum driven photocatalytic hydrogen evolution and environmental remediation. Ceram. Int. 2021, 47, 8849–8858. [Google Scholar] [CrossRef]
- Dong, L.; Wang, P.; Yu, H. EDTA-assisted synthesis of amorphous BiSx nanodots for improving photocatalytic hydrogen-evolution rate of TiO2. J. Alloys Compd. 2021, 887, 161425. [Google Scholar] [CrossRef]
- Liu, R.; Fang, S.Y.; Dong, C.D.; Tsai, K.C.; Yang, W.D. Enhancing hydrogen evolution of water splitting under solar spectra using Au/TiO2 heterojunction photocatalysts. Int. J. Hydrogen Energy 2021, 46, 28462–28473. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Cao, T.M.; Balayeva, N.O.; Pham, V.V. Thermal treatment of polyvinyl alcohol for coupling MoS2 and TiO2 nanotube arrays toward enhancing photoelectrochemical water splitting performance. Catalysts 2021, 11, 857. [Google Scholar] [CrossRef]
- Zhang, M.; Zhong, W.; Xu, Y.; Xue, F.; Fan, J.; Yu, H. Photoinduced synthesis of ultrasmall amorphous NiWSx nanodots for boosting photocatalytic H2-evolution activity of TiO2. J. Phys. Chem. Solids. 2021, 149, 109796. [Google Scholar] [CrossRef]
- Dai, X.; Feng, S.; Wu, W.; Zhou, Y.; Ye, Z.; Cao, X.; Wang, Y.; Yang, C. Photocatalytic hydrogen evolution and antibiotic degradation by S-scheme ZnCO2S4/TiO2. Int. J. Hydrogen Energy 2022, 47, 25104–25116. [Google Scholar] [CrossRef]
- Qiu, T.; Wang, L.; Zhou, B.; Zhu, Y.; Zhuang, C.; Liu, Q.; Shen, Q.; Xiong, Y.; Zhou, Y.; Zou, Z. Molybdenum Sulfide Quantum Dots Decorated on TiO2 for Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 702–709. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xian, Q.; Zhao, Y. Direct Z-scheme TiO2-ZnIn2S4 nanoflowers for cocatalyst-free photocatalytic water splitting. Appl. Catal. B 2021, 291, 120126. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y.; Pan, R.; Hao, Q.; Wu, Y.; van Ree, T.; Holze, R. Regulating Graphitic Carbon Nitride/Cocatalyst by an Amorphous MoS2 Conformal Multifunctional Intermediate Layer for Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2021, 4, 13288–13296. [Google Scholar] [CrossRef]
- Mao, L.; Lu, B.; Shi, J.; Zhang, Y.; Kang, X.; Chen, Y.; Jin, H.; Guo, L. Rapid high-temperature hydrothermal post treatment on graphitic carbon nitride for enhanced photocatalytic H2 evolution. Catal. Today, 2022; in press. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, J.; Zhang, C.; Cao, X. MoS2 and Fe2O3 co-modify g-C3N4 to improve the performance of photocatalytic hydrogen production. Sci. Rep. 2022, 12, 3261. [Google Scholar] [CrossRef]
- Wang, J.; Guan, Z.; Huang, J.; Li, Q.; Yang, J. Enhanced photocatalytic mechanism for the hybrid gC3N4/MoS2 nanocomposite. J. Mater. Chem. A 2014, 2, 7960–7966. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Zhao, J.; Wang, H.; Liu, R. Accelerated exciton dissociation and electron extraction across the metallic sulfide-carbon nitride ohmic interface for efficient photocatalytic hydrogen production. J. Mater. Chem. A 2021, 9, 16522–16531. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, J.; Wang, X.; Hong, X.; Fan, J.; Yu, H. Sulfur-mediated photodeposition synthesis of NiS cocatalyst for boosting H2-evolution performance of g-C3N4 photocatalyst. Chin. J. Catal. 2021, 42, 37–45. [Google Scholar] [CrossRef]
