1. Introduction
NO
x is a series of reactive gases, including NO
2, NO, and N
2O. Considerable NO
x emissions due to human activities cause significant environmental problems, such as acid rain, the ozone hole, and photochemical smog [
1,
2,
3,
4]. Currently, selective catalytic reduction using NH
3 (NH
3-SCR) technology is widely used in thermal power plants, industrial boilers, and other fixed-source flue gas-control [
5], owing to its high efficiency and low cost. A catalyst is essential for NH
3-SCR technology. Metal oxide catalysts are widely used in NH
3-SCR for their excellent denitrification performance and availability. Currently, V
2O
5/TiO
2 and V
2O
5-WO
3/TiO
2 catalysts are widely used. Because they need a relatively high reaction temperature of above 300 °C, so the process is usually performed in the gas upstream [
6]. An SCR denitrification device is commonly located in front of the dust removal and desulfurization device, which results in the catalyst being easily inactivated by the poisoning of SO
2 and dust. Therefore, low-temperature SCR technology is attractive. Owing to its advantages of low energy consumption and low impact of SO
2 and dust, SCR technology has gradually attracted the attention of researchers [
7,
8,
9,
10].
To date, a series of low-temperature metal oxide catalysts (e.g., cerium (Ce), cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), nickel (Ni), and vanadium (V)) have been studied. Of these, manganese oxide (MnO
x) has various oxidation states, a high valence state, and characteristic crystal form, and it shows excellent NO conversion and N
2 selectivity. Pena et al. [
11] showed that MnO
x/TiO
2 had the highest activity compared to Co, Cr, Cu, Fe, Mn, Ni, and V oxides supported on TiO
2 at a low temperature. Among the characteristic crystal forms of MnO
x, the active components of the catalysts MnO
2 and Mn
2O
3 show the highest NO conversion and N
2 selectivity [
12]. However, residual sulfur dioxide after desulfurization has a strong poisoning effect on metal oxidation catalysts at low temperatures. Thus, it is important to clarify the poisoning mechanism of the catalyst. There are different opinions about the poisoning mechanism of SO
2. Kijlstra et al. [
13] speculated that the main reason for the decrease in the activity of the MnO
x/Al
2O
3 catalyst after passing SO
2 was the sulfation of the active center atom, Mn, to form manganese sulfate. Luo et al. [
14] believed that an SO
2-induced decrease in the MnO
x/MWCNTs catalyst’s activity was attributed, in part, to the formation of ammonium sulfate on the catalyst’s surface that blocks the catalyst’s pores and is also attributed, in part, to the sulfation of the active center atom, Mn, to form MnSO
4. Qi and Yang [
15] thought that the main reason for MnO
x/TiO
2 deactivation is the competitive adsorption of SO
2 and NO. This deactivation is reversible, and the activity of the catalyst can be completely restored after removing SO
2.
The structural formula of palygorskite (PG) is Mg
5Si
8O
20(OH)
2(OH
2)
4·4H
2O. In our previous study, MnO
x/PG showed a high NO
x conversion rate at a low temperature window of 100–300 °C [
16]. Because of its easily available raw materials, abundant channel structure, and large specific surface area, MnO
x/PG has a good industrialization prospect. However, its NO
x conversion rate is easily reduced under low concentrations of SO
2. Currently, as there are no unanimous conclusions on the poisoning mechanism of Mn-based catalysts, our group explored the poisoning mechanism of MnO
x/PG. Zhang et al. [
17] determined the poisoning mechanism of MnO
x/PG catalysts by simulating the conditions of poisoning and the recovery of thermal regeneration. They thought that the reason for the deactivation of the catalyst was the collaborative effect of ammonium sulfate and metal sulfate and that the deposition of ammonium sulfate was the main reason for the deactivation of the catalyst at low temperatures. The addition of Ce enables MnO
x/PG to resist sulfur [
18]. The abovementioned researchers believed that the fundamental reason for the resistance to Ce is to inhibit MnSO
4 production. As a transition metal, Ce easily combines with SOx to form stable Ce
2(SO
4)
3, which protects the sulfation of Mn. The formation of MnSO
4 is an important reason for the deactivation of the catalyst above 150 °C. However, there is no clear conclusion about the deactivation mechanism of the Mn
8/PG catalysts. Therefore, it is necessary to explore the cause of low-temperature SCR catalyst inactivation caused by SO
2 so as to further find a solution to SCR-catalyst sulfide poisoning. In addition to ammonium sulfate salts and ammonium sulfite salts, the effect of metal sulfate salts on the deactivation of catalysts is controversial. In this study, the reasons why SO
2 poisoning leads to a decrease in the catalytic activity and water-washed regeneration leads to the recovery of activity were studied under ammonia-free conditions, combined with specific surface-area and pore-size determination (BET), elemental analysis (EA), X-ray diffraction (XRD), temperature-programmed reduction (TPR), temperature-programmed desorption (TPD), and temperature-programmed surface reaction (TPSR). After discussing the mechanisms of SO
2-caused poisoning and water-washed regeneration in depth, we proposed a possible explanation for the catalyst deactivation by SO
2 and regeneration of the deactivation catalyst by a water-washing process.
2. Results and Discussion
2.1. Elemental Analysis, Specific Surface-Area and Pore-Volume Determination, and X-ray Diffraction
To explore the composition of soluble sulfur compounds and their effect on the physical environment of the catalyst surface, elemental and BET analysis tests were performed.
Table 1 shows that the S content of the Mn
8/PG-S catalyst is significantly higher than that of fresh the Mn
8/PG catalyst. A negligible portion of sulfur was observed in the Mn
8/PG catalyst due to the measurement accuracy of the method or impurities in the instrument. The S content on the catalyst surface was significantly decreased after water-washed regeneration, which indicates that the accumulation and removal of S from the catalyst were the key factors for the deactivation and regeneration of the catalyst.
The BET results show that the specific surface area and pore volume of the catalyst decreased after the SO2-pretreated poisoning at 250 °C; the specific surface area and pore volume decreased by 24% and 3%, respectively. Clearly, the formation of sulfur blocked pores and reduced the specific surface area, which was an important factor that inhibited the SCR activity of the Mn8/PG-S catalyst. The specific surface area and pore volume of the Mn8/PG-S-W catalyst considerably increased compared to that of the Mn8/PG-S catalyst. Thus, the SCR activity of the Mn8/PG-S-W catalyst recovered even more than that of fresh samples. This suggests that sulfur compounds on the catalyst were efficiently removed by the water-washing process, which resulted in a large extent of recovery of specific surface area. A small portion of sulfur was observed in the Mn8/PG catalyst due to the measurement accuracy of the method or impurities in the instrument because no sulfur-containing precursors were used during the preparation of the catalyst.
To explore the changes in the crystal shape of the catalyst before and after the SO
2-pretreated poisoning and water-washed regeneration, XRD tests were performed on the three catalysts. The XRD patterns of the three catalysts are shown in
Figure 1. All of the catalysts obtained by different treatments maintained the primary structure of palygorskite.
The main forms of manