Synthesis of Ce0.1La0.9MnO3 Perovskite for Degradation of Endocrine-Disrupting Chemicals under Visible Photons
Abstract
:1. Introduction
2. Results
2.1. Characterization Techniques
2.2. Photocatalytic Degradation Study
2.2.1. Degradation of 4-n-Nonylphenol (NP) and Bisphenol A (BPA)
2.2.2. Optimization Studies
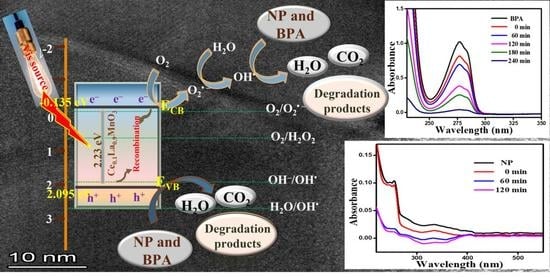
2.2.3. Proposed Photocatalytic Mechanism
2.2.4. FTIR Analysis
2.2.5. Recyclability and Photostability Study
3. Materials and Methods
3.1. Chemical Reagents
3.2. Synthesis of Perovskite-Type Ce0.1La0.9MnO3 (CLMO) Nanostructures
3.3. Characterization Details
3.4. Photocatalytic Activity Experimental Setup
3.5. Kinetics of Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, C.; Huang, X.; Wang, H.; Shi, H.; Zhao, G. Mechanism Investigation on the Enhanced Photocatalytic Oxidation of Nonylphenol on Hydrophobic TiO2 Nanotubes. J. Hazard. Mater. 2020, 382, 121017. [Google Scholar] [CrossRef]
- An, J.; Huang, M.; Wang, M.; Chen, J.; Wang, P. Removal of Nonylphenol by Using Fe-Doped NaBiO3 Compound as an Efficient Visible-Light-Heterogeneous Fenton-like Catalyst. Environ. Technol. 2019, 40, 3003–3016. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Liu, H.; Li, J.; Chen, Q.; Ma, D. Influence of Post-Treatment Temperature of TNTa Photoelectrodes on Photoelectrochemical Properties and Photocatalytic Degradation of 4-Nonylphenol. J. Solid State Chem. 2013, 199, 49–55. [Google Scholar] [CrossRef]
- Di Gioia, D.; Sciubba, L.; Bertin, L.; Barberio, C.; Salvadori, L.; Frassinetti, S.; Fava, F. Nonylphenol Polyethoxylate Degradation in Aqueous Waste by the Use of Batch and Continuous Biofilm Bioreactors. Water Res. 2009, 43, 2977–2988. [Google Scholar] [CrossRef] [PubMed]
- Naya, S.; Nikawa, T.; Kimura, K.; Tada, H. Rapid and Complete Removal of Nonylphenol by Gold Nanoparticle/Rutile Titanium (IV) Oxide Plasmon Photocatalyst. ACS Catal. 2013, 3, 903–907. [Google Scholar] [CrossRef]
- Giger, W.; Brunner, P.H.; Schaffner, C. 4-Nonylphenol in Sewage Sludge: Accumulation of Toxic Metabolites from Nonionic Surfactants. Science 1984, 225, 623–625. [Google Scholar] [CrossRef] [Green Version]
- Noorimotlagh, Z.; Kazeminezhad, I.; Jaafarzadeh, N.; Ahmadi, M.; Ramezani, Z. Improved Performance of Immobilized TiO2 under Visible Light for the Commercial Surfactant Degradation: Role of Carbon Doped TiO2 and Anatase/Rutile Ratio. Catal. Today 2020, 348, 277–289. [Google Scholar] [CrossRef]
- Naderi, M.; Zargham, D.; Asadi, A.; Bashti, T.; Kamayi, K. Short-Term Responses of Selected Endocrine Parameters in Juvenile Rainbow Trout (Oncorhynchus mykiss) Exposed to 4-Nonylphenol. Toxicol. Ind. Health 2015, 31, 1218–1228. [Google Scholar] [CrossRef]
- Duan, P.; Hu, C.; Butler, H.J.; Quan, C.; Chen, W.; Huang, W.; Tang, S.; Zhou, W.; Yuan, M.; Shi, Y.; et al. Effects of 4-Nonylphenol on Spermatogenesis and Induction of Testicular Apoptosis through Oxidative Stress-Related Pathways. Reprod. Toxicol. 2016, 62, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Wang, A.; Zhang, Y.; Tian, H. Effects of Nonylphenol on Immune Function of Female Sprague-Dawley Rats. Toxicol. Environ. Chem. 2013, 95, 658–668. [Google Scholar] [CrossRef]