- Lin, B.; Zhou, Y.; Xu, B.; Zhu, C.; Tang, W.; Niu, Y.; Di, J.; Song, P.; Zhou, J.; Luo, X.; et al. 2D PtS nanorectangles/gC3N4 nanosheets with a metal sulfide–support interaction effect for high-efficiency photocatalytic H2 evolution. Mater. Horizons 2021, 8, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Song, L.; Li, X.; Chen, L.; Li, X.; Sun, J.; Zhang, X.; Wang, Y.; Tian, X. Interfacial Engineering of the Platinum/Molybdenum Disulfide/graphitic Carbon Nitride Composite for Enhanced Photocatalytic Hydrogen Production. ACS Appl. Energy Mater. 2022, 5, 8800–8811. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Shao, X.; Zhao, X.; Ghufran, S.; Wang, L.; Wu, R.; Guo, J.; Yang, C. Cooperative effects of zinc–nickel sulfides as a dual cocatalyst for the enhanced photocatalytic hydrogen evolution activity of g-C3N4. J. Environ. Chem. Eng. 2022, 10, 107216. [Google Scholar] [CrossRef]
- Lei, W.; Wang, F.; Pan, X.; Ye, Z.; Lu, B. Z-scheme MoO3-2D SnS nanosheets heterojunction assisted g-C3N4 composite for enhanced photocatalytic hydrogen evolutions. Int. J. Hydrogen Energy 2022, 47, 10877–10890. [Google Scholar] [CrossRef]
- Li, J.; Wu, W.; Li, Y.; Zhang, H.; Xu, X.; Jiang, Y.; Lin, K. In Situ Synthesized Rodlike MoS2 as a Cocatalyst for Enhanced Photocatalytic Hydrogen Evolution by Graphitic Carbon Nitride without a Noble Metal. ACS Appl. Energy Mater. 2021, 4, 11836–11843. [Google Scholar] [CrossRef]
- Li, J.; Cao, W.; Li, Y.; Xu, X.; Jiang, Y.; Lin, K. In Situ Synthesis of Hybrid-Phase WS2 with S Defects as a Cocatalyst for the g-C3N4-Based Photocatalytic Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 9463–9470. [Google Scholar] [CrossRef]
- Ma, X.; Lei, Z.; Wang, C.; Fu, Z.; Hu, X.; Fan, J.; Liu, E. Fabrication of P-doped Co9S8/g-C3N4 heterojunction for excellent photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 36781–36791. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Fan, J.; Sun, W.; Liu, E. S-scheme bimetallic sulfide ZnCO2S4/g-C3N4 heterojunction for photocatalytic H2 evolution. Ceram. Int. 2021, 47, 30194–30202. [Google Scholar] [CrossRef]
- Wang, W.; Tao, Y.; Du, L.; Wei, Z.; Yan, Z.; Chan, W.K.; Lian, Z.; Zhu, R.; Phillips, D.L.; Li, G. Femtosecond time-resolved spectroscopic observation of long-lived charge separation in bimetallic sulfide/g-C3N4 for boosting photocatalytic H2 evolution. Appl. Catal. B 2021, 282, 119568. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Wang, W.; He, H.; Deng, L.; Zhang, Y.; Huang, J.; Zhao, N.; Yu, G.; Liu, Y.N. Design of well-defined shell-core covalent organic frameworks/metal sulfide as an efficient Z-scheme heterojunction for photocatalytic water splitting. Chem. Sci. 2021, 12, 16065–16073. [Google Scholar] [CrossRef]
- Jin, Z.; Gong, H.; Li, H. Visible-light-driven two dimensional metal-organic framework modified manganese cadmium sulfide for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 603, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Xiang, Z.; Liu, D.; Yang, Q. Bimetallic MOF-Derived Sulfides with Heterojunction Interfaces Synthesized for Photocatalytic Hydrogen Evolution. Ind. Eng. Chem. Res. 2021, 60, 11439–11449. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, H.; Li, Y.; Fang, J.; Chen, C. Ce-based organic framework enhanced the hydrogen evolution ability of ZnCdS photocatalyst. Int. J. Hydrogen Energy 2022, 47, 962–970. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, H.; Wang, X.; Li, L.; Zhang, J.; Zhang, H.; Li, Y.F.; Dai, W.L. Robust hollow tubular ZnIn2S4 modified with embedded metal-organic-framework-layers: Extraordinarily high photocatalytic hydrogen evolution activity under simulated and real sunlight irradiation. Appl. Catal. B 2021, 298, 120632. [Google Scholar] [CrossRef]
- Lian, Z.; Li, Z.; Wu, F.; Zhong, Y.; Liu, Y.; Wang, W.; Zi, J.; Yang, W. Photogenerated hole traps in metal-organic-framework photocatalysts for visible-light-driven hydrogen evolution. Commun. Chem 2022, 5, 93. [Google Scholar] [CrossRef]
- Cao, A.; Zhang, M.; Su, X.; Romanovski, V.; Chu, S. In Situ Fabrication of NiS2-Decorated Graphitic Carbon Nitride/Metal-Organic Framework Nanostructures for Photocatalytic H2 Evolution. ACS Appl. Nano Mater. 2022, 5, 5416–5424. [Google Scholar] [CrossRef]
- Ghosh, A.; Karmakar, S.; Rahimi, F.A.; Roy, R.S.; Nath, S.; Gautam, U.K.; Maji, T.K. Confinement Matters: Stabilization of CdS Nanoparticles inside a Postmodified MOF toward Photocatalytic Hydrogen Evolution. ACS Appl. Mater. 2022, 14, 25220–25231. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, J.; Liu, L.; Li, X.; Liang, Q.; Li, Z. Self-Assembled Zeolitic Imidazolate Framework/CdS Hollow Microspheres with Efficient Charge Separation for Enhanced Photocatalytic Hydrogen Evolution. Inorg. Chem 2022, 61, 10598–10608. [Google Scholar] [CrossRef]
- Cheng, L.; Yu, Y.; Huang, R.; Shi, X. Lollipop-shaped Co9S8/CdS nanocomposite derived from zeolitic imidazolate framework-67 for the photocatalytic hydrogen production. Int. J. Hydrogen Energy 2021, 46, 31288–31299. [Google Scholar] [CrossRef]
- Xi, X.; Dang, Q.; Wang, G.; Chen, W.; Tang, L. ZIF-67-derived flower-like ZnIn2S4@CoS2 heterostructures for photocatalytic hydrogen production. New J. Chem. 2021, 45, 20289–20295. [Google Scholar] [CrossRef]
- Pang, X.; Xue, S.; Zhou, T.; Xu, Q.; Lei, W. 2D/2D nanohybrid of Ti3C2 MXene/WO3 photocatalytic membranes for efficient water purification. Ceram. Int. 2022, 48, 3659–3668. [Google Scholar] [CrossRef]
- Fang, H.; Cai, J.; Li, H.; Wang, J.; Li, Y.; Zhou, W.; Mao, K.; Xu, Q. Fabrication of Ultrathin Two-Dimensional/Two-Dimensional MoS2/ZnIn2S4 Hybrid Nanosheets for Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2022, 5, 8232–8240. [Google Scholar] [CrossRef]
- Mamiyev, Z.Q.; Balayeva, N.O. Optical and structural studies of ZnS nanoparticles synthesized via chemical in situ technique. Chem. Phys. Lett. 2016, 646, 69–74. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Askerova, O.O.; Azizov, A.A.; Alosmanov, R.M.; Eyvazova, G.M.; Muradov, M.B. Synthesis of CuS and PbS nanocrystals on the basis of PE/NBR polymer/elastomeric composites for their applications. Compos. B. Eng. 2013, 53, 391–394. [Google Scholar] [CrossRef]
- Guo, J.; Liang, Y.; Liu, L.; Hu, J.; Wang, H.; An, W.; Cui, W. Core-shell structure of sulphur vacancies-CdS@CuS: Enhanced photocatalytic hydrogen generation activity based on photoinduced interfacial charge transfer. J. Colloid Interface Sci. 2021, 600, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bie, C.; He, B.; Zhu, B.; Zhang, L.; Cheng, B. 0D/2D NiS/CdS nanocomposite heterojunction photocatalyst with enhanced photocatalytic H2 evolution activity. Appl. Surf. Sci. 2021, 554, 149622. [Google Scholar] [CrossRef]
