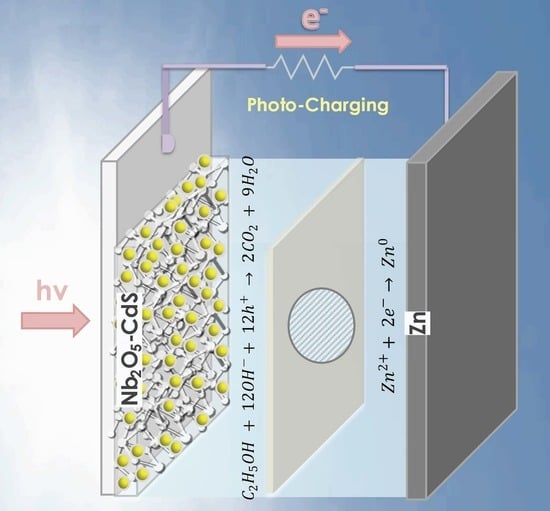
Photo-Charging a Zinc-Air Battery Using a Nb2O5-CdS Photoelectrode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Electrodes
2.2. Photocharging of the Zinc-Air Battery
3. Materials and Methods
3.1. Photoelectrode Construction
3.2. Morphological and Photoelectrochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, Q.; Lai, Y.; Jiang, L.; Liu, F.; Hao, X.; Wang, L.; Green, M.A. Integrated Photorechargeable Energy Storage System: Next-Generation Power Source Driving the Future. Adv. Energy Mater. 2020, 10, 1903930. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Tian, Z.; Sun, J. Solar-Driven Integrated Energy Systems: State of the Art and Challenges. J. Power Sources 2020, 478, 228762. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Guo, S.; Zhou, H. Solar Energy Storage in the Rechargeable Batteries. Nano Today 2017, 16, 46–60. [Google Scholar] [CrossRef]
- Yan, N.F.; Gao, X.P. Photo-Assisted Rechargeable Metal Batteries for Energy Conversion and Storage. Energy Environ. Mater. 2021, 5, 439–451. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. The Impact of Nano-Scaled Materials on Advanced Metal-air Battery Systems. Nano Energy 2013, 2, 468–480. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Metal-Air Batteries: Will They Be the Future Electrochemical Energy Storage Device of Choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Liu, Y.; Li, N.; Wu, S.; Liao, K.; Zhu, K.; Yi, J.; Zhou, H. Reducing the Charging Voltage of a Li-O2 Battery to 1.9 v by Incorporating a Photocatalyst. Energy Environ. Sci. 2015, 8, 2664–2667. [Google Scholar] [CrossRef]
- Liu, Y.; Li, N.; Liao, K.; Li, Q.; Ishida, M.; Zhou, H. Lowering the Charge Voltage of Li-O2 Batteries: Via an Unmediated Photoelectrochemical Oxidation Approach. J. Mater. Chem. A 2016, 4, 12411–12415. [Google Scholar] [CrossRef]
- Feng, Y.; Xue, H.; Wang, T.; Gong, H.; Gao, B.; Xia, W.; Jiang, C.; Li, J.; Huang, X.; He, J. Enhanced Li2O2 Decomposition in Rechargeable Li-O2 Battery by Incorporating WO3 Nanowire Array Photocatalyst. ACS Sustain. Chem. Eng. 2019, 7, 5931–5939. [Google Scholar] [CrossRef]
- Tan, P.; Xiao, X.; Dai, Y.; Cheng, C.; Ni, M. Photo-Assisted Non-Aqueous Lithium-Oxygen Batteries: Progress and Prospects. Renew. Sustain. Energy Rev. 2020, 127, 109877. [Google Scholar] [CrossRef]
- Grande, L.; Paillard, E.; Hassoun, J.; Park, J.B.; Lee, Y.J.; Sun, Y.K.; Passerini, S.; Scrosati, B. The Lithium/Air Battery: Still an Emerging System or a Practical Reality? Adv. Mater. 2015, 27, 784–800. [Google Scholar] [CrossRef]
