Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin
Abstract
:1. Introduction
1.1. Antibiotics Occurrence in Aqueous Environment
1.2. Sulfamethoxazole, Trimethoprim and Ciprofloxacin as Antibiotics of Great Concern
1.3. Antimicrobial Resistance as a Global Health Crisis
1.4. Pathways of Antibiotics Release into Aquatic Environment
2. Water Treatment Techniques for Degradation of Antibiotics
2.1. Advanced Oxidation Processes
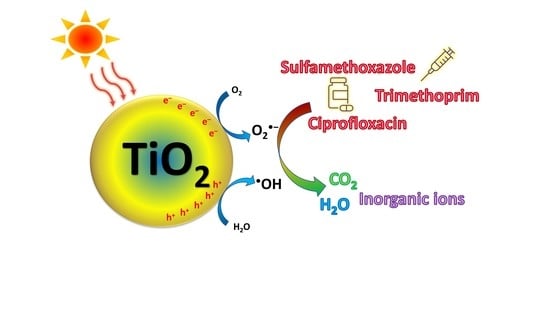
2.2. Heterogeneous Photocatalysis over TiO2
- Adsorption of organic pollutants on TiO2 surface;
- The photocatalytic degradation of the adsorbed organic pollutants via oxidation-reduction reactions with photogenerated electrons, holes and reactive species (depicted in Figure 5);
- Desorption of degradation products.
2.3. Doping and Modification of TiO2
3. Parameters Affecting Efficiency of Photocatalytic Degradation over TiO2-Based Photocatalysts
3.1. Effect of Antibiotic Concentration
3.2. Effect of Catalyst Concentration
3.3. Effect of pH
3.4. Effect of Presence of Inorganic Ions
3.5. Effect of Presence of Natural Organic Matter
3.6. Effect of Reaction Media
3.7. Effect of Dissolved Oxygen and Oxidants
3.8. Effect of Light Source
4. Degradation Pathways of Antibiotics
5. Ecotoxicity of Photoproducts
6. Challenges and Future Research Needs
- Huge variety of TiO2-based materials with unique features have been synthesized, as well as many different techniques having been reported to synthesize efficient TiO2-based photocatalysts. However, as the operational cost is crucial for practical applications, there is still a strong need for simple and cost-effective synthesis and modification processes to decrease the cost of the photocatalytic process. This can be achieved by implementing low-cost synthesis methods or by using low-cost materials, or both. The introduction of multiple cheaper modificators might be more cost-effective than introducing one expensive dopant, while retaining high photocatalytic activity. For example, low-cost alternatives to photocatalysts modified with expensive and scarce noble metals could be TiO2-doped with cocatalysts like Ni and Cu [154,155] or Co and Ni [156]; Mo-doped TiO2 [157], graphene-doped TiO2 [158], Cu nanowires decorated with TiO2 [159].
- Special emphasis should be placed on the development of TiO2-based photocatalysts active under solar light. Considering that high energy costs are associated with the usage of visible and ultraviolet light sources, solar photocatalysis is not only a cost-efficient solution, but also a sustainable one. Besides, further cost reduction can be achieved through the development of highly stable photocatalysts that can be easily separated from aqueous solution (immobilized/supported catalysts) and successfully reused over multiple cycles;
- Further research is needed in studying the potential of photocatalytic process over TiO2-based materials for the successful removal not only of antibiotics, but also of ARB and ARGs from wastewaters;
- In order to fulfill industrial application, photocatalytic tests should be carried out on real wastewater samples. The composition of wastewater is complicated: it contains a mixture of different pharmaceuticals and other organic pollutants, as well as inorganic substances. This decreases the degradation efficiency of antibiotics compared to the degradation efficiency of a single antibiotic in purified water. Moreover, there is a need to design and develop pilot plant installations that work under solar irradiation and in the continuous mode of operation. These are essential requirements for industrial treatment systems;
- Further investigations should be performed on the efficiency of combined methods. Heterogeneous photocatalysis combined with conventional treatment methods, for example with biological treatment, should be studied in order to develop a technically feasible and cost-effective solution for wastewater treatment;
- Assessment of eco-toxicity should be an essential part of degradation tests over TiO2-based photocatalysts. The complete mineralization of antibiotics remains a challenge, and incomplete mineralization leads to the formation of various intermediates and by-products, sometimes more toxic than the parent compound. Degradation mechanisms require deeper studies.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | antimicrobial resistance |
| AOPs | advanced oxidation processes |
| APIs | active pharmaceutical ingredients |
| ARB | antibiotic resistant bacteria |
| ARGs | antibiotic resistant genes |
| BET | Brunauer-Emmett-Teller |
| CB | conduction band |
| CIP | ciprofloxacin |
| COD | chemical oxygen demand |
| DOC | dissolved organic carbon |
| NOM | natural organic matter |
| NPOC | non-purgeable organic carbon |
| PZC | point of zero charge |
| SEM | scanning electron microscopy |
| SMX | sulfamethoxazole |
| TEM | transmission electron microscopy |
| TMP | trimethoprim |
| TOC | total organic carbon |
| UV | ultraviolet |
| VB | valence band |
| WWTP | wastewater treatment plant |
| XRD | X-ray diffraction |
References
- OECD. Pharmaceutical Residues in Freshwater: Hazards and Policy Responses; OECD Publishing: Paris, France, 2019; ISBN 9789264776333. [Google Scholar]
- Chavoshani, A.; Hashemi, M.; Mehdi Amin, M.; Ameta, S.C. Pharmaceuticals as emerging micropollutants in aquatic environments. In Micropollutants and Challenges; Elsevier Inc.: Philadelphia, PA, USA, 2020; pp. 35–90. ISBN 9780128186121. [Google Scholar]
- Aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment-Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Tit, D.M.; Behl, T.; Aleya, L.; Zaha, D.C. Aspects of excessive antibiotic consumption and environmental influences correlated with the occurrence of resistance to antimicrobial agents. Curr. Opin. Environ. Sci. Health 2021, 19, 100224. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use—Present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Szymańska, U.; Wiergowski, M.; Sołtyszewski, I.; Kuzemko, J.; Wiergowska, G.; Woźniak, M.K. Presence of antibiotics in the aquatic environment in Europe and their analytical monitoring: Recent trends and perspectives. Microchem. J. 2019, 147, 729–740. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Antibiotics traces in the aquatic environment: Persistence and adverse environmental impact. Curr. Opin. Environ. Sci. Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment-occurrence and environmental implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Menz, J.; Olsson, O.; Kümmerer, K. Antibiotic residues in livestock manure: Does the EU risk assessment sufficiently protect against microbial toxicity and selection of resistant bacteria in the environment? J. Hazard. Mater. 2019, 379, 120807. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.P.; Kumar, S.; Giri, B.S.; Kim, K.H. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies. J. Clean. Prod. 2019, 234, 1484–1505. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef]
- Sabri, N.A.; van Holst, S.; Schmitt, H.; van der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Fate of antibiotics and antibiotic resistance genes during conventional and additional treatment technologies in wastewater treatment plants. Sci. Total Environ. 2020, 741, 140199. [Google Scholar] [CrossRef]
- Prashanth, V.; Jayasree, P.; Rajput, P.; Remya, N. Solar photocatalysis and its application for emerging contaminant removal from wastewater. In Advanced Oxidation Processes for Effluent Treatment Plants; Elsevier Inc.: Philadelphia, PA, USA, 2021; pp. 69–85. ISBN 9780128210116. [Google Scholar]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef]
- Johnson, A.C.; Keller, V.; Dumont, E.; Sumpter, J.P. Assessing the concentrations and risks of toxicity from the antibiotics ciprofloxacin, sulfamethoxazole, trimethoprim and erythromycin in European rivers. Sci. Total Environ. 2015, 511, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Kötke, D.; Gandrass, J.; Xie, Z.; Ebinghaus, R. Prioritised pharmaceuticals in German estuaries and coastal waters: Occurrence and environmental risk assessment. Environ. Pollut. 2019, 255, 113161. [Google Scholar] [CrossRef]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S. A review on contamination and removal of sulfamethoxazole from aqueous solution using cleaner techniques: Present and future perspective. J. Clean. Prod. 2020, 250, 119553. [Google Scholar] [CrossRef]
- Sarafraz, M.; Sadeghi, M.; Yazdanbakhsh, A.; Amini, M.M.; Sadani, M.; Eslami, A. Enhanced photocatalytic degradation of ciprofloxacin by black Ti3+/N-TiO2 under visible LED light irradiation: Kinetic, energy consumption, degradation pathway, and toxicity assessment. Process Saf. Environ. Prot. 2020, 137, 261–272. [Google Scholar] [CrossRef]
- Ryan, C.C.; Tan, D.T.; Arnold, W.A. Direct and indirect photolysis of sulfamethoxazole and trimethoprim in wastewater treatment plant effluent. Water Res. 2011, 45, 1280–1286. [Google Scholar] [CrossRef]
- Oros-Ruiz, S.; Zanella, R.; Prado, B. Photocatalytic degradation of trimethoprim by metallic nanoparticles supported on TiO2-P25. J. Hazard. Mater. 2013, 263, 28–35. [Google Scholar] [CrossRef]
- Paul, T.; Dodd, M.C.; Strathmann, T.J. Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: Transformation products and residual antibacterial activity. Water Res. 2010, 44, 3121–3132. [Google Scholar] [CrossRef]