- Wintgens, T.; Gallenkemper, M.; Melin, T. Occurrence and Removal of Endocrine Disrupters in Landfill Leachate Treatment Plants. Water Sci. Technol. 2003, 48, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.; Böhmer, W.; Müller, J.; Rüdel, H.; SchrÖter-Kermani, C. Retrospective Monitoring of Alkylphenols and Alkylphenol Monoethoxylates in Aquatic Biota from 1985 to 2001: Results from the German Environmental Specimen Bank. Environ. Sci. Technol. 2004, 38, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Česen, M.; Lenarčič, K.; Mislej, V.; Levstek, M.; Kovačič, A.; Cimrmančič, B.; Uranjek, N.; Kosjek, T.; Heath, D.; Dolenc, M.S.; et al. The Occurrence and Source Identification of Bisphenol Compounds in Wastewaters. Sci. Total Environ. 2018, 616, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xue, J.; Yao, H.; Wu, Q.; Venkatesan, A.K.; Halden, R.U.; Kannan, K. Occurrence and Estrogenic Potency of Eight Bisphenol Analogs in Sewage Sludge from the US EPA Targeted National Sewage Sludge Survey. J. Hazard. Mater. 2015, 299, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-H.; Zhang, X.-M.; Wang, F.; Gao, C.-J.; Chen, D.; Palumbo, J.R.; Guo, Y.; Zeng, E.Y. Occurrence of Bisphenol S in the Environment and Implications for Human Exposure: A Short Review. Sci. Total Environ. 2018, 615, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Garay-Rodríguez, L.F.; Zermeño, B.; López de la O, K.A.; Leyva, E.; Moctezuma, E. Photocatalytic Degradation of Bisphenol A: Kinetic Studies and Determination of the Reaction Pathway. J. Appl. Res. Technol. 2018, 16, 334–345. [Google Scholar]
- Guo, J.; Dai, Y.; Chen, X.; Zhou, L.; Liu, T. Synthesis and Characterization of Ag3PO4/LaCoO3 Nanocomposite with Superior Mineralization Potential for Bisphenol A Degradation under Visible Light. J. Alloys Compd. 2017, 696, 226–233. [Google Scholar] [CrossRef]
- Colombo, A.; Cappelletti, G.; Ardizzone, S.; Biraghi, I.; Bianchi, C.L.; Meroni, D.; Pirola, C.; Spadavecchia, F. Bisphenol A Endocrine Disruptor Complete Degradation Using TiO2 Photocatalysis with Ozone. Environ. Chem. Lett. 2012, 10, 55–60. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The Adverse Health Effects of Bisphenol A and Related Toxicity Mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Erjavec, B.; Hudoklin, P.; Perc, K.; Tišler, T.; Dolenc, M.S.; Pintar, A. Glass Fiber-Supported TiO2 Photocatalyst: Efficient Mineralization and Removal of Toxicity/Estrogenicity of Bisphenol A and Its Analogs. Appl. Catal. B Environ. 2016, 183, 149–158. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Moon, H.-B.; Yamashita, N.; Yun, S.; Kannan, K. Bisphenol Analogues in Sediments from Industrialized Areas in the United States, Japan, and Korea: Spatial and Temporal Distributions. Environ. Sci. Technol. 2012, 46, 11558–11565. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-P.; Ding, H.-Y.; Cao, X.-Y.; Ding, Q.-Y. Sorption Behavior of Nonylphenol on Marine Sediments: Effect of Temperature, Medium, Sediment Organic Carbon and Surfactant. Mar. Pollut. Bull. 2011, 62, 2362–2369. [Google Scholar] [CrossRef]
- Kostura, B.; Škuta, R.; Plachá, D.; Kukutschová, J.; Matýsek, D. Mg–Al–CO3 Hydrotalcite Removal of Persistent Organic Disruptor—Nonylphenol from Aqueous Solutions. Appl. Clay Sci. 2015, 114, 234–238. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, X.; Sun, Y.; Ai, Y.; Wang, X. Adsorption of 4-n-Nonylphenol and Bisphenol-A on Magnetic Reduced Graphene Oxides: A Combined Experimental and Theoretical Studies. Environ. Sci. Technol. 2015, 49, 9168–9175. [Google Scholar] [CrossRef]
- Yu, K.S.H.; Wong, A.H.Y.; Yau, K.W.Y.; Wong, Y.S.; Tam, N.F.Y. Natural Attenuation, Biostimulation and Bioaugmentation on Biodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) in Mangrove Sediments. Mar. Pollut. Bull. 2005, 51, 1071–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Cheng, Y.; Zhang, T.; Ji, F.; Xu, X. Treatment of Pharmaceutical Wastewater Using Interior Micro-Electrolysis/Fenton Oxidation-Coagulation and Biological Degradation. Chemosphere 2016, 152, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, Y.; Sun, W.; Xie, S.; Liu, Y. Nonylphenol Biodegradation in River Sediment and Associated Shifts in Community Structures of Bacteria and Ammonia-Oxidizing Microorganisms. Ecotoxicol. Environ. Saf. 2014, 106, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kuramitz, H.; Saitoh, J.; Hattori, T.; Tanaka, S. Electrochemical Removal of P-Nonylphenol from Dilute Solutions Using a Carbon Fiber Anode. Water Res. 2002, 36, 3323–3329. [Google Scholar] [CrossRef]
- Ning, B.; Graham, N.J.D.; Zhang, Y. Degradation of Octylphenol and Nonylphenol by Ozone—Part II: Indirect Reaction. Chemosphere 2007, 68, 1173–1179. [Google Scholar] [CrossRef]
- Ahmadi, M.; Motlagh, H.R.; Jaafarzadeh, N.; Mostoufi, A.; Saeedi, R.; Barzegar, G.; Jorfi, S. Enhanced Photocatalytic Degradation of Tetracycline and Real Pharmaceutical Wastewater Using MWCNT/TiO2 Nano-Composite. J. Environ. Manag. 2017, 186, 55–63. [Google Scholar] [CrossRef]
- Jafry, H.R.; Liga, M.V.; Li, Q.; Barron, A.R. Simple Route to Enhanced Photocatalytic Activity of P25 Titanium Dioxide Nanoparticles by Silica Addition. Environ. Sci. Technol. 2011, 45, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Pelizzetti, E.; Minero, C.; Maurino, V.; Sclafani, A.; Hidaka, H.; Serpone, N. Photocatalytic Degradation of Nonylphenol Ethoxylated Surfactants. Environ. Sci. Technol. 1989, 23, 1380–1385. [Google Scholar] [CrossRef]
- Wei, T.; Fan, Z.; Zhao, G. Enhanced Adsorption and Degradation of Nonylphenol on Electron-Deficient Centers of Photocatalytic Surfaces. Chem. Eng. J. 2020, 388, 124168. [Google Scholar] [CrossRef]
- Kohtani, S.; Hiro, J.; Yamamoto, N.; Kudo, A.; Tokumura, K.; Nakagaki, R. Adsorptive and Photocatalytic Properties of Ag-Loaded BiVO4 on the Degradation of 4-n-Alkylphenols under Visible Light Irradiation. Catal. Commun. 2005, 6, 185–189. [Google Scholar] [CrossRef]
- Ashar, A.; Iqbal, M.; Bhatti, I.A.; Ahmad, M.Z.; Qureshi, K.; Nisar, J.; Bukhari, I.H. Synthesis, Characterization and Photocatalytic Activity of ZnO Flower and Pseudo-Sphere: Nonylphenol Ethoxylate Degradation under UV and Solar Irradiation. J. Alloys Compd. 2016, 678, 126–136. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, C.; Li, X.; Zhang, P.; Sun, Y.; Su, C.; Zhang, X.; Ren, J.; Liu, Y. Carbon-Modified BiVO 4 Microtubes Embedded with Ag Nanoparticles Have High Photocatalytic Activity under Visible Light. Nanoscale 2012, 4, 7501–7508. [Google Scholar] [CrossRef]
- Ge, L. Novel Pd/BiVO4 Composite Photocatalysts for Efficient Degradation of Methyl Orange under Visible Light Irradiation. Mater. Chem. Phys. 2008, 107, 465–470. [Google Scholar] [CrossRef]