- Tian, J.; Xue, W.; Li, M.; Sun, T.; Hu, X.; Fan, J.; Liu, E. Amorphous CoSx decorated Cd0.5Zn0.5S with a bulk-twinned homojunction for efficient photocatalytic hydrogen evolution. Catal. Sci. Technol. 2022, 12, 3165–3174. [Google Scholar] [CrossRef]
- Liu, S.; Ma, Y.; Chi, D.; Sun, Y.; Chen, Q.; Zhang, J.; He, Z.; He, L.; Zhang, K.; Liu, B. Hollow heterostructure CoS/CdS photocatalysts with enhanced charge transfer for photocatalytic hydrogen production from seawater. Int. J. Hydrogen Energy 2022, 47, 9220–9229. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Xue, C.; Sun, Y.; Wu, C.; Shao, G.; Zhang, P. Controllable construction of hierarchically CdIn2S4/CNFs/Co4S3 nanofiber networks towards photocatalytic hydrogen evolution. J. Chem. Eng. 2021, 419, 129213. [Google Scholar] [CrossRef]
- Zhao, H.; Fu, H.; Yang, X.; Xiong, S.; Han, D.; An, X. MoS2/CdS rod-like nanocomposites as high-performance visible light photocatalyst for water splitting photocatalytic hydrogen production. Int. J. Hydrogen Energy 2022, 47, 8247–8260. [Google Scholar] [CrossRef]
- Li, P.; Liu, M.; Li, J.; Guo, J.; Zhou, Q.; Zhao, X.; Wang, S.; Wang, L.; Wang, J.; Chen, Y.; et al. Atomic heterojunction-induced accelerated charge transfer for boosted photocatalytic hydrogen evolution over 1D CdS nanorod/2D ZnIn2S4 nanosheet composites. J. Colloid Interface Sci. 2021, 604, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Chen, W.; Shen, R.; Jiang, Z.; Zhang, P.; Liu, W.; Li, X. Regulating interfacial morphology and charge-carrier utilization of Ti3C2 modified all-sulfide CdS/ZnIn2S4 S-scheme heterojunctions for effective photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 112, 85–95. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, Y.G.; Chen, J.; Huang, C.Y.; Hsieh, S.C.; Wu, S.Y. Ionic liquid/surfactant-hydrothermal synthesis of dendritic PbS@CuS core-shell photocatalysts with improved photocatalytic performance. Appl. Surf. Sci. 2021, 546, 149106. [Google Scholar] [CrossRef]
- Fan, H.T.; Wu, Z.; Liu, K.C.; Liu, W.S. Fabrication of 3D CuS@ZnIn2S4 hierarchical nanocages with 2D/2D nanosheet subunits p-n heterojunctions for improved photocatalytic hydrogen evolution. J. Chem. Eng. 2022, 433, 134474. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Jin, Z. Rational design of a cobalt sulfide/bismuth sulfide S-scheme heterojunction for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 592, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, W.; Yang, Y.; Liu, S.; Zhu, C.; Tian, Q. Core-shell Cu1.94S-MnS nanoheterostructures synthesized by cation exchange for enhanced photocatalytic hydrogen evolution. CrystEngComm 2021, 23, 6291–6299. [Google Scholar] [CrossRef]
- Zhou, H.; Xiong, H.; Zhang, R.; Zhang, L.; Zhang, L.; Li, L.; Zhang, W.; Zhu, Z.; Qiao, Z.A. A General Polymer-Oriented Acid-Mediated Self-Assembly Approach toward Crystalline Mesoporous Metal Sulfides. Small 2021, 17, 2100428. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Tzschoppe, M.; Huck, C.; Pucci, A.; Pfnür, H. Plasmon Standing Waves by Oxidation of Si (553)-Au. J. Phys. Chem. C 2019, 123, 9400–9406. [Google Scholar] [CrossRef] [Green Version]