- Jia, A.; Wan, Y.; Xiao, Y.; Hu, J. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 2012, 46, 387–394. [Google Scholar] [CrossRef]
- Haddad, T.; Kümmerer, K. Characterization of photo-transformation products of the antibiotic drug Ciprofloxacin with liquid chromatography-tandem mass spectrometry in combination with accurate mass determination using an LTQ-Orbitrap. Chemosphere 2014, 115, 40–46. [Google Scholar] [CrossRef]
- Kumar, M.; Jaiswal, S.; Sodhi, K.K.; Shree, P.; Singh, D.K.; Agrawal, P.K.; Shukla, P. Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environ. Int. 2019, 124, 448–461. [Google Scholar] [CrossRef]
- Girardi, C.; Greve, J.; Lamshöft, M.; Fetzer, I.; Miltner, A.; Schäffer, A.; Kästner, M. Biodegradation of ciprofloxacin in water and soil and its effects on the microbial communities. J. Hazard. Mater. 2011, 198, 22–30. [Google Scholar] [CrossRef]
- Cortes, L.G.; Marinov, D.; Sanseverino, I.; Cuenca, A.N.; Niegowska, M.; Rodriguez, E.P.; Lettieri, T. Selection of Substances for the 3rd Watch List under the Water Framework Directive; Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Savin, M.; Bierbaum, G.; Hammerl, J.A.; Heinemann, C.; Parcina, M.; Sib, E.; Voigt, A.; Kreyenschmidt, J. Antibiotic-resistant bacteria and antimicrobial residues in wastewater and process water from German pig slaughterhouses and their receiving municipal wastewater treatment plants. Sci. Total Environ. 2020, 727, 138788. [Google Scholar] [CrossRef]
- Bartolomeu, M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Wastewater chemical contaminants: Remediation by advanced oxidation processes. Photochem. Photobiol. Sci. 2018, 17, 1573–1598. [Google Scholar] [CrossRef] [PubMed]
- Li, L.G.; Huang, Q.; Yin, X.; Zhang, T. Source tracking of antibiotic resistance genes in the environment—Challenges, progress, and prospects. Water Res. 2020, 185, 116127. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Rodriguez, L.; Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Puma, G.L.; Malato, S. Treatment of emerging contaminants in wastewater treatment plants (WWTP) effluents by solar photocatalysis using low TiO2 concentrations. J. Hazard. Mater. 2012, 211–212, 131–137. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Wei, Q.; Wei, D. A critical review on antibiotics and hormones in swine wastewater: Water pollution problems and control approaches. J. Hazard. Mater. 2020, 387, 121682. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Giri, B.S.; Shukla, P.; Gupta, P. Recent advancement in remediation of synthetic organic antibiotics from environmental matrices: Challenges and perspective. Bioresour. Technol. 2021, 319, 124161. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Mohamed Saheed, M.S.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Janani, F.Z.; Elhalil, A.; Mahjoubi, F.Z.; Barka, N. Removal of emerging pharmaceutical pollutants: A systematic mapping study review. J. Environ. Chem. Eng. 2020, 8, 104251. [Google Scholar] [CrossRef]
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic degradation of pharmaceutical and pesticide compounds (PPCs) using doped TiO2 nanomaterials: A review. Water-Energy Nexus 2020, 3, 46–61. [Google Scholar] [CrossRef]
- Majumdar, A.; Pal, A. Recent advancements in visible-light-assisted photocatalytic removal of aqueous pharmaceutical pollutants. Clean Technol. Environ. Policy 2020, 22, 11–42. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Ma, Y.L.; Zhang, J.T.; Fan, N.S.; Huang, B.C.; Jin, R.C. A critical review of antibiotic removal strategies: Performance and mechanisms. J. Water Process Eng. 2020, 38, 101681. [Google Scholar] [CrossRef]
- Fawzi Suleiman Khasawneh, O.; Palaniandy, P. Photocatalytic Degradation of Pharmaceuticals Using TiO2 Based Nanocomposite Catalyst-Review. Civ. Environ. Eng. Reports 2019, 29, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Hiller, C.X.; Hübner, U.; Fajnorova, S.; Schwartz, T.; Drewes, J.E. Antibiotic microbial resistance (AMR) removal efficiencies by conventional and advanced wastewater treatment processes: A review. Sci. Total Environ. 2019, 685, 596–608. [Google Scholar] [CrossRef]
- Anjali, R.; Shanthakumar, S. Insights on the current status of occurrence and removal of antibiotics in wastewater by advanced oxidation processes. J. Environ. Manag. 2019, 246, 51–62. [Google Scholar] [CrossRef]
- Serhiienko, A.O.; Dontsova, T.A.; Yanushevska, O.I.; Nahirniak, S.V.; Hosseini-Bandegharaei, A. Ceramic Membranes: New Trends and Prospects. Water Water Purif. Technol. Sci. Tech. News 2020, 27, 4–31. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Lee, C.M.; Palaniandy, P.; Dahlan, I. Pharmaceutical residues in aquatic environment and water remediation by TiO2 heterogeneous photocatalysis: A review. Environ. Earth Sci. 2017, 76, 611. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Liu, J.; Shangguan, W. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Balogun, H.A.; Sambudi, N.S.; Bilad, M.R.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review. Process Saf. Environ. Prot. 2021, 146, 220–256. [Google Scholar] [CrossRef]
- Kutuzova, A.; Dontsova, T. Synthesis, characterization and properties of titanium dioxide obtained by hydrolytic method. In Proceedings of the IEEE 7th International Conference on Nanomaterials: Applications and Properties, NAP 2017 IEEE, Odessa, Ukraine, 10–15 September 2017; pp. 01NNPT02-1–01NNPT02-5. [Google Scholar]
- Sviderskyi, A.; Nahirniak, S.; Yashchenko, T.; Dontsova, T.; Kalinowski, S. Properties of TiO2 and SnO2 in a State of Different Dispersion and Morphology. In Proceedings of the IEEE 8th International Conference Nanomaterials: Application & Properties (NAP), Zatoka, Ukraine, 9–14 September 2018; pp. 1–4. [Google Scholar]
- Dontsova, T.A.; Kutuzova, A.S.; Bila, K.O.; Kyrii, S.O.; Kosogina, I.V.; Nechyporuk, D.O. Enhanced Photocatalytic Activity of TiO2/SnO2 Binary Nanocomposites. J. Nanomater. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Kutuzova, A.S.; Dontsova, T.A. Characterization and properties of TiO2–SnO2 nanocomposites, obtained by hydrolysis method. Appl. Nanosci. 2019, 9, 873–880. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Wen, J.; Li, X.; Liu, W.; Fang, Y.; Xie, J.; Xu, Y. Photocatalysis fundamentals and surface modification of TiO2 nanomaterials. Cuihua Xuebao Chin. J. Catal. 2015, 36, 2049–2070. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M.C. Recent developments and challenges in practical application of visible–light–driven TiO2–based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef]
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. TiO2–SnO2 Nanocomposites: Effect of Acid–Base and Structural-Adsorption Properties on Photocatalytic Performance. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3060–3072. [Google Scholar] [CrossRef]
- Abdurahman, M.H.; Abdullah, A.Z.; Shoparwe, N.F. A comprehensive review on sonocatalytic, photocatalytic, and sonophotocatalytic processes for the degradation of antibiotics in water: Synergistic mechanism and degradation pathway. Chem. Eng. J. 2020, 127412. [Google Scholar] [CrossRef]
- Kutuzova, A.; Dontsova, T. TiO2-SnO2 Nanocomposites Obtained by Hydrothermal Method. In Proceedings of the IEEE 8th International Conference on Nanomaterials: Applications & Properties, Zatoka, Ukraine, 9–14 September 2018; pp. 1–5. [Google Scholar]
- Bila, K.; Dontsova, T.; Kutuzova, A. Effect of Precursor Type on Physico-chemical and Photocatalytic Properties of TiO2-SnO2 Nanocomposites. In Proceedings of the IEEE 10th International Conference on “Nanomaterials: Applications and Properties”, NAP 2020 IEEE, Sumy, Ukraine, 9–13 November 2020; pp. 02NEE01-1–02NEE01-4. [Google Scholar]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2- and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Tiwari, A.; Shukla, A.; Lalliansanga; Tiwari, D.; Lee, S.M. Nanocomposite thin films Ag0(NP)/TiO2 in the efficient removal of micro-pollutants from aqueous solutions: A case study of tetracycline and sulfamethoxazole removal. J. Environ. Manag. 2018, 220, 96–108. [Google Scholar] [CrossRef]
- Alfred, M.O.; Omorogie, M.O.; Bodede, O.; Moodley, R.; Ogunlaja, A.; Adeyemi, O.G.; Günter, C.; Taubert, A.; Iermak, I.; Eckert, H.; et al. Solar-active clay-TiO2 nanocomposites prepared via biomass assisted synthesis: Efficient removal of ampicillin, sulfamethoxazole and artemether from water. Chem. Eng. J. 2020, 398, 125544. [Google Scholar] [CrossRef]
- Ioannidou, E.; Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Solar photocatalytic degradation of sulfamethoxazole over tungsten—Modified TiO2. Chem. Eng. J. 2017, 318, 143–152. [Google Scholar] [CrossRef]
- Cai, Q.; Hu, J. Decomposition of sulfamethoxazole and trimethoprim by continuous UVA/LED/TiO2 photocatalysis: Decomposition pathways, residual antibacterial activity and toxicity. J. Hazard. Mater. 2017, 323, 527–536. [Google Scholar] [CrossRef]
- Karim, A.V.; Shriwastav, A. Degradation of ciprofloxacin using photo, sono, and sonophotocatalytic oxidation with visible light and low-frequency ultrasound: Degradation kinetics and pathways. Chem. Eng. J. 2020, 392, 124853. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, Y.; Yuan, X.; Zou, D.; Fang, J.; Jiang, L.; Zhang, J.; Yang, H.; Xiao, Z. MXene Ti3C2 derived Z–scheme photocatalyst of graphene layers anchored TiO2/g–C3N4 for visible light photocatalytic degradation of refractory organic pollutants. Chem. Eng. J. 2020, 394, 124921. [Google Scholar] [CrossRef]