- Cao, S.-W.; Yin, Z.; Barber, J.; Boey, F.Y.C.; Loo, S.C.J.; Xue, C. Preparation of Au-BiVO4 Heterogeneous Nanostructures as Highly Efficient Visible-Light Photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, G.; Jimmy, C.Y. Enhanced Photo-Fenton Degradation of Rhodamine B Using Graphene Oxide--Amorphous FePO4 as Effective and Stable Heterogeneous Catalyst. J. Colloid Interface Sci. 2015, 448, 460–466. [Google Scholar] [CrossRef]
- Liang, M.; Yang, Z.; Yang, Y.; Mei, Y.; Zhou, H.; Yang, S. One-Step Introduction of Metallic Bi and Non-Metallic C in Bi2WO6 with Enhanced Photocatalytic Activity. J. Mater. Sci. Mater. Electron. 2019, 30, 1310–1321. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, X.; Zhang, H.; Yang, B.; Xiao, K.; Guo, T.; Zhang, J.; Shao, H.; Wang, Y.; Yu, G. MOF-Derived Nitrogen Doped Carbon Modified g-C3N4 Heterostructure Composite with Enhanced Photocatalytic Activity for Bisphenol A Degradation with Peroxymonosulfate under Visible Light Irradiation. Appl. Catal. B Environ. 2018, 233, 35–45. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Gao, J.; Hojamberdiev, M.; Zhu, R.; Wei, X.; Guo, Q.; Liu, P. Enhanced Photocatalytic Activity of Bi4Ti3O12 Nanosheets by Fe3+-Doping and the Addition of Au Nanoparticles: Photodegradation of Phenol and Bisphenol A. Appl. Catal. B Environ. 2017, 200, 72–82. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, H.; Gao, G.; Liu, L.; Chen, W. Facet-Dependent Catalytic Activity of Nanosheet-Assembled Bismuth Oxyiodide Microspheres in Degradation of Bisphenol A. Environ. Sci. Technol. 2015, 49, 6240–6248. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Truyen, D.H.; Kim, T.H.; Bark, C.W. Characteristics of Perovskites ReNiO3 (Re = La and Nd) Prepared by Solid State Reaction in the Ambient of Oxygen. J. Nanosci. Nanotechnol. 2020, 20, 4239–4243. [Google Scholar] [CrossRef]
- Wang, L.; Ma, T.; Dai, S.; Ren, T.; Chang, Z.; Dou, L.; Fu, M.; Li, X. Experimental Study on the High Performance of Zr Doped LaCoO3 for Solar Thermochemical CO Production. Chem. Eng. J. 2020, 389, 124426. [Google Scholar] [CrossRef]
- De Lima, R.K.C.; Batista, M.S.; Wallau, M.; Sanches, E.A.; Mascarenhas, Y.P.; Urquieta-González, E.A. High Specific Surface Area LaFeCo Perovskites—Synthesis by Nanocasting and Catalytic Behavior in the Reduction of NO with CO. Appl. Catal. B Environ. 2009, 90, 441–450. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Li, Y.; Chen, L.; Shu, Z.; Chen, H.; Shi, J. High Surface Area Mesoporous LaFexCo1−xO3 Oxides: Synthesis and Electrocatalytic Property for Oxygen Reduction. Dalton Trans. 2013, 42, 9448–9452. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, P.; Tang, H. Visible-Light Photocatalytic Degradation of Bisphenol A on NaBiO3 Nanosheets in a Wide PH Range: A Synergistic Effect between Photocatalytic Oxidation and Chemical Oxidation. Chem. Eng. J. 2016, 291, 149–160. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Zeng, G.-M. An in Depth Mechanism Insight of the Degradation of Multiple Refractory Pollutants via a Novel SrTiO3/BiOI Heterojunction Photocatalysts. J. Catal. 2017, 356, 283–299. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Yang, K.; Tang, W.; Liu, H.; Yang, J.; Yue, R.; Chen, Y. Effects of Cerium Incorporation on the Catalytic Oxidation of Benzene over Flame-Made Perovskite La1−XCexMnO3 Catalysts. Particuology 2015, 19, 60–68. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic Combustion of VOCs on Non-Noble Metal Catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ahmad, N.; Alam, M.; Adil, S.F.; Ramay, S.M.; Albadri, A.; Ahmad, A.; Al-Enizi, A.M.; Alrayes, B.F.; Assal, M.E.; et al. Physico-Chemical Properties and Catalytic Activity of the Sol-Gel Prepared Ce-Ion Doped LaMnO3 Perovskites. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasdi, M.; Alamdari, H.; Royer, S.; Adnot, A. Electrical and CO Gas Sensing Properties of Nanostructured La1−XCexCoO3 Perovskite Prepared by Activated Reactive Synthesis. Sens. Actuators B Chem. 2011, 156, 147–155. [Google Scholar] [CrossRef]
- Zhang-Steenwinkel, Y.; Beckers, J.; Bliek, A. Surface Properties and Catalytic Performance in CO Oxidation of Cerium Substituted Lanthanum-Manganese Oxides. Appl. Catal. A Gen. 2002, 235, 79–92. [Google Scholar] [CrossRef]
- Royer, S.; Alamdari, H.; Duprez, D.; Kaliaguine, S. Oxygen Storage Capacity of La1- XA′ XBO3 Perovskites (with A′ = Sr, Ce; B= Co, Mn)—Relation with Catalytic Activity in the CH4 Oxidation Reaction. Appl. Catal. B Environ. 2005, 58, 273–288. [Google Scholar] [CrossRef]
- Patil, S.; Seal, S.; Guo, Y.; Schulte, A.; Norwood, J. Role of Trivalent La and Nd Dopants in Lattice Distortion and Oxygen Vacancy Generation in Cerium Oxide Nanoparticles. Appl. Phys. Lett. 2006, 88, 243110. [Google Scholar] [CrossRef] [Green Version]
- Lakshmi, R.V.; Bera, P.; Hiremath, M.; Dubey, V.; Kundu, A.K.; Barshilia, H.C. Structural, Magnetic, and Dielectric Properties of Solution Combustion Synthesized LaFeO3, LaFe0.9Mn0.1O3, and LaMnO3 Perovskites. Phys. Chem. Chem. Phys. 2022, 24, 5462–5478. [Google Scholar] [CrossRef]
- Asamoto, M.; Harada, N.; Iwamoto, Y.; Yamaura, H.; Sadaoka, Y.; Yahiro, H. Catalytic Activity of Multi-Metallic Perovskite-Type Oxide Prepared by the Thermal Decomposition of Heteronuclear Cyano Complex, Sm [FexCo1−x(CN)6]·nH2O. Top. Catal. 2009, 52, 823–827. [Google Scholar] [CrossRef]
- Weifan, C.; Fengsheng, L.; Leili, L.; Yang, L. One-Step Synthesis of Nanocrytalline Perovskite LaMnO3 Powders via Microwave-Induced Solution Combustion Route. J. Rare Earths 2006, 24, 782–787. [Google Scholar] [CrossRef]
- Maridevaru, M.C.; Wu, J.J.; Viswanathan Mangalaraja, R.; Anandan, S. Ultrasonic-Assisted Preparation of Perovskite-Type Lanthanum Nickelate Nanostructures and Its Photocatalytic Properties. ChemistrySelect 2020, 5, 7947–7958. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, X.; Ma, L.; Xu, X. Rational Construction of Z-Scheme Ag2CrO4/g-C3N4 Composites with Enhanced Visible-Light Photocatalytic Activity. Appl. Surf. Sci. 2016, 390, 357–367. [Google Scholar] [CrossRef]
- Ismael, M.; Wu, Y.; Taffa, D.H.; Bottke, P.; Wark, M. Graphitic Carbon Nitride Synthesized by Simple Pyrolysis: Role of Precursor in Photocatalytic Hydrogen Production. New J. Chem. 2019, 43, 6909–6920. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute Electronegativity and Hardness: Application to Inorganic Chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Selvam, N.; Vijaya, J.J.; Kennedy, L.J. Effect of Ce Doping on Structural, Optical and Photocatalytic Properties of ZnO Nano-Structures. J. Nanosci. Nanotechnol. 2014, 14, 2317–2324. [Google Scholar] [CrossRef]