- Himstedt, R.; Rusch, P.; Hinrichs, D.; Kodanek, T.; Lauth, J.; Kinge, S.; Siebbeles, L.D.A.; Dorfs, D. Localized Surface Plasmon Resonances of Various Nickel Sulfide Nanostructures and Au-Ni3S2 Core-Shell Nanoparticles. Chem. Mater. 2017, 29, 7371–7377. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Sanna, S.; Ziese, F.; Dues, C.; Tegenkamp, C.; Pfnür, H. Plasmon Localization by H Induced Band Switching. J. Phys. Chem. C 2019, 124, 958–967. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Tegenkamp, C.; Pfnür, H. Plasmon localization by adatoms in gold atomic wires on Si (775). J. Phys. Condens. Matter 2021, 33, 205001. [Google Scholar] [CrossRef] [PubMed]
- Faucheaux, J.A.; Stanton, A.L.D.; Jain, P.K. Plasmon Resonances of Semiconductor Nanocrystals: Physical Principles and New Opportunities. J. Phys. Chem. Lett. 2014, 5, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Mamiyev, Z.; Fink, C.; Holtgrewe, K.; Pfnür, H.; Sanna, S. Enforced Long-Range Order in 1D Wires by Coupling to Higher Dimensions. Phys. Rev. Lett. 2021, 126, 106101. [Google Scholar] [CrossRef]
- Ingram, D.B.; Linic, S. Water Splitting on Composite Plasmonic-Metal/Semiconductor Photoelectrodes: Evidence for Selective Plasmon-Induced Formation of Charge Carriers near the Semiconductor Surface. J. Am. Chem. Soc. 2011, 133, 5202–5205. [Google Scholar] [CrossRef]
- Chen, W.B.; Hu, L.Y.; Meng, F.; Tang, L.; Liang, S.; Li, J.B. Dual-plasmon-induced photocatalytic performance enhancement in Au-PbS-CdS nanodumbbells with double Au caps on the ends. Opt. Mater. 2021, 117, 111210. [Google Scholar] [CrossRef]
- Liu, Y.T.; Lu, M.Y.; Perng, T.P.; Chen, L.J. Plasmonic enhancement of hydrogen production by water splitting with CdS nanowires protected by metallic TiN overlayers as highly efficient photocatalysts. Nano Energy 2021, 89, 106407. [Google Scholar] [CrossRef]
- Tu, C.Y.; Wu, J.M. Localized surface plasmon resonance coupling with piezophototronic effect for enhancing hydrogen evolution reaction with Au@MoS2 nanoflowers. Nano Energy 2021, 87, 106131. [Google Scholar] [CrossRef]
- Yang, J.L.; He, Y.L.; Ren, H.; Zhong, H.L.; Lin, J.S.; Yang, W.M.; Li, M.D.; Yang, Z.L.; Zhang, H.; Tian, Z.Q.; et al. Boosting Photocatalytic Hydrogen Evolution Reaction Using Dual Plasmonic Antennas. ACS Catal. 2021, 11, 5047–5053. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, J.; Zeng, R.; Xing, F.; Huang, C. Schottky barrier tuning via surface plasmon and vacancies for enhanced photocatalytic H2 evolution in seawater. Appl. Catal. B 2022, 310, 121321. [Google Scholar] [CrossRef]
- Mandari, K.K.; Son, N.; Kim, Y.S.; Kang, M. Plasmonic quaternary heteronanostructures (HNSs) for improved solar light utilization, spatial charge separation, and stability in photocatalytic hydrogen production. J. Colloid Interface Sci. 2021, 582, 720–731. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Yang, F.; Zhang, Y.; Yan, L.; Li, K.; Guo, H.; Yan, J.; Lin, J. Construction of Au/g-C3N4/ZnIn2S4 plasma photocatalyst heterojunction composite with 3D hierarchical microarchitecture for visible-light-driven hydrogen production. Int. J. Hydrogen Energy 2022, 47, 2900–2913. [Google Scholar] [CrossRef]
- Ren, B.; Luan, Q.; Ma, L.; Ding, Y.; Ma, D.; Cao, X.; Guo, Y.; Guan, R.; Chen, Q. Amorphous domain induced LSPR Zn-Cr-In-S solid solution with enhanced visible photocatalytic H2 production. Mater. Chem. Phys. 2022, 285, 126100. [Google Scholar] [CrossRef]
- Simon, T.; Carlson, M.T.; Stolarczyk, J.K.; Feldmann, J. Electron Transfer Rate vs Recombination Losses in Photocatalytic H2 Generation on Pt-Decorated CdS Nanorods. ACS Energy Lett. 2016, 1, 1137–1142. [Google Scholar] [CrossRef]