- Liu, Y.; He, P.; Zhou, H. Rechargeable Solid-State Li–Air and Li–S Batteries: Materials, Construction, and Challenges. Adv. Energy Mater. 2018, 8, 1701602. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc-air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Z.; Karahan, H.E.; Shao, Q.; Wei, L.; Chen, Y. Recent Advances in Materials and Design of Electrochemically Rechargeable Zinc-air Batteries. Small 2018, 14, 1801929. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent Advances in Zinc-Air Batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [Green Version]
- Du, D.; Zhao, S.; Zhu, Z.; Li, F.; Chen, J. Photo-Excited Oxygen Reduction and Oxygen Evolution Reactions Enable a High-Performance Zn-air Battery. Angew. Chem.—Int. Ed. 2020, 59, 18140–18144. [Google Scholar] [CrossRef]
- Lv, J.; Abbas, S.C.; Huang, Y.; Liu, Q.; Wu, M.; Wang, Y.; Dai, L. A Photo-Responsive Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. Nano Energy 2018, 43, 130–137. [Google Scholar] [CrossRef]
- Mathur, A.; Kaushik, R.; Halder, A. Visible-Light-Driven Photo-Enhanced Zinc-Air Batteries Using Synergistic Effect of Different Types of MnO2nanostructures. Catal. Sci. Technol. 2020, 10, 7352–7364. [Google Scholar] [CrossRef]
- Andrade, T.S.; Pereira, M.C.; Lianos, P. High Voltage Gain in Photo-Assisted Charging of a Metal-Air Battery. J. Electroanal. Chem. 2020, 878, 114559. [Google Scholar] [CrossRef]
- Andrade, T.S.; Dracopoulos, V.; Pereira, M.C.; Lianos, P. Unmediated Photoelectrochemical Charging of a Zn-Air Battery: The Realization of the Photoelectrochemical Battery. J. Electroanal. Chem. 2020, 878, 114709. [Google Scholar] [CrossRef]
- Andrade, T.S.; Sena, I.C.; de Oliveira, L.C.A.; Lianos, P.; Pereira, M.C. Decreasing the Charging Voltage of a Zinc-Air Battery Using a Bifunctional W:BiVO4/V2O5 Photoelectrode and Sulfite as a Sacrificial Agent. Mater. Today Commun. 2021, 28, 9–12. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, Y.; Liu, J.; Liu, B.; Chen, X.; Ding, J.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Utilizing Solar Energy to Improve the Oxygen Evolution Reaction Kinetics in Zinc-air Battery. Nat. Commun. 2019, 10, 4767. [Google Scholar] [CrossRef] [Green Version]
- Tomon, C.; Sarawutanukul, S.; Duangdangchote, S.; Krittayavathananon, A.; Sawangphruk, M. Photoactive Zn-Air Batteries Using Spinel-Type Cobalt Oxide as a Bifunctional Photocatalyst at the Air Cathode. Chem. Commun. 2019, 55, 5855–5858. [Google Scholar] [CrossRef]
- Sarawutanukul, S.; Tomon, C.; Duangdangchote, S.; Phattharasupakun, N.; Sawangphruk, M. Rechargeable Photoactive Zn-Air Batteries Using NiCo2S4 as an Efficient Bifunctional Photocatalyst towards OER/ORR at the Cathode. Batter. Supercaps 2020, 3, 541–547. [Google Scholar] [CrossRef]
- Su, K.; Liu, H.; Gao, Z.; Fornasiero, P.; Wang, F. Nb2O5-Based Photocatalysts. Adv. Sci. 2021, 8, 2003156. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, X.; Ye, L.; Chi Edman Tsang, S. Nanostructured Nb2O5 Catalysts. Nano Rev. 2012, 3, 17631. [Google Scholar] [CrossRef] [Green Version]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Oliveira, H.S.; Mayrink, G.; Mansur, H.S.; Mansur, A.A.P.; Moreira, R.L. One-Pot Synthesis of CdS@Nb2O5 Core–Shell Nanostructures with Enhanced Photocatalytic Activity. Appl. Catal. B Environ. 2014, 152–153, 403–412. [Google Scholar] [CrossRef]