- Maridevaru, M.C.; Anandan, S.; Aljafari, B.; Wu, J.J. LaCoxFe1−XO3 (0 ≤ X ≤ 1) Spherical Nanostructures Prepared via Ultrasonic Approach as Photocatalysts. Ultrason. Sonochem. 2021, 80, 105824. [Google Scholar] [CrossRef] [PubMed]
- Maridevaru, M.C.; Aljafari, B.; Anandan, S.; Ashokkumar, M. Synergistic Impacts of Sonolysis Aided Photocatalytic Degradation of Water Pollutant over Perovskite-Type CeNiO3 Nanospheres. New J. Chem. 2022, 46, 10117–10127. [Google Scholar] [CrossRef]
- Paramanik, L.; Reddy, K.H.; Sultana, S.; Parida, K. Architecture of Biperovskite-Based LaCrO3/PbTiO3 p-n Heterojunction with a Strong Interface for Enhanced Charge Anti-Recombination Process and Visible Light-Induced Photocatalytic Reactions. Inorg. Chem. 2018, 57, 15133–15148. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Ahmad, I.; Zheng, Y. Fourier Transform Infrared Spectroscopy of “Bisphenol A”. J. Spectrosc. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Photocatalytic degradation of nonylphenol by immobilized TiO2 in dual layer hollow fibre membranes. Chem. Eng. J. 2015, 269, 255–261. [Google Scholar]
- Altin, I. CuO-TiO2/graphene ternary nanocomposite for highly efficient visible-light-driven photocatalytic degradation of bisphenol A. J. Mol. Struct. 2022, 1252, 132199. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, N.; Cheng, G.; Guo, H.; Shen, Z.; Yang, L.; Zhao, Y.; Alsaedi, A.; Hayat, T.; Wang, X. preparing a photocatalytic Fe doped TiO2/rGO for enhanced bisphenol A and its analogues degradation in water sample. Appl. Surf. Sci. 2020, 505, 144640. [Google Scholar]
- García-Díaz, E.; Zhang, D.; Li, Y.; Verduzco, R.; Alvarez, P.J.J. TiO2 microspheres with cross-linked cyclodextrin coating exhibit improved stability and sustained photocatalytic degradation of bisphenol A in secondary effluent. Water Res. 2020, 183, 116095. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Ashokkumar, M. Sonochemical synthesis of Au-TiO2 nanoparticles for the sonophotocatalytic degradation of organic pollutants in aqueous environment. Ultrason. Sonochemistry 2009, 16, 316–320. [Google Scholar] [CrossRef] [PubMed]













| Degradation Efficiency and First-Order Rate Kinetics | |||||
|---|---|---|---|---|---|
| 4-n-Nonylphenol Degraded in 120 min | Bisphenol A Degraded in 240 min | ||||
| Sample | Degradation Efficiency (%) | Rate Constant (min−1) | Sample | Degradation Efficiency (%) | Rate Constant (min−1) |
| Photo | 14.8 | 2.93 × 10−3 | Photo | 5.6 | 2.19 × 10−4 |
| CLMO | 13 | 1.25 × 10−3 | CLMO | 12.4 | 6.47 × 10−4 |
| CLMO + Photo | 92 | 2.26 × 10−2 | CLMO + Photo | 94 | 2.78 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maridevaru, M.C.; Naceruddin, A.H.; Aljafari, B.; Anandan, S. Synthesis of Ce0.1La0.9MnO3 Perovskite for Degradation of Endocrine-Disrupting Chemicals under Visible Photons. Catalysts 2022, 12, 1258. https://doi.org/10.3390/catal12101258
Maridevaru MC, Naceruddin AH, Aljafari B, Anandan S. Synthesis of Ce0.1La0.9MnO3 Perovskite for Degradation of Endocrine-Disrupting Chemicals under Visible Photons. Catalysts. 2022; 12(10):1258. https://doi.org/10.3390/catal12101258
Chicago/Turabian StyleMaridevaru, Madappa C., Afreen Hooriya Naceruddin, Belqasem Aljafari, and Sambandam Anandan. 2022. "Synthesis of Ce0.1La0.9MnO3 Perovskite for Degradation of Endocrine-Disrupting Chemicals under Visible Photons" Catalysts 12, no. 10: 1258. https://doi.org/10.3390/catal12101258