- Hassani, A.; Khataee, A.; Karaca, S. Photocatalytic degradation of ciprofloxacin by synthesized TiO2 nanoparticles on montmorillonite: Effect of operation parameters and artificial neural network modeling. J. Mol. Catal. A Chem. 2015, 409, 149–161. [Google Scholar] [CrossRef]
- Xie, X.; Li, S.; Zhang, H.; Wang, Z.; Huang, H. Promoting charge separation of biochar-based Zn-TiO2/pBC in the presence of ZnO for efficient sulfamethoxazole photodegradation under visible light irradiation. Sci. Total Environ. 2019, 659, 529–539. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, R.; Guo, J.; Li, Y.; Zhu, J.; Xie, X. TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere 2017, 185, 351–360. [Google Scholar] [CrossRef]
- Kim, J.R.; Kan, E. Heterogeneous photocatalytic degradation of sulfamethoxazole in water using a biochar-supported TiO2 photocatalyst. J. Environ. Manag. 2016, 180, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Chiang, L.F.; Doong, R.A. Enhanced photocatalytic degradation of sulfamethoxazole by visible-light-sensitive TiO2 with low Cu addition. Sep. Purif. Technol. 2015, 156, 1003–1010. [Google Scholar] [CrossRef]
- Abellán, M.N.; Giménez, J.; Esplugas, S. Photocatalytic degradation of antibiotics: The case of sulfamethoxazole and trimethoprim. Catal. Today 2009, 144, 131–136. [Google Scholar] [CrossRef]
- Yuan, R.; Zhu, Y.; Zhou, B.; Hu, J. Photocatalytic oxidation of sulfamethoxazole in the presence of TiO2: Effect of matrix in aqueous solution on decomposition mechanisms. Chem. Eng. J. 2019, 359, 1527–1536. [Google Scholar] [CrossRef]
- Nasuhoglu, D.; Yargeau, V.; Berk, D. Photo-removal of sulfamethoxazole (SMX) by photolytic and photocatalytic processes in a batch reactor under UV-C radiation (λmax = 254 nm). J. Hazard. Mater. 2011, 186, 67–75. [Google Scholar] [CrossRef]
- Gong, H.; Chu, W. Determination and toxicity evaluation of the generated products in sulfamethoxazole degradation by UV/CoFe2O4/TiO2. J. Hazard. Mater. 2016, 314, 197–203. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.; Choi, Y.; Kim, S.; Lee, S.; Lee, S.; Choi, W.; Lee, J. Heterogeneous photocatalytic treatment of pharmaceutical micropollutants: Effects of wastewater effluent matrix and catalyst modifications. Appl. Catal. B Environ. 2014, 147, 8–16. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, R.; Choi, H.; Bhattacharjee, C. Involvement of process parameters and various modes of application of TiO2 nanoparticles in heterogeneous photocatalysis of pharmaceutical wastes—A short review. RSC Adv. 2014, 4, 57250–57266. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.; Zhou, Z.; Shang, Y.; Zhuang, X. Photocatalytic degradation of sulfonamides by Bi2O3-TiO2/PAC ternary composite: Mechanism, degradation pathway. J. Water Process Eng. 2020, 36, 101335. [Google Scholar] [CrossRef]
- Mourid, E.H.; El Mouchtari, E.M.; El Mersly, L.; Benaziz, L.; Rafqah, S.; Lakraimi, M. Development of a new recyclable nanocomoposite LDH-TiO2 for the degradation of antibiotic sulfamethoxazole under UVA radiation: An approach towards sunlight. J. Photochem. Photobiol. A Chem. 2020, 396, 112530. [Google Scholar] [CrossRef]
- Długosz, M.; Zmudzki, P.; Kwiecień, A.; Szczubiałka, K.; Krzek, J.; Nowakowska, M. Photocatalytic degradation of sulfamethoxazole in aqueous solution using a floating TiO2-expanded perlite photocatalyst. J. Hazard. Mater. 2015, 298, 146–153. [Google Scholar] [CrossRef]
- De Matos Rodrigues, M.H.; Rodrigues de Sousa, P.A.; Borges, K.C.M.; de Melo Coelho, L.; de Fátima Gonçalves, R.; Teodoro, M.D.; Vilella da Motta, F.; Maribondo do Nascimento, R.; Júnior, M.G. Enhanced degradation of the antibiotic sulfamethoxazole by heterogeneous photocatalysis using Ce0,8Gd0,2O2-δ/TiO2 particles. J. Alloys Compd. 2019, 808, 2–10. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, Z.; Greaves, J.; Cooper, W.J.; Song, W. Trimethoprim: Kinetic and mechanistic considerations in photochemical environmental fate and AOP treatment. Water Res. 2012, 46, 1327–1336. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.; Ye, C.; Wang, A.; Wei, W.; Hu, D.; Qiu, R.; Yan, K. Facile synthesis of Z-scheme composite of TiO2 nanorod/g-C3N4 nanosheet efficient for photocatalytic degradation of ciprofloxacin. J. Clean. Prod. 2020, 253, 120055. [Google Scholar] [CrossRef]
- Gad-Allah, T.A.; Ali, M.E.M.; Badawy, M.I. Photocatalytic oxidation of ciprofloxacin under simulated sunlight. J. Hazard. Mater. 2011, 186, 751–755. [Google Scholar] [CrossRef]
- Li, S.; Hu, J. Transformation products formation of ciprofloxacin in UVA/LED and UVA/LED/TiO2 systems: Impact of natural organic matter characteristics. Water Res. 2018, 132, 320–330. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Zhu, M. Green synthesis of 3D tripyramid TiO2 architectures with assistance of aloe extracts for highly efficient photocatalytic degradation of antibiotic ciprofloxacin. Appl. Catal. B Environ. 2020, 260, 118149. [Google Scholar] [CrossRef]
- Huang, X.; Yang, W.; Zhang, G.; Yan, L.; Zhang, Y.; Jiang, A.; Xu, H.; Zhou, M.; Liu, Z.; Tang, H.; et al. Alternative synthesis of nitrogen and carbon co-doped TiO2 for removing fluoroquinolone antibiotics in water under visible light. Catal. Today 2021, 361, 11–16. [Google Scholar] [CrossRef]
- Du, J.; Ma, S.; Yan, Y.; Li, K.; Zhao, F.; Zhou, J. Corn-silk-templated synthesis of TiO2 nanotube arrays with Ag3PO4 nanoparticles for efficient oxidation of organic pollutants and pathogenic bacteria under solar light. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 237–249. [Google Scholar] [CrossRef]
- Gan, Y.; Zhang, M.; Xiong, J.; Zhu, J.; Li, W.; Zhang, C.; Cheng, G. Impact of Cu particles on adsorption and photocatalytic capability of mesoporous Cu@TiO2 hybrid towards ciprofloxacin antibiotic removal. J. Taiwan Inst. Chem. Eng. 2019, 96, 229–242. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Novel Magnetic Carbon Nanotube-TiO2 Composites for Solar Light Photocatalytic Degradation of Pharmaceuticals in the Presence of Natural Organic Matter. J. Water Process Eng. 2019, 31, 100836. [Google Scholar] [CrossRef]
- Carbajo, J.; Jiménez, M.; Miralles, S.; Malato, S.; Faraldos, M.; Bahamonde, A. Study of application of titania catalysts on solar photocatalysis: Influence of type of pollutants and water matrices. Chem. Eng. J. 2016, 291, 64–73. [Google Scholar] [CrossRef]
- Malesic-Eleftheriadou, N.; Evgenidou, E.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of antibiotics in aqueous media by using new synthesized bio-based poly (ethylene terephthalate)-TiO2 photocatalysts. Chemosphere 2019, 234, 746–755. [Google Scholar] [CrossRef]
- Porcar-Santos, O.; Cruz-Alcalde, A.; López-Vinent, N.; Zanganas, D.; Sans, C. Photocatalytic degradation of sulfamethoxazole using TiO2 in simulated seawater: Evidence for direct formation of reactive halogen species and halogenated by-products. Sci. Total Environ. 2020, 736, 139605. [Google Scholar] [CrossRef]
- Yang, C.C.; Huang, C.L.; Cheng, T.C.; Lai, H.T. Inhibitory effect of salinity on the photocatalytic degradation of three sulfonamide antibiotics. Int. Biodeterior. Biodegrad. 2015, 102, 116–125. [Google Scholar] [CrossRef]
- Xekoukoulotakis, N.P.; Drosou, C.; Brebou, C.; Chatzisymeon, E.; Hapeshi, E.; Fatta-Kassinos, D.; Mantzavinos, D. Kinetics of UV-A/TiO2 photocatalytic degradation and mineralization of the antibiotic sulfamethoxazole in aqueous matrices. Catal. Today 2011, 161, 163–168. [Google Scholar] [CrossRef]
- Diao, Z.H.; Xu, X.R.; Jiang, D.; Liu, J.J.; Kong, L.J.; Li, G.; Zuo, L.Z.; Wu, Q.H. Simultaneous photocatalytic Cr(VI) reduction and ciprofloxacin oxidation over TiO2/Fe0 composite under aerobic conditions: Performance, durability, pathway and mechanism. Chem. Eng. J. 2017, 315, 167–176. [Google Scholar] [CrossRef]
- Khan, S.A.; Arshad, Z.; Shahid, S.; Arshad, I.; Rizwan, K.; Sher, M.; Fatima, U. Synthesis of TiO2/Graphene oxide nanocomposites for their enhanced photocatalytic activity against methylene blue dye and ciprofloxacin. Compos. Part B Eng. 2019, 175, 107120. [Google Scholar] [CrossRef]
- Huerta-Aguilar, C.A.; García Gutiérrez, Y.S.; Thangarasu, P. Crystal plane directed interaction of TiO2 [1 0 1] with AgNPs [1 1 1] silver nanoparticles enhancing solar light induced photo-catalytic oxidation of ciprofloxacin: Experimental and theoretical studies. Chem. Eng. J. 2020, 394, 124286. [Google Scholar] [CrossRef]
- Pablos, C.; Marugán, J.; van Grieken, R.; Serrano, E. Emerging micropollutant oxidation during disinfection processes using UV-C, UV-C/H2O2, UV-A/TiO2 and UV-A/TiO2/H2O2. Water Res. 2013, 47, 1237–1245. [Google Scholar] [CrossRef]
- Biancullo, F.; Moreira, N.F.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Castro-Silva, S.M.; Silva, A.M.T. Heterogeneous photocatalysis using UVA-LEDs for the removal of antibiotics and antibiotic resistant bacteria from urban wastewater treatment plant effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Salma, A.; Thoröe-Boveleth, S.; Schmidt, T.C.; Tuerk, J. Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Berk, D. Characterization and mechanistic study of Mo+6 and V+5 codoped TiO2 as a photocatalyst. J. Photochem. Photobiol. A Chem. 2014, 294, 96–109. [Google Scholar] [CrossRef]
- Wang, F.; Yu, X.; Ge, M.; Wu, S. One-step synthesis of TiO2/γ-Fe2O3/GO nanocomposites for visible light-driven degradation of ciprofloxacin. Chem. Eng. J. 2020, 384, 2–9. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Z. Ag-SrTiO3/TiO2 composite nanostructures with enhanced photocatalytic activity. Mater. Res. Bull. 2019, 118, 110492. [Google Scholar] [CrossRef]
- Jahdi, M.; Mishra, S.B.; Nxumalo, E.N.; Mhlanga, S.D.; Mishra, A.K. Smart pathways for the photocatalytic degradation of sulfamethoxazole drug using F-Pd co-doped TiO2 nanocomposites. Appl. Catal. B Environ. 2020, 267, 118716. [Google Scholar] [CrossRef]
- Kowalska, K.; Maniakova, G.; Carotenuto, M.; Sacco, O.; Vaiano, V.; Lofrano, G.; Rizzo, L. Removal of carbamazepine, diclofenac and trimethoprim by solar driven advanced oxidation processes in a compound triangular collector based reactor: A comparison between homogeneous and heterogeneous processes. Chemosphere 2020, 238, 124665. [Google Scholar] [CrossRef]
- Manasa, M.; Chandewar, P.R.; Mahalingam, H. Photocatalytic degradation of ciprofloxacin & norfloxacin and disinfection studies under solar light using boron & cerium doped TiO2 catalysts synthesized by green EDTA-citrate method. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Eskandarian, M.R.; Choi, H.; Fazli, M.; Rasoulifard, M.H. Effect of UV-LED wavelengths on direct photolytic and TiO2 photocatalytic degradation of emerging contaminants in water. Chem. Eng. J. 2016, 300, 414–422. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Jiang, W.; Mkaouar, A.R.; Xu, P. Comparison study on photocatalytic oxidation of pharmaceuticals by TiO2-Fe and TiO2-reduced graphene oxide nanocomposites immobilized on optical fibers. J. Hazard. Mater. 2017, 333, 162–168. [Google Scholar] [CrossRef]
- Durán-Álvarez, J.C.; Avella, E.; Ramírez-Zamora, R.M.; Zanella, R. Photocatalytic degradation of ciprofloxacin using mono- (Au, Ag and Cu) and bi- (Au-Ag and Au-Cu) metallic nanoparticles supported on TiO2 under UV-C and simulated sunlight. Catal. Today 2016, 266, 175–187. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Pretali, L.; Irastorza, E.A.; Fasani, E.; Albini, A. Photolytic and photocatalytic degradation of fluoroquinolones in untreated river water under natural sunlight. Appl. Catal. B Environ. 2012, 119–120, 32–39. [Google Scholar] [CrossRef]
- Borowska, E.; Gomes, J.F.; Martins, R.C.; Quinta-Ferreira, R.M.; Horn, H.; Gmurek, M. Solar photocatalytic degradation of sulfamethoxazole by TiO2 modified with noble metals. Catalysts 2019, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Wang, Y.; Sun, F.; Wang, R.; Zhou, Y. Novel mpg-C3N4/TiO2 nanocomposite photocatalytic membrane reactor for sulfamethoxazole photodegradation. Chem. Eng. J. 2018, 337, 183–192. [Google Scholar] [CrossRef]
- Sirtori, C.; Agüera, A.; Gernjak, W.; Malato, S. Effect of water-matrix composition on Trimethoprim solar photodegradation kinetics and pathways. Water Res. 2010, 44, 2735–2744. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Gar Alalm, M.; Fujii, M.; Ookawara, S.; Ohno, T. Photocatalytic degradation of trimethoprim using S-TiO2 and Ru/WO3/ZrO2 immobilized on reusable fixed plates. J. Water Process Eng. 2020, 33, 3–10. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Carbone, F.; Giancotti, V.; Baiocchi, C. Characterization of intermediate compounds formed upon photoinduced degradation of quinolones by high-performance liquid chromatography/high-resolution multiple-stage mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1533–1552. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Peng, Q.; Zhou, L.; Tan, X.; Jiang, L.; Tang, C.; Wang, H.; Liu, S.; Wang, Y.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, S.; Wang, Y.; Sun, X.; Gao, Y.; Gao, B. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO2 nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interface Sci. 2018, 527, 202–213. [Google Scholar] [CrossRef]
- Gan, Y.; Wei, Y.; Xiong, J.; Cheng, G. Impact of post-processing modes of precursor on adsorption and photocatalytic capability of mesoporous TiO2 nanocrystallite aggregates towards ciprofloxacin removal. Chem. Eng. J. 2018, 349, 1–16. [Google Scholar] [CrossRef]
- Murgolo, S.; Yargeau, V.; Gerbasi, R.; Visentin, F.; El Habra, N.; Ricco, G.; Lacchetti, I.; Carere, M.; Curri, M.L.; Mascolo, G. A new supported TiO2 film deposited on stainless steel for the photocatalytic degradation of contaminants of emerging concern. Chem. Eng. J. 2017, 318, 103–111. [Google Scholar] [CrossRef]
- Silva, A.R.; Martins, P.M.; Teixeira, S.; Carabineiro, S.A.C.; Kuehn, K.; Cuniberti, G.; Alves, M.M.; Lanceros-Mendez, S.; Pereira, L. Ciprofloxacin wastewater treated by UVA photocatalysis: Contribution of irradiated TiO2 and ZnO nanoparticles on the final toxicity as assessed by Vibrio fischeri. RSC Adv. 2016, 6, 95494–95503. [Google Scholar] [CrossRef]
- Xing, X.; Du, Z.; Zhuang, J.; Wang, D. Removal of ciprofloxacin from water by nitrogen doped TiO2 immobilized on glass spheres: Rapid screening of degradation products. J. Photochem. Photobiol. A Chem. 2018, 359, 23–32. [Google Scholar] [CrossRef]
- Polliotto, V.; Pomilla, F.R.; Maurino, V.; Marcì, G.; Bianco Prevot, A.; Nisticò, R.; Magnacca, G.; Paganini, M.C.; Ponce Robles, L.; Perez, L.; et al. Different approaches for the solar photocatalytic removal of micro-contaminants from aqueous environment: Titania vs. hybrid magnetic iron oxides. Catal. Today 2019, 328, 164–171. [Google Scholar] [CrossRef]
- Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, K.T.; Le, P.H. TiO2 and Au-TiO2 nanomaterials for rapid photocatalytic degradation of antibiotic residues in aquaculture wastewater. Materials 2019, 12, 2434. [Google Scholar] [CrossRef] [Green Version]
- Tsiampalis, A.; Frontistis, Z.; Binas, V.; Kiriakidis, G.; Mantzavinos, D. Degradation of sulfamethoxazole using iron-doped titania and simulated solar radiation. Catalysts 2019, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Martini, J.; Orge, C.A.; Faria, J.L.; Pereira, M.F.R.; Soares, O.S.G.P. Catalytic advanced oxidation processes for sulfamethoxazole degradation. Appl. Sci. 2019, 9, 2652. [Google Scholar] [CrossRef] [Green Version]
- Liang, R.; Van Leuwen, J.C.; Bragg, L.M.; Arlos, M.J.; Li Chun Fong, L.C.M.; Schneider, O.M.; Jaciw-Zurakowsky, I.; Fattahi, A.; Rathod, S.; Peng, P.; et al. Utilizing UV-LED pulse width modulation on TiO2 advanced oxidation processes to enhance the decomposition efficiency of pharmaceutical micropollutants. Chem. Eng. J. 2019, 361, 439–449. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.S.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.P.; et al. Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters. Appl. Catal. B Environ. 2018, 224, 810–824. [Google Scholar] [CrossRef]
- Arlos, M.J.; Hatat-Fraile, M.M.; Liang, R.; Bragg, L.M.; Zhou, N.Y.; Andrews, S.A.; Servos, M.R. Photocatalytic decomposition of organic micropollutants using immobilized TiO2 having different isoelectric points. Water Res. 2016, 101, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Ramasundaram, S.; Seid, M.G.; Choe, J.W.; Kim, E.J.; Chung, Y.C.; Cho, K.; Lee, C.; Hong, S.W. Highly reusable TiO2 nanoparticle photocatalyst by direct immobilization on steel mesh via PVDF coating, electrospraying, and thermal fixation. Chem. Eng. J. 2016, 306, 344–351. [Google Scholar] [CrossRef]
- Murgolo, S.; Petronella, F.; Ciannarella, R.; Comparelli, R.; Agostiano, A.; Curri, M.L.; Mascolo, G. UV and solar-based photocatalytic degradation of organic pollutants by nano-sized TiO2 grown on carbon nanotubes. Catal. Today 2015, 240, 114–124. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Puca, M.; Pérez Bravo, J.; Bafico, J.; Campo Dall Orto, V.; Copello, G.J. Dual adsorbent-photocatalytic keratin-TiO2 nanocomposite for trimethoprim removal from wastewater. New J. Chem. 2020, 44, 10964–10972. [Google Scholar] [CrossRef]
- Paredes, L.; Murgolo, S.; Dzinun, H.; Dzarfan Othman, M.H.; Ismail, A.F.; Carballa, M.; Mascolo, G. Application of immobilized TiO2 on PVDF dual layer hollow fibre membrane to improve the photocatalytic removal of pharmaceuticals in different water matrices. Appl. Catal. B Environ. 2019, 240, 9–18. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Dong, X.; Zhang, X.; Chen, T.; Zheng, S.; Sun, Z. Tuning and controlling photocatalytic performance of TiO2/kaolinite composite towards ciprofloxacin: Role of 0D/2D structural assembly. Adv. Powder Technol. 2020, 31, 1241–1252. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Hildebrand, J.P.; Taffa, D.H.; Wark, M.; Kamonsuangkasem, K.; Chirawatkul, P.; Wantala, K. Visible light-induced degradation of antibiotic ciprofloxacin over Fe–N–TiO2 mesoporous photocatalyst with anatase/rutile/brookite nanocrystal mixture. J. Photochem. Photobiol. A Chem. 2020, 391, 112371. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Lu, Q.; Wang, Q.; Ma, Z. TiOF2/TiO2 composite nanosheets: Effect of hydrothermal synthesis temperature on physicochemical properties and photocatalytic activity. J. Taiwan Inst. Chem. Eng. 2019, 96, 214–222. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, J.; Zeng, D.; Yu, C.; Huang, W.; Yang, K.; Liu, X.; Liu, H. Binary-phase TiO2 modified Bi2MoO6 crystal for effective removal of antibiotics under visible light illumination. Mater. Res. Bull. 2019, 112, 336–345. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kidkhunthod, P.; Chanlek, N.; Soontaranon, S.; Wantala, K. High anatase purity of nitrogen-doped TiO2 nanorice particles for the photocatalytic treatment activity of pharmaceutical wastewater. Appl. Surf. Sci. 2019, 478, 1–14. [Google Scholar] [CrossRef]