- Xue, W.; Sun, H.; Hu, X.; Bai, X.; Fan, J.; Liu, E. UV-VIS-NIR-induced extraordinary H2 evolution over W18O49/Cd0.5Zn0.5S: Surface plasmon effect coupled with S-scheme charge transfer. Chin. J. Catal. 2022, 43, 234–245. [Google Scholar] [CrossRef]
- Zhong, W.; Wu, X.; Liu, Y.; Wang, X.; Fan, J.; Yu, H. Simultaneous realization of sulfur-rich surface and amorphous nanocluster of NiS1+x cocatalyst for efficient photocatalytic H2 evolution. Appl. Catal. B 2021, 280, 119455. [Google Scholar] [CrossRef]
















| Photocatalyst | Type/Junction | Rate (µmol ) | QYE (%) | Incident Light (, nm) | Ref. |
|---|---|---|---|---|---|
| WN/CdS | Schottky (p-n) | 24,130 | 19.8 | >420 | [55] |
| CdS@Ni2P | n-n | 287 | - | 480 | [53] |
| NiWO4/CdS nanosheets | Z-scheme | 26,430 | 22 | 420 | [54] |
| PtP2@C/CdS (10 wt%) | - | 9760 | 41.67 | 420 | [56] |
| 3 wt% WC/CdS | Schottky | 9180 | 14.3 | 420 | [59] |
| red-P/Co9S8 | Schottky | 4362 | - | UV-Vis | [119] |
| 25 wt%-ZnCo2S44/g-C3N4 | S-scheme | 6619 | - | Lab-solar | [120] |
| CuNiS/g-C3N4 | p-n | 752.8 | - | 800 | [121] |
| T-COF/CdS | Z-scheme | 500 | 37.8 | 365 | [122] |
| S/Ni-MOF-74 | type-II | 7104 | >420 | [123] | |
| CdS@NiS/MOFs | p-n | 42,700 | 13.23 | 450 | [124] |
| ZnCdS/UIO-66(Ce) | type-II | 3958 | - | UV-Vis | [125] |
| ZnIn2S4-MOF layer | - | 28,200 | 22.67 | 350 | [126] |
| Pt@NH2-UiO-66/CdS | type-II | 38,000 | 40.3 | 400 | [127] |
| CN/amorphous-MoS2/Pt | Schottky | 5830 | 8.51 | 400 | [107] |
| CoSx-rGO/TiO2 (10%) | - | 256.97 | 14.62 | 365 | [98] |
| TiO2@CuS | LSPR-Schottky | 2467 | 13.4/3.7 | UV-vis/near-IR | [99] |
| BiSx/TiO2 (1.0 wt%) | - | 803.2 | 3.86 | 365 | [100] |
| NiWSx-ND/TiO2 (3 wt%) | Schottky | 4580 | 13 | 365 | [103] |
| ZnCo2S4/TiO2 | S-scheme | 5580 | 11.5 | 420 | [104] |
| amorphous MoO2/TiO2 | co-catalyst | 880.3 | - | UV-Vis | [105] |
| TiO2-ZnIn2S4 | direct Z-scheme | 214.9 | 36.7/11.6 | UV/Vis | [106] |
| MoS2/Fe2O3/g-C3N4 | Z-scheme | 7820 | - | Vis | [109] |
| Co3S4/g-CN | Ohmic | 536.0 | 7.55 | 400 | [111] |
| NiS@g-C3N4-30 | co-catalyst | 3297 | - | Vis | [42] |
| NiS/g-C3N4 | co-catalyst | 244 | - | 420 | [112] |
| PtS/g-C3N4 nanosheets | co-catalyst | 1072.6 | 45.7 | 420 | [113] |
| Pt/MoS2/g-C3N4 | co-catalyst | 1595.3 | 30.9 | 435 | [114] |
| ZnS-NiS2/g-C3N4 | dual co-catalyst | 302.7 | 1.8 | >420 | [115] |
| 2D SnS/g-C3N4 nanosheets | Z-scheme | 818.93 | 0.55 | Solar-simulator | [116] |
| NixPB-rGO/CdS | p-n | 5790 | 9 | 420 | [52] |
| MoS2 /ZnIn2S4 | co-catalyst | 221.71 | 11.8 | 420 | [134] |
| CdS-SV@CuS(5%) | - | 1654.53 | 6.51 | 450 | [137] |
| W18O49/Cd0.5Zn0.5S | S-scheme | 147,700 | 45.3 | <460 | [166] |
| MoS2@MoO3 | S-scheme | 12,416.8 | 8.43 | 500 | [92] |
| CdSe/CuInS2 microflowers | p-n | 10,610.37 | 48.97 | 420 | [72] |
| 3% Ni-doped S | - | 108,300 | - | 420 | [73] |
| a-NiCuSx/TiO2 (3:1) | - | 427.9 | 34.67 | UV | [167] |
| Co9S8@CdIn2S4 | type-I | 4604 | - | Vis | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. https://doi.org/10.3390/catal12111316
Mamiyev Z, Balayeva NO. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts. 2022; 12(11):1316. https://doi.org/10.3390/catal12111316
Chicago/Turabian StyleMamiyev, Zamin, and Narmina O. Balayeva. 2022. "Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances" Catalysts 12, no. 11: 1316. https://doi.org/10.3390/catal12111316