- Zu, D.; Song, H.; Wang, Y.; Chao, Z.; Li, Z.; Wang, G.; Shen, Y.; Li, C.; Ma, J. One-Pot in-Situ Hydrothermal Synthesis of CdS/Nb2O5/Nb2C Heterojunction for Enhanced Visible-Light-Driven Photodegradation. Appl. Catal. B Environ. 2020, 277, 119140. [Google Scholar] [CrossRef]
- Yue, Z.; Liu, A.; Zhang, C.; Huang, J.; Zhu, M.; Du, Y.; Yang, P. Noble-Metal-Free Hetero-Structural CdS/Nb2O5/N-Doped-Graphene Ternary Photocatalytic System as Visible-Light-Driven Photocatalyst for Hydrogen Evolution. Appl. Catal. B Environ. 2017, 201, 202–210. [Google Scholar] [CrossRef]
- Andrade, T.S.; Dracopoulos, V.; Keramidas, A.; Pereira, M.C.; Lianos, P. Charging a vanadium redox battery with a photo (catalytic) fuel cell. Sol. Energy Mater. Sol. Cells 2021, 221, 110889. [Google Scholar] [CrossRef]
- Katsoufis, P.; Mylona, V.; Politis, C.; Avgouropoulos, G.; Lianos, P. Study of Some Basic Operation Conditions of an Al-Air Battery Using Technical Grade Commercial Aluminum. J. Power Sources 2020, 450, 227624. [Google Scholar] [CrossRef]
- Katsoufis, P.; Katsaiti, M.; Mourelas, C.; Andrade, T.S.; Dracopoulos, V.; Politis, C.; Avgouropoulos, G.; Lianos, P. Study of a Thin Film Aluminum-Air Battery. Energies 2020, 13, 1447. [Google Scholar] [CrossRef] [Green Version]
- Keramidas, A.D.; Hadjithoma, S.; Drouza, C.; Andrade, T.S.; Lianos, P. Four Electron Selective O2reduction by a Tetranuclear Vanadium(IV/V)/Hydroquinonate Catalyst: Application in the Operation of Zn-Air Batteries. New J. Chem. 2022, 46, 470–479. [Google Scholar] [CrossRef]
- Bu, D.; Batmunkh, M.; Zhang, Y.; Li, Y.; Qian, B.; Lan, Y.; Hou, X.; Li, S.; Jia, B.; Song, X.M.; et al. Rechargeable Sunlight-Promoted Zn-Air Battery Constructed by Bifunctional Oxygen Photoelectrodes: Energy-Band Switching between ZnO/Cu2O and ZnO/CuO in Charge-Discharge Cycles. Chem. Eng. J. 2022, 433, 133559. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, T.S.; Neto, A.R.S.; Nogueira, F.G.E.; Oliveira, L.C.A.; Pereira, M.C.; Lianos, P. Photo-Charging a Zinc-Air Battery Using a Nb2O5-CdS Photoelectrode. Catalysts 2022, 12, 1240. https://doi.org/10.3390/catal12101240
Andrade TS, Neto ARS, Nogueira FGE, Oliveira LCA, Pereira MC, Lianos P. Photo-Charging a Zinc-Air Battery Using a Nb2O5-CdS Photoelectrode. Catalysts. 2022; 12(10):1240. https://doi.org/10.3390/catal12101240
Chicago/Turabian StyleAndrade, Tatiana S., Antero R. S. Neto, Francisco G. E. Nogueira, Luiz C. A. Oliveira, Márcio C. Pereira, and Panagiotis Lianos. 2022. "Photo-Charging a Zinc-Air Battery Using a Nb2O5-CdS Photoelectrode" Catalysts 12, no. 10: 1240. https://doi.org/10.3390/catal12101240