- Feng, X.; Wang, P.; Hou, J.; Qian, J.; Ao, Y.; Wang, C. Significantly enhanced visible light photocatalytic efficiency of phosphorus doped TiO2 with surface oxygen vacancies for ciprofloxacin degradation: Synergistic effect and intermediates analysis. J. Hazard. Mater. 2018, 351, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Teixeira, S.; Mora, H.; Blasse, L.M.; Martins, P.M.; Carabineiro, S.A.C.; Lanceros-Méndez, S.; Kühn, K.; Cuniberti, G. Photocatalytic degradation of recalcitrant micropollutants by reusable Fe3O4/SiO2/TiO2 particles. J. Photochem. Photobiol. A Chem. 2017, 345, 27–35. [Google Scholar] [CrossRef]
- Eckert, H.; Bobeth, M.; Teixeira, S.; Kühn, K.; Cuniberti, G. Modeling of photocatalytic degradation of organic components in water by nanoparticle suspension. Chem. Eng. J. 2015, 261, 67–75. [Google Scholar] [CrossRef]
- Spanu, D.; Minguzzi, A.; Recchia, S.; Shahvardanfard, F.; Tomanec, O.; Zboril, R.; Schmuki, P.; Ghigna, P.; Altomare, M. An operando x-ray absorption spectroscopy study of a NiCu-TiO2 photocatalyst for H2 evolution. ACS Catal. 2020, 10, 8293–8302. [Google Scholar] [CrossRef]
- Majeed, I.; Nadeem, M.A.; Hussain, E.; Waterhouse, G.I.N.; Badshah, A.; Iqbal, A.; Nadeem, M.A.; Idriss, H. On the Synergism between Cu and Ni for Photocatalytic Hydrogen Production and their Potential as Substitutes of Noble Metals. ChemCatChem 2016, 8, 3146–3155. [Google Scholar] [CrossRef]
- Lin, J.D.; Yan, S.; Huang, Q.D.; Fan, M.T.; Yuan, Y.Z.; Tan, T.T.Y.; Liao, D.W. TiO2 promoted by two different non-noble metal cocatalysts for enhanced photocatalytic H2 evolution. Appl. Surf. Sci. 2014, 309, 188–193. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, J.; Bai, Y.; Liang, X.; Wang, T.; Wang, C. Facile synthesis of Mo-doped TiO2 for selective photocatalytic CO2 reduction to methane: Promoted H2O dissociation by Mo doping. J. CO2 Util. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Thakare, S.R.; Mate, V.R.; Urkude, K.; Gawande, S.B. Graphene-TiO2-polyaniline nanocomposite: A new green and efficient catalyst as a alternative for noble metal and NaBH4 induced the reduction of 4-nitro phenol. FlatChem 2020, 22, 100179. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, P.; Zhu, W.; Li, G.; Zhang, D.; Li, H. Copper Nanowires: A Substitute for Noble Metals to Enhance Photocatalytic H2 Generation. Nano Lett. 2015, 15, 4853–4858. [Google Scholar] [CrossRef]









| Antibiotic | Sulfamethoxazole | Trimethoprim | Ciprofloxacin |
|---|---|---|---|
| Number of countries where antibiotic was detected | 47 | 29 | 20 |
| Average concentration (µg/L) | 0.095 | 0.037 | 18.99 |
| Maximum concentration (µg/L) | 53.8 | 13.6 | 6500 |
| Antibiotic | Sulfamethoxazole | Trimethoprim | Ciprofloxacin |
|---|---|---|---|
| Class | Sulfonamides | Diaminopyrimidines | Fluoroquinolones |
| Molecular formula | C10H11N3O3S | C14H18N4O3 | C17H18FN3O3 |
| Molecular weight (g/mol) | 253.28 | 290.32 | 331.34 |
| pKa1, pKa2 | 1.7; 5.6 [23] | 3.2; 6.7 [23] | 5.9; 8.9 [24] |
| Solubility in water (mg/L) [8] | 610 | 400 | 30000 |
| Technology | Description | Advantages | Drawbacks |
|---|---|---|---|
| Adsorption | A mass transfer process of accumulation of chemicals from the liquid phase into the solid phase of adsorbent [23,42]. A wide variety of adsorbents is utilized (clays and minerals, metal oxides, polymers, nanocomposites, carbon nanotubes; activated carbon and biochars are among the most commonly used) [23,46]. | High removal efficiency [35,45,47] | Complex and expensive production of some adsorbents [46] |
| Short treatment period and simple operation [43,46] | High material costs and high regeneration costs of the used adsorbent [23,43,47] | ||
| Low operation costs [23,41] | Nondestructive process, production of secondary waste [35,41,47] | ||
| Membrane filtration | Physical process of chemicals separation using synthetic membranes [42]. Only nanofiltration and reverse osmosis can be efficiently applied to antibiotics removal [46]. | High removal efficiency [42,45] | High costs of operation and maintenance [35,43] |
| Fast and simple process with no chemicals involved [50,51] | Membrane fouling [50,51] | ||
| Not suitable for large volumes of wastewater [45,51] | |||
| Nondestructive process [41,45] | |||
| Advanced oxidation processes (AOPs) | Processes based on utilization of highly reactive chemical species that are efficient in oxidizing and mineralizing organic compounds [35,41]. | High efficiency of pollutants degradation and possibility of complete mineralization in a short period of time [43,46,47] | High operation costs [43,46]. In case of incomplete mineralization post-treatment is required to remove toxicity [43] |
| Include environmentally friendly, safe and sustainable processes [52] | |||
| Disinfection properties [43] | |||
| AOPs: Ozonation | Ozone molecule O3 has high oxidation capability (E0 = 2.07 V), and therefore is able to efficiently oxidize organic pollutants [41,42]. | On-site generation of ozone [43] | High operation and energy costs [45,47,49] |
| No waste production [49] | Possible formation of harmful by-products [35,46,47] | ||
| Limited pollutant mineralization [43] | |||
| AOPs: Fenton process (homogeneous) | Fenton reagent consisting of Fe2+ and H2O2 produces highly reactive hydroxyl radicals •OH that oxidize organic pollutants [49,53]. | Fast, effective and safe process [42,49] | Reaction is limited to acidic conditions pH 2.8–3.5 [41,43,53] |
| Easy operation [53] | Large volumes of ferrous sludge produced [42,43,53] | ||
| Possible mineralization of pollutants [43] | Complicated recovery of Fe2+/Fe3+ ions [23] | ||
| Iron is abundant and non-toxic [42,49] | |||
| AOPs: Electrochemical processes | Processes of oxidizing organic substances using electric current [53]. Most widely employed anode materials include graphite and TiO2, as well as Ti-based alloys, Ru and Ir oxides [41]. | Easy to operate, safe and highly efficient process [23,43] | High operation costs [23,42,45] |
| No chemical reagents required and no generation of secondary wastes [23,41] | Expensive electrodes [41], short electrode life time, electrode fouling [23] | ||
| Suitable for waste waters with high concentrations of pharmaceuticals [45] | It is required that wastewater is highly conductive (otherwise electrolytes should be added) [23,41,42] | ||
| Mass transfer resistance [41] | |||
| Applicable to wastewater with low flow rate [45] | |||
| Water oxidation occurs faster than oxidation of organic pollutants [42] | |||
| AOPs: Ultrasonication | Processes employing sound waves for formation, growth and collapse of bubbles in liquid media [23]. | No chemical reagents required [43] | High operation costs [43] |
| Destruction and corrosion of reactor metallic surface [23] | |||
| AOPs: Radiation assisted catalytic reaction | Processes employing electromagnetic radiation (for example, microwaves, x-rays, gamma rays) to form highly reactive species (•OH, e−, •H, H2, H2O2, H3O+) that oxidize organic pollutants [23,53]. | Fast and energy-efficient process [23] | Toxicity of intermediates in mineralization process should be considered [23] |
| No chemical reagents required [23] | Various factors affect degradation efficiency (dose of radiation, pH, water matrix composition) [51] | ||
| AOPs: Catalytic wet peroxide oxidation | Processes of pollutants degradation in aqueous media through catalytic H2O2 reduction to OH− and •OH under extreme pressures and temperatures [42]. | Fast and efficient process [23] | High operation and material costs [23] |
| Some substances (containing nitro functional groups and halogens) are difficult to degrade [42] | |||
| AOPs: Photolysis | Processes employing light (artificial or natural) for the generation of reactive species and subsequent degradation of organic pollutants [41]. | Cost-effective process [41] | Lowest degradation efficiency among AOPs [45] |
| Applicable only to photo-sensitive pollutants [41] | |||
| AOPs: Photocatalysis | Degradation of organic contaminants using semiconducting materials (photocatalysts) and light (artificial or natural) [42]. | Easy to operate, highly efficient and environmentally friendly process [18,42,51] | Fast recombination of photogenerated charge carriers decreases process efficiency [42] |
| Performed under ambient temperature and pressure and utilizes atmospheric oxygen as oxidant [23,44] | Limited visible light response [42] | ||
| Complete mineralization of organic pollutants is possible, no waste generation [42,43] | Laboratory scale [43] | ||
| Efficient recovery and reuse of photocatalysts is possible [52,54] | Not applicable to water with high concentrations of organic pollutants [42,51] | ||
| Losses of photocatalyst under long-term operation [51] | |||
| Toxicity of by-products should be considered [42] |
| Catalyst | Characteristics | Process conditions | Performance | Ref. |
|---|---|---|---|---|
| Sulfamethoxazole | ||||
| Kaolinite Clay-TiO2- ZnWO4 and agrowaste (carica papaya seeds or musa paradisiaca peels) nanocomposite | Particle size: 62–257 nm (SEM)Band gap: 2.56–2.89 eV | Medium: Ultrapure water Ant. Conc.: 0.05 gL−1 Cat. Conc: 0.05 gL−1 pH: 6.8Light source: sunlight irradiation (10 AM–5 PM) Cat.: TZPP5 (based on musa paradisiaca peels and calcined at 500 °C) | Photodegradation: 60% (60 min) Mineralization: 50% (60 min) COD k(1st) = 0.0227 min−1 t1/2 = 30.48 minStability: over 4 cycles | [73] |
| LDH-TiO2 (Zn2-Al-CO3/TiO2 P25) | - | Ant. Conc.: 0.02 gL−1 Cat. Conc.: 0.5 gL−1 pH: 10Light source: 300 W UV-A lamp (300–400 nm) with high pressure tungsten filament Cat.: LDH-TiO2 (10% TiO2) | Photodegradation: 100% (360 min) Mineralization: 100% (144 h) COD Stability: 90.5% after 5 cycles | [90] |
| TiO2 P25 (Evonik, Germany) | Particle size: 21 nm Composition: 80% anatase, 20% rutile BET surface: 35–65 m2g−1 Band gap: 3.2 eV | Medium: deionized water Ant. Conc.: 0.001 gL−1 Cat. Conc.: 0.1 gL−1 pH: 6.0 Light source: solar simulation chamber with a 1.5 kW Xenon lamp (290–400 nm) Temperature: 20 °C | Photodegradation: 100% (120 min) k(1st) = 0.041 min−1 | [104] |
| F-Pd co-doped TiO2 | Particle size: 5–25 nm (TEM) Band gap: 0.54–3.26 eV | Medium: deionized water Ant. Conc.: 0.03 gL−1 Cat. Conc.: 1 gL−1 Light source: direct sunlight irradiation Temperature: 29–31 °C Cat.: FPd-TiO2 (10 mol.% Pd) | Photodegradation: 98.4% (40 min) Mineralization: 93% (40 min) TOC | [116] |
| Bi2O3-TiO2/PAC (powdered activated carbon) | Crystallite size: 48 nm (XRD) Band gap: 2.58 eV | Medium: deionized water Ant. Conc.: 0.02 gL−1 Cat. Conc.: 0.2 gL−1 Light source: solar simulator (300 W Xe arc lamp) | Photodegradation: 100% (30 min) Stability: 90.4% after 5 cycles | [89] |
| TiO2 P25 | Particle size: 21 nm Composition: 80% anatase, 20% rutile BET surface: ~50 m2g−1 Band gap: 3.2 eV | Ant. Conc.: 0.04 gL−1 Cat. Conc.: 0.024 gL−1 pH: 6.0 Light source: UV-C (254 nm), light intensity 15 mW cm−2Temperature: 25 °C | Photodegradation: 95.0% (120 min) Mineralization: 66% (120 min) TOC | [84] |
| TiO2 | BET surface: 52 m2 g−1Band gap: 3.08 eV | Ant. Conc.: 100 µgL−1 Cat. Conc.: 0.2 gL−1 Light source: solar simulator with Xenon lamp (UV irradiance 30 Wm−2) | Photodegradation: 100% (30 min) | [134] |
| Au-TNWs/TNAs (Au nanoparticle-decorated TiO2 nanowires on TiO2 nanotube arrays) | Composition: 100% anatase Crystallite size: 21.3–24.7 nm (XRD) | Medium: blank wastewater samples with 0.1% (v.v) formic acid Ant. Conc.: 500 ng/mL, 30mL solutions Light source: UV-VIS 100 W Xenon lamp, 120 mWcm−2 Temperature: 32–33 °C | Photodegradation: k(1st) = 1.05 min−1 | [135] |
| Fe/TiO2 | Composition: 100% anatase Crystallite size: 27 nm (XRD) Band gap: 3 eV | Medium: ultrapure water Ant. Conc.: 234 µgL−1 Cat. Conc.: 1 gL−1 pH: 6 Light source: solar simulator with 100 W Xenon lamp Cat.: 0.04 mol.% Fe | Photodegradation: 95% (90 min) k(1st) = 0.029 min−1 Stability: 5 cycles (55%) | [136] |
| TiO2 | Composition: 93% anatase, 7% rutile Crystallite size: 8.91 nm anatase; 14.7 nm rutile (XRD) BET surface: 134 m2g−1 | Medium: ultrapure water Ant. Conc.: 0.03 gL−1 Cat. Conc.: 0.5 gL−1 pH: 5.1 Light source: medium pressure mercury vapour lamp (UV-vis: λ > 350 nm; 50 mWcm−2) | Photodegradation: 100% (120 min) Mineralization: 40% (180 min) TOC | [137] |
| TiO2/CNT (carbon nanotubes), 10% CNT | Composition: 97% anatase, 3% rutile Crystallite size: 14.6 nm anatase; 40.7 nm rutile (XRD) BET surface: 142 m2g−1 | Photodegradation: 100% (120 min) Mineralization: 70% (180 min) TOC Stability: 3 cycles | ||
| Pt/TiO2 P25 (1.0 wt.% Pt) | Composition: 80% anatase, 20% rutile Crystallite size: 220 nm anatase, 216 nm rutile (XRD) BET surface: ~50 m2g−1 Band gap: 3.18 eV | Medium: ultrapure water Ant. Conc.: 0.001 gL−1 Cat. Conc.: 0.05 gL−1 Light source: natural sunlight | Photodegradation: 90% (30 min) k(1st) = 0.076 min−1 t1/2 = 9.1 min Mineralization: 29±10% (60 min) DOC, Ccat = ~25 mgL−1 Ecotoxicity: Lepidium sativum, no phytotoxicity | [123] |
| Pd/TiO2 P25 (1.0 wt.% Pd) | Composition: 89% anatase, 11% rutile Crystallite size: 206 nm anatase, 180 nm rutile (XRD) BET surface: ~50 m2g−1 Band gap: 2.92 eV | Photodegradation: 100% (10 min) k(1st) = 0.521 min−1 t1/2 =1.3 min Mineralization: 45 ± 2% (60 min) DOC, Ccat = ~25 mgL−1 Ecotoxicity: Lepidium sativum, no phytotoxicity | ||
| Ce0.8Gd0.2O2-δ/TiO2 | Particle size: 7–20 nm (TEM) Composition: 55.78% ceria; 27.01% rutile; 17.21% anatase Crystallite size: 72.94 nm TiO2; 19.71 nm Ce0.8Gd0.2O2-δ (XRD) BET surface: 5.11 m2g−1 Band gap: 2.84 eV | Ant. Conc.: 0.025 gL−1 Cat. Conc.: 0.1 gL−1 Light source: 15 W mercury UV lamp | Photodegradation: 97% (120 min) k(2nd) = 0.2959 mg−1min−1Stability: 5 cycles | [92] |
| Biobased-PET-TiO2 P25 composite films | TiO2 P25:Particle size: 20–30 nm Composition: 80% anatase; 20% rutile BET surface: 56 m2g−1 PZC: 6.3–6.8 | Medium: ultrapure water Ant. Conc.: 0.001 gL−1 (for each antibiotic, mixture of eight) Cat. Conc.: 0.05 gL−1 Light source: solar simulator with xenon lamp (1.5 kW, 500 Wm−2) Cat.: PET-10%-TiO2 (10 wt.% of TiO2) | Photodegradation: 98% (6 h) k(1st) = 0.015 min−1 t1/2 = 46.2 min Stability: 5 cycles | [103] |
| Zn-TiO2/pBC (reed straw biochar) | Crystallite size: 9.4 nm (XRD) BET surface: 169.12 m2g−1 | Medium: ultrapure water Ant. Conc.: 0.01 gL−1 Cat. Conc.: 1.25 gL−1 pH: 4Light source: 50 W Xenon lamp with 420 nm cutoff filter (visible) Temperature: 25 °C Cat.: Zn10-TiO2/pBC (10 wt.% Zn) | Photodegradation: 80.81% (3 h) k(1st) = 0.0085 min−1 t1/2 = 81 min Mineralization: 56.13% (3 h) COD Stability: 5 cycles (77.41%) | [79] |
| Magnetic carbon nanotube-TiO2 P25 (MCNT-TiO2) composites | BET surface: 151 m2g−1 | Medium: ultrapure water Ant. Conc.: 150 μgL−1 Cat. Conc.: 0.1 gL−1 pH: 7.0±0.2 Light source: solar simulator, 1000 Wm−2 Temperature: 26±3 °C Cat.: MCNT-TiO2 (1:5 mass ratio) | Photodegradation: 92% (30 min) k(1st) = 0.05 min−1 Stability: 5 cycles | [101] |
| TiO2 P25 | Composition: 80% anatase, 20% rutile | Medium: secondary urban wastewater Ant. Conc.: 100 µgL−1 Cat. Conc.: 1 gL−1 Light source: four 9 W UVA-LEDs, 381 nm | Photodegradation: 100% SMX (30 min) k(1st) = 0.2126 min−1 Disinfection: total heterotrophs, E. coli and enterococci | [111] |
| Porous titanium-titanium dioxide (PTT) substrates | Composition: anatase Band gap: 3.0 eV Isoelectric point: 6.0 | Medium: ultrapure water Ant. Conc.: 300 mL of 2 µgL−1 (eighteen pharmaceuticals) pH: ~5 Light source: UV-LEDs, 1.7×10−3 W, 365 nm | Photodegradation: 72.74% (300 min) k(1st) = 0.00435 min−1 | [138] |
| Graphene-based TiO2 P25 (TiO2-rGO) | BET surface: 44.761 m2g−1 | Medium: membrane bioreactor-treated urban wastewater Ant. Conc.: 100 μgL−1 (three compounds) Cat. Conc.: 0.1 gL−1 pH: 5.2–6.2 Light source: solar simulator with 1 kW Xenon lamp, 63 Wm−2Temperature: 25 ± 1 °C Cat.: TiO2-rGO-PH | Photodegradation: 50 ± 3% (60 min) Disinfection: E. coli complete inactivation (180 min) | [139] |
| Ag0(NP)/TiO2 thin films | Particle size: 10–15 nm (TEM) BET surface: 12.02 m2g−1 | Medium: purified water Ant. Conc.: 50 mL of 1.0 mgL−1 pH: 6.0 Light source: 9 W UVA 360 nm Temperature: 25 ± 1 °C Cat.: Ag0(NP)/TiO2 (B) | Photodegradation: 57% (120 min) k(1st) = 0.0067 min−1 Mineralization: 30.2% (120 min) NPOC Stability: 6 cycles | [72] |
| TiO2 supported on reed straw biochar TiO2/pBC | Composition: 100% anatase Crystallite size: 10.1 nm BET surface: 102.16 m2g−1 | Medium: ultrapure water Ant. Conc.: 0.01 gL−1 Cat. Conc.: 1.25 gL−1 pH: 4.0 Light source: 50 W xenon lamp with visible light filter Temperature: 25 °C Cat.: TiO2/pBC (300) | Photodegradation: 91.27% (3 h) k(1st) = 0.0130 min−1 t1/2 = 53.32 minMineralization: 57.44% (3 h) CODStability: 5 cycles | [80] |
| TiO2 P25-WO3 | Composition: 77% anatase, 23% rutile Crystallite size: 25 nm anatase, 78 nm rutile (XRD) BET surface: 32 m2g−1 Band gap: 3.0 eV | Medium: ultrapure water Ant. Conc.: 350 µgL−1 Cat. Conc.: 0.250 gL−1 Light source: solar simulator with 100 W xenon lamp, 420 nm cut-off filter Temperature: 25 °C Cat.: 4% W-P25(700) | Photodegradation: 100% (60 min) solar irradiation 25% (120 min) visible light k(1st) = 0.133 min−1 Mineralization: 28% (6 h), 20 mgL−1 SMX with 1 gL−1 4% W-P25(700) Ecotoxicity: Escherichia coli, Enterococcus faecalis | [74] |
| TiO2 P25-Fe immobilized on optical fibers | Grain size: 7.41 nm (XRD)Composition: 54% anatase, 46% rutile Band gap: 2.40 eV | Medium: deionized water Ant. Conc.: 0.005 gL−1 Cat. Dosage: 30 pieces of 10 cm photocatalyst-coated SOFs pH: 6.0 Light source: visible light source (halogen lamp 150 W) Temperature: 23 °C | Photodegradation: 35% (6 h) k(1st) = 0.082 min−1 | [120] |
| TiO2 P25-reduced graphene oxide (TiO2-rGO) immobilized on optical fibers | Grain size: 6.52 nm (XRD)Composition: 69% anatase, 31% rutile Band gap: 2.85 eV | Photodegradation: 35% (6 h) k(1st) = 0.079 min−1 | ||
| CoFe2O4/TiO2 (+TiO2 P25) | - | Medium: ultrapure water Ant. Conc.: 100 µM Cat. Conc.: 0.5 gL−1 Light source: photo-chemical reactor with twelve mercury lamps (350 nm) | Photodegradation: 100% (5 h) Mineralization: 50% (5 h) TOC Ecotoxicity: green alga Chlorella vulgaris, brine shrimp Artemia salina | [86] |
| Biochar/TiO2 | Composition: anatase | Medium: pure water Ant. Conc.: 0.01 gL−1 Cat. Conc.: 5 gL−1 pH: 4.0 Light source: UV-C, 15 W, 254 nm Temperature: 293 K | Photodegradation: 75% (3 h) Mineralization: 65% (3 h) COD Ecotoxicity: Daphnia magna, E.Coli | [81] |
| TiO2 (TiEt-450) | Hydrodynamic particle size: 3.0 µm Composition: 100% anatase BET surface: 43 m2g−1 Isoelectric point: 7.1 Band gap: 3.22 eV | Medium: deionized water Ant. Conc.: 100 µgL−1 of each contaminant (mixture of five, 500 µgL−1 in total) Cat. Conc.: 0.5 gL−1 pH: natural Light source: solar radiation pilot plant Temperature: ambient | Photodegradation: 100% (30 min) k(1st) = 0.095 min−1 | [102] |
| TiO2 P25 | Particle size: 30 nm Composition: 70% anatase, 30% rutile BET surface: 50 ± 15 m2g−1 Band gap: 3.15 eV | Medium: high purity water Ant. Conc.: 0.02 gL−1 Cat. Conc.: 0.5 gL−1 pH: natural Light source: UV-LEDs: 365 ± 10 nm, 300 ± 5 nm, and 260 ± 10 nm Temperature: 25 °C | Photodegradation: 58% (3 h) UV-A 85% (3 h) UV-B 100% (3 h) UV-C Mineralization:35.1% (3 h) UV-A 40.5% (3 h) UV-B 59.9% (3 h) UV-C | [119] |
| TiO2 immobilized on porous supports: quartz fiber filters (QFT) or porous titanium sheets (PTT) | Composition: anatase Isoelectric point: 4 (QFT), 6 (PTT) Band gap: 3.18 eV (QFT), 3.0 eV (PTT) | Medium: ultrapure water Ant. Conc.: 300 mL of 2.0 µgL−1 pH: 4.5–5 Light source: UV-LED 365 nm, 1.7 mW, 0.13 mW/cm2 Temperature: 24 ± 2 °C Cat.: TiO2 PTT | Photodegradation: k(1st) = 0.0069 min−1 Stability: 2 cycles | [140] |
| TiO2 P25 immobilized on PVDF-coated steel mesh (SM-TiO2) | - | Ant. Conc.: 10 µM Cat. Conc.: 0.02 gL−1 pH: 6.8–7.0 Light source: six blacklight blue lamps, 4 W, 350–400 nm | Photodegradation: 100% (120 min) k(1st) = 0.0568 min−1 Mineralization: 15% (180 min) TOC Stability: 20 cycles | [141] |
| Floating TiO2-expanded perlite (EP-TiO2-773) | - | Medium: deionized water Ant. Conc.: 0.1 gL−1 Cat. Conc.: 3.33 gL−1 pH: 10 Light source: photoreactor with six (8 W each) lamps, 316–400 nm | Photodegradation: k(0st) = 4.57×10−6 min−1 | [91] |
| Nano-sized TiO2 supported on single wall carbon nanotubes (SWCNTs/TiO2) | Composition: anatase | Medium: ultrapure water Ant. Conc.: 200–500 µgL−1 Cat. Conc.: 0.1 gL−1 Light source: low pressure mercury lamp, 17 W, 254 nm, 0.1 Wcm−2 | Photodegradation: k(1st) = 0.42 min−1 Stability: 5 cycles | [142] |
| Cu–TiO2 P25 | Particle size: 21 ± 4 nm (TEM) BET surface: 52–59 m2g−1 | Medium: oxygen-saturated waterAnt. Conc.: 0.004 gL−1 Cat. Conc.: 1 gL−1 pH: 5.2 Light source: eight 8 W lamps, 77 mWcm−2, 460 nm visible light Cat.: Cu–TiO2 P25 (0.045 wt.% Cu) | Photodegradation: 100% (90 min) k(1st) = 0.0506 min−1 Stability: 4 cycles | [82] |
| TiO2 P25 | Particle size: 21 nm | Medium: distilled water Ant. Conc.: 0.05 gL−1 Cat. Conc.: 0.1 gL−1 Light source: UVA 15 W, 360 nm, 1.15 mWcm−2 Temperature: 25 ± 2 °C | Photodegradation: 92.3% (510 min) t1/2 = 132 ± 5 min Mineralization: 34% (510 min) CODEcotoxicity: Vibrio fischeri | [105] |
| TiO2 P25 | - | Medium: purified water Ant. Conc.: 400 ppb each (mixture of two antibiotics) Cat. Conc.: 0.05 gL−1 pH: 5.6 Light source: thirty two 1W UVA/LED chips, peak emission at 365 nm Temperature: 25 °C | Photodegradation: 91% (20 min) Disinfection: Escherichia coli Ecotoxicity: Vibrio fischeri | [75] |
| Trimethoprim | ||||
| TiO2 P25 | - | Medium: purified water Ant. Conc.: 400 ppb each (mixture of two antibiotics) Cat. Conc.: 0.05 gL−1 pH: 5.6 Light source: thirty two 1W UVA/LED chips, peak emission at 365 nm Temperature: 25 °C | Photodegradation: 96% (20 min) Disinfection: Escherichia coli Ecotoxicity: Vibrio fischeri | [75] |
| Biobased-PET-TiO2 P25 composite films | TiO2 P25: Particle size: 20–30 nm Composition: 80% anatase; 20% rutile BET surface: 56 m2g−1 ZPC: 6.3–6.8 | Medium: ultrapure water Ant. Conc.: 0.001 gL−1 (for each antibiotic, mixture of eight) Cat. Conc.: 0.05 gL−1 Light source: solar simulator with xenon lamp (1.5 kW, 500 Wm−2) Cat.: PET-10%-TiO2 (10 wt.% of TiO2) | Photodegradation: 90% (6 h) k(1st) = 0.007 min−1 t1/2 = 99.0 min Stability: 5 cycles | [103] |
| Keratin-TiO2 nanocomposite | Composition: 85% anatase; 15% rutile | Ant. Conc.: 0.172 mM Cat. Conc.: 1 gL−1 Light source: Xenon lamp, simulated solar light, 28 kLux Temperature: 25 °C Cat.: K-TiO2 10% | Photodegradation: 100% (4 h) Stability: 4 cycles | [143] |
| S-TiO2 | Particle size: 12–22 nm (TEM) Composition: anatase; brookite | Ant. Conc.: 0.01 gL−1 Cat. Conc.: 0.5 gL−1 pH: 7.0 Light source: UV-Vis 400 W metal halide lamp | Photodegradation: 98.2% (4 h) Stability: 5 cycles (for immobilized catalyst) | [126] |
| TiO2 P25 | Composition: 80% anatase, 20% rutile | Medium: secondary urban wastewater Ant. Conc.: 100 µgL−1 Cat. Conc.: 1 gL−1 Light source: 4 UVA-LEDs, 9 W, 381 nm | Photodegradation: 100% (60 min) k(1st) = 0.1171 min−1 Disinfection: total heterotrophs, E. coli and enterococci | [111] |
| Nitrogen-doped TiO2 immobilized on polystyrene spheres (N-TiO2) | Composition: anatase Band gap: 2.5 eV | Medium: distilled water Ant. Conc.: 200 µgL−1 Cat. Conc.: 160.74 gL−1 pH: 6.13–6.38 (not adjusted) Light source: natural sunlight Temperature: 25.7–36.1 °C (not adjusted) | Photodegradation: 100% (150 min) k(1st) = 0.0167 min−1 t1/2 = 42 min | [117] |
| Porous titanium-titanium dioxide (PTT) substrates | Composition: anatase Band gap: 3.0 eV Isoelectric point: 6.0 | Medium: ultrapure water Ant. Conc.: 300 mL of 2 µgL−1 (eighteen pharmaceuticals) pH: ~5 Light source: UV-LEDs, 1.7×10−3 W, 365 nm | Photodegradation: 31.78% (300 min) k(1st) = 0.00132 min−1 | [138] |
| Nano-sized TiO2 supported on single wall carbon nanotubes (SWCNTs/TiO2) | Composition: anatase | Medium: ultrapure water Ant. Conc.: 200–500 µgL−1 Cat. Conc.: 0.1 gL−1 Light source: low pressure mercury lamp, 17 W, 254 nm, 0.1 Wcm−2 | Photodegradation: k(1st) = 0.075 min−1 Stability: 5 cycles | [142] |
| Immobilized TiO2 P25 on poly(vinylidene fluoride) (PVDF) dual layer hollow fibre membrane | Composition: anatase, rutile BET surface: 50 m2g−1 | Medium: ground water and secondary wastewater effluent Ant. Conc.: 200–400 μgL−1 Cat. Conc.: 0.057 gL−1 Light source: low-pressure mercury UV lamp, 40 W, 254 nm | Photodegradation: k(1st) = 0.045 min−1 (ground water) k(1st) = 0.095 min−1 (secondary WW effluent)Stability: 5 cycles | [144] |
| TiO2 film deposited on stainless steel mesh (nanoTiO2-SS) | - | Medium: filtered ground water Ant. Conc.: 200–400 µgL−1 Cat. Conc.: 0.1 gL−1 pH: 7.85 Light source: 40 W Hg low pressure UV lamp, 254 nm, 50 mWcm−2 Temperature: 24.8 °C | Photodegradation: k(1st) = 0.107 min−1 Ecotoxicity: AMES Fluctuation, Fish Embryo, Green alga Selenastrum capricornutum, Daphnia magna. Vibrio fischeri Stability: 10 cycles | [131] |
| Ciprofloxacin | ||||
| Black Ti3+/N-TiO2 P25 (b-N-TiO2) | Particle size: < 100 nm (FE-SEM) Composition: anatase BET surface: 100 m2g−1 PZC: 7.9 Band gap: 2.0 eV | Medium: ultrapure water Ant. Conc.: 0.5 mgL−1 Cat. Conc.: 0.43 gL−1 pH: 6.7 Light source: 5 W visible LED lamp, 550 nm | Photodegradation: 100% (70 min) k(1st) = 0.0778 min−1 Mineralization: 82% (140 min) TOC Ecotoxicity: Daphnia magna Stability: 5 cycles | [24] |
| 3D tripyramid TiO2 architectures | Particle size: 10 nm (TEM) Composition: anatase BET surface: 84 m2g−1 Band gap: 3.2 eV | Ant. Conc.: 32.6 μM Cat. Conc.: 0.1 gL−1 Light source: UV-vis light | Photodegradation: 90% (60 min) k(1st) = 0.0403 min−1 Stability: 5 cycles | [97] |
| TiO2 nanorod/g-C3N4 nanosheet (TiO2 nanorod-CN) | Composition: anatase ZPC: 6.3 Band gap: 2.95 eV | Ant. Conc.: 15 µmolL−1 Cat. Conc.: 0.2 gL−1 pH: 6.3 Light source: simulated sunlight irradiation, 500 W Xenon lamp Cat.: 30 wt.% g-C3N4 | Photodegradation: 93.4% (60 min) k(1st) = 0.0389 min−1 | [94] |
| TiO2-Ag NPs | Particle size: 80–100 nm (SEM)Composition: >90% anatase, rutile (XRD) Grain size: 51.56 nm anatase, 21.87 nm rutile (XRD) Band gap: 3.26–3.30 eV | Ant. Conc.: 1.0 mM Cat. Conc.: 0.001 gL−1 pH: 7.0 Light source: 120 W UV Hg lamp; natural sunlight | Photodegradation: 85.21% UV light k = 1.53 mMs−1 75.58% visible light k = 1.20 mMs−1 | [109] |
| TiO2 P25 (Acros) and γ-Fe2O3 co-doped graphene oxide (GO) nanosheets(TiO2/γ-Fe2O3/GO) | Composition: anatase, rutile Band gap: 2.43 eV | Ant. Conc.: 0.01 gL−1 Cat. Conc.: 0.4 gL−1 pH: 6.6 Light source: 300 W xenon lamp, 420 nm cutoff filter Cat.: 0.03TiO2/γ-Fe2O3/GO | Photodegradation: 99% (140 min) k(1st) = 0.019 min−1 Stability: 4 cycles | [114] |
| TiO2/kaolinite | Crystallite size: 16.631 nm (XRD) Composition: anatase BET surface: 60.21 m2g−1 Band gap: 3.18 eV | Medium: deionized water Ant. Conc.: 20 ppm Cat. Conc.: 2 gL−1 Light source: high-pressure mercury lamp Cat.: TK-6.0 (58.54% TiO2) | Photodegradation: 93.14% (40 min) k(1st) = 0.04549 min−1 Stability: 4 cycles | [145] |
| Fe–N–TiO2 | Particle size: ~25 nm (HR-TEM) Composition: 61.1% anatase, 21.4% rutile, 17.5% brookite Crystallite size: 28.1 nm anatase, 28.7 nm rutile, 34.3 nm brookite (XRD) BET surface: 90.2 m2g−1 Band gap: 2.7 eV | Ant. Conc.: 0.02 gL−1 Cat. Conc.: 1 gL−1 Light source: 12 W LED daylight lamps (visible) Temperature: ambient Cat.: 2.5%N–1.5%Fe | Photodegradation: 67.72% (6 h) k(1st) = 0.00552 min−1 Mineralization: 49.07% (6 h) TOC | [146] |
| N-TiO2 | Particle size: 15 ± 0.56 nm (TEM) Composition: anatase, rutile Crystallite size: 12–18 nm (XRD) BET surface: 24.59 m2g−1 PZC: 5.76 Band gap: 2.84 eV | Medium: distilled water Ant. Conc.: 0.01 gL−1 Cat. Conc.: 0.5 gL−1 pH: 7.0 Light source: three 14 W blue LEDs, 457 nm Cat.: Ti:N molar ratio 1:1 | Photodegradation: 55% (180 min) Mineralization: 24% (180 min) TOC | [76] |
| B-TiO2 P25 | Particle size: 61.8 nm (nanoparticle size analyzer) 25–34 nm (TEM) Composition: anatase, rutile Crystallite size: 19.82 nm anatase, 26.24 nm rutile (XRD) BET surface: 30.1 m2g−1 Band gap: 2.89 eV | Ant. Conc.: 0.01 gL−1 Cat. Conc.: 1 gL−1 pH: 7.0 Light source: natural sunlight Temperature: 33 °C Cat.: 1B-TiO2 (1 at.% B) | Photodegradation: 93.16% (180 min) k(1st) = 0.0249 min−1 Mineralization: 93% (180 min) COD Disinfection: 95–99.99% efficiency against E.coli Stability: 3 cycles | [118] |
| Ce-TiO2 P25 | Particle size: 89.5 nm (nanoparticle size analyzer), 19–39 nm (TEM)Composition: anatase, rutile Crystallite size: 16.95 nm anatase, 23.21 nm rutile (XRD) BET surface: 41.5 m2g−1 Band gap: 2.50 eV | Ant. Conc.: 0.01gL−1 Cat. Conc.: 0.5 gL−1 pH: 7.0 Light source: natural sunlight Temperature: 33 °C Cat.: 1Ce-TiO2 (1 at.% Ce) | Photodegradation: 93.22% (180 min) k(1st) = 0.0266 min−1 Mineralization: 92% (180 min) COD Disinfection: 95–99.99% efficiency against E.coli Stability: 3 cycles | [118] |
| Graphene/TiO2/g–C3N4 (GTOCN) | Particle size: 227.18 nm BET surface: 26.41 m2g−1 PZC: 4.16 | Medium: ultrapure water Ant. Conc.: 0.003 gL−1 Cat. Conc.: 0.6 gL−1 Light source: 300 W Xenon lamp (>400 nm), 300 mWcm−2 Cat.: GTOCN3 (40 mg Ti3C2) | Photodegradation: 61.7% (60 min) k(1st) = 0.01675 min−1 Mineralization: 41.8% (60 min) TOC Stability: 3 cycles | [77] |
| TiO2 nanotube arrays (TiO2 NTAs) with Ag3PO4 nanoparticles | Composition: anatase BET surface: 4.7 m2g−1 Band gap: < 3.25 eV | Ant. Conc.: 0.01 gL−1 Cat. Conc.: 1 gL−1 Light source: 300 W Xenon lamp, visible light, 200 mWcm−2Cat.: 0.6Ag3PO4/TiO2 (Ag3PO4:TiO2 mass ratio 0.6:1) | Photodegradation: 85.3% (60 min) k(1st) = 0.02499 min−1 Disinfection: E. coli 100% (120 min) Stability: 3 cycles | [99] |
| Nitrogen and carbon co-doped TiO2 nano-catalysts (NCD-TiO2) | Particle size: 9 nm (HR-TEM) Composition: anatase Crystallite size: 8.8 nm (XRD) BET surface: 116.5 m2g−1 Band gap: 2.94 eV | Medium: ultrapure water Ant. Conc.: 75 µM Cat. Conc.: 1 gL−1 pH: 5.7 Light source: four 8 W fluorescent lamps with UV light filter, 11.58 mWcm−2 Cat.: NCD200-430 (N:Ti molar ratio 2:1 calcined at 430 °C) | Photodegradation: 68.7% (120 min) k(1st) = 0.0093 min−1 | [98] |
| TiO2/Graphene oxide | Composition: anatase Crystallite size: 12.5 nm (XRD) BET surface: 91.25 m2g−1 Band gap: 2.47 eV | Medium: distilled water Ant. Conc.: 0.005 gL−1 Cat. Conc.: 0.5 gL−1 Light source: visible light Cat.: TiO2/GO (8%) | Photodegradation: 96.73% (60 min) Stability: 6 cycles | [108] |
| TiOF2/TiO2 nanosheets | Composition: anatase Crystallite size: 26.2 nm (XRD) BET surface: 119 m2g−1 Band gap: 3.285 eV | Ant. Conc.: 0.02 gL−1 Cat. Conc.: 1 gL−1 Light source: 300 W Xenon lamp (UV+visible) Cat.: S-160 (hydrothermal treatment at 160 °C) | Photodegradation: 95.3% (90 min) k(1st) = 0.034 min−1 | [147] |
| Cu-TiO2 | Particle size: 10 nm Cu (TEM) 200–400 nm Cu (SEM) Composition: anatase BET surface: 170.15 m2g−1 Band gap: 3.0 eV | Medium: deionized water Ant. Conc.: 0.08 gL−1 Cat. Conc.: 0.25 gL−1 Light source: 500 W Xenon lamp (simulated sunlight) Cat.: 0.1-Cu-TiO2 (weight ratio of Cu) | Photodegradation: 97% (4 h) k(1st) = 0.63 min−1Stability: 6 cycles | [100] |
| Ag-SrTiO3/TiO2 (SrTiO3 nanocubes supported on TiO2 nanosheets with Ag nanoparticles deposited on both) | Particle size: 30 nm Ag (SEM) Composition: anatase BET surface: 28.3 m2g−1 | Ant. Conc.: 0.02 gL−1 Cat. Conc.: 0.4 gL−1 Light source: 300 W Xenon lamp (simulated sunlight) | Photodegradation: 97.6% (60 min) k(1st) = 0.07 min−1 | [115] |
| TiO2-modified Bi2MoO6 nanocrystals TiO2/Bi2MoO6 | Particle size: 20 nm Bi2MoO6, 12.4 nm rutile, 6.1 nm diameter and 19.1 nm length rod-like anatase (TEM) Composition: Bi2MoO6, rutile, anatase BET surface: 17.7 m2g−1 Band gap: 2.60–2.68 eV | Ant. Conc.: 0.01 gL−1 Cat. Conc.: 0.6 gL−1 Light source: 350 W Xenon lamp, λ ≥ 420 nm Cat.: TiO2(0.41 wt%)/Bi2MoO6 | Photodegradation: 88% (150 min) k(1st) = 0.008 min−1 | [148] |
| N–TiO2 | Particle size: 180 nm length and 50 nm width (FIB/FESEM) Composition: anatase BET surface: 42.70 m2g−1 Band gap: 3.17 eV | Medium: deionized water Ant. Conc.: 20 ppm Cat. Conc.: 1 gL−1 pH: 5.5 Light source: three 20 W UV-A lamps 365 nm, 0.493 mWcm−2 Cat.: 12.5% N | Photodegradation: 94.29% (420 min) Mineralization: 66.31% (420 min) TOC | [149] |
| Graphitized mesoporous carbon (GMC)-TiO2 | Particle size: 15 nm (TEM)Composition: anatase Crystallite size: 12 nm (XRD) BET surface: 286 m2g−1 | Medium: deionized water Ant. Conc.: 0.015 gL−1 Cat. Conc.: 0.35 gL−1 Light source: 14 W UV lamp, 254 nm | Photodegradation: 100% (90 min) k(1st) = 0.102 min−1 Mineralization: 100% (120 min) TOC Ecotoxicity: Vibrio fischeri | [129] |
| Mesoporous nano-TiO2 | Composition: anatase Crystallite size: 13.5 nm (XRD) BET surface: 191.4 m2g−1 Band gap: 2.95 eV | Medium: deionized water Ant. Conc.: 0.16 gL−1 Cat. Conc.: 0.25 gL−1 Light source: 500 W Xe lamp, 200–1000 nm Cat.: TiO2 (hydr)–hydrothermal post-treatment | Photodegradation: 96.05% (6 h) k(1st) = 0.45 min−1 Mineralization: 76.66% (6 h) TOC Ecotoxicity: Staphylococcus aureus | [130] |
| P-doped TiO2 | Particle size: 12 nm (TEM)Composition: anatase BET surface: 88.54 cm2g−1 Band gap: 3.02 eV | Ant. Conc.: 5 ppm Cat. Conc.: 0.5 gL−1 Light source: visible light Cat.: PT-50 (50 mg of NaH2PO2) | Photodegradation: 100% (60 min) k(1st) = 0.065 min−1 Mineralization: 72.7% (60 min) TOC | [150] |
| g-C3N4/TiO2/kaolinite | Composition: anatase Crystallite size: 14.21 nm TiO2 (XRD) BET surface: 51.596 m2g−1 Band gap: 2.72 eV | Medium: deionized water Ant. Conc.: 10 ppm Cat. Conc.: 2 gL−1 Light source: Xenon lamp with 400 nm cut-off filter, 90 mWcm−2 | Photodegradation: 92% (240 min) k(1st) = 0.00813 min−1 Disinfection: Stapheylococcus aureus Stability: 4 cycles | [151] |
| N-TiO2 immobilized on glass spheres | Composition: anatase Crystallite size: 5.69 nm BET surface: 140.47 m2g−1 Band gap: 2.84 eV | Ant. Conc.: 0.02 gL−1 Cat. Conc.: 3 gL−1 Light source: 500 W Xe lamp, λ > 420 nm Cat.: N/Ti weight ratio 0.34% | Photodegradation: 93.5% (90 min) k(1st) = 0.02859 min−1 Ecotoxicity: Toxicity Estimation Software Tool (T.E.S.T.) Stability: 5 cycles | [133] |
| TiO2 P25 | Particle size: 30 nm Composition: 80% anatase, 20% rutile BET surface: 50 ± 15 m2g−1 | Medium: deionized water Ant. Conc.: 0.02 gL−1 Cat. Conc.: 0.1 gL−1 pH: 6.00 ± 0.10 Light source: twelve 3 W UVA/LED lamps, 365 nm, 10mWcm−2 | Photodegradation: k(1st) = 0.2217 ± 0.0179 min−1 Mineralization:76% (240 min) TOC | [96] |
| TiO2 P25/Fe0 | - | Medium: double distilled water Ant. Conc.: 0.03 gL−1 Cat. Conc.: 1 gL−1 pH: 3.0 Light source: 10 W UV lamp, 254 nm, 2.0 Wm−2 | Photodegradation: 94.6% (60 min) Stability: 5 cycles | [107] |
| Fe3O4/SiO2/TiO2 | Particle size: 293 ± 81 nm (SEM) Composition: anatase, rutile BET surface: 19 m2g−1 PZC: 3–5 Band gap: 2.8 eV | Medium: Millipore water Ant. Conc.: 0.005 gL−1 Cat. Conc.: 1 gL−1 pH: 5.5 Light source: six 8 W blacklight blue lamps, 365 nm, 1.6–1.7 mWcm−2 | Photodegradation: 95% (90 min) k(1st) = 0.032 min−1 Stability: 5 cycles | [152] |
| TiO2 P25 immobilized on glass plates | Composition: 80% anatase, 20% rutile BET surface: 48.3 m2g−1 | Medium: deionized water Ant. Conc.: 60 µmolL−1 Cat. Conc.: 0.75 gL−1 (3.8 × 48 cm rectangle glass plate with 20.5 gm−2 TiO2) pH: 9 Light source: 15 W UV-C lamp, 254 nm | Photodegradation: 100% (120 min) k(1st) = 4.0×10−4 s−1 | [112] |
| Mono- (Au, Ag and Cu) and bi-metallic Au–Ag and Au–Cu nanoparticles deposited on TiO2 | Particle size: 2.5–4 nm Composition: anatase, brookite BET surface: 52–64 m2g−1 PZC: 6.3 Band gap: 3.1–3.19 eV | Medium: tridistilled water Ant. Conc.: 0.03 gL−1 Cat. Conc.: 0.5 gL−1 Light source: 15 W UV-C low pressure Hg lamp, 254 nm, 44 Wm−2; solar simulator with a 1500 W Xenon lamp, 500 Wm−2 Temperature: 25 °C (<35 °C under simulated sunlight) Cat.: 1.5 wt.% for Au and Ag, 1.0 wt.% for Cu (mono-metallic); 1.0 wt.% for Au and 0.5 wt.% for Ag and Cu (bi-metallic) | Photodegradation: 100% (90 min) UV-C k(1st) = 0.06 min−1 1.5% Au/TiO2 k(1st) = 0.117 min−1 1.5% Ag/TiO2 k(1st) = 0.072 min−1 1.0% Cu/TiO2 k(1st) = 0.053 min−1 Au-Ag/TiO2 k(1st) = 0.099 min−1 Au-Cu/TiO2 100% (240 min) simulated sunlight k(1st) = 0.042 min−1 1.5% Au/TiO2 k(1st) = 0.04 min−1 1.5% Ag/TiO2 k(1st) = 0.023 min−1 1.0% Cu/TiO2 k(1st) = 0.021 min−1 Au-Ag/TiO2 k(1st) = 0.022 min−1 Au-Cu/TiO2Mineralization: 100% (180 min) TOC (UV-C) 100% (360 min, bi-metallic) TOC (simulated sunlight) Ecotoxicity: V. fischeri, E. coli Stability: 3 cycles | [121] |
| TiO2 P25 | Particle size: 30 nm BET surface: 35–65 m2g−1 | Medium: ultra-pure water Ant. Conc.: 300 µgL−1 Cat. Conc.: 1 gL−1 Light source: UV-A, 365 nm, 1.6–1.7 mWcm−2 | Photodegradation: 100% (6 min) Ecotoxicity: Vibrio fischeri | [132] |
| TiO2/MMT (montmorillonite) | Particle size: 40–60 nm Composition: anatase BET surface: 53.058 m2g−1 PZC: 8.4 | Ant. Conc.: 0.02 gL−1 Cat. Conc.: 0.1 gL−1 pH: 5.0 Light source: 16 W UV-C lamp | Photodegradation: 61.7% (120 min) k(1st) = 0.0069 min−1 t1/2 = 100.46 min Stability: 5 cycles | [78] |
| TiO2 P25 | BET surface: 56 m2g−1 | Ant. Conc.: 12.5 µM Cat. Conc.: 1 gL−1 Light source: six 8 W mercurial fluorescent tubes, UVA 365 nm, 18–19 Wm−2 | Photodegradation: 100% (30 min) | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts 2021, 11, 728. https://doi.org/10.3390/catal11060728
Kutuzova A, Dontsova T, Kwapinski W. Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin. Catalysts. 2021; 11(6):728. https://doi.org/10.3390/catal11060728
Chicago/Turabian StyleKutuzova, Anastasiya, Tetiana Dontsova, and Witold Kwapinski. 2021. "Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, Trimethoprim and Ciprofloxacin" Catalysts 11, no. 6: 728. https://doi.org/10.3390/catal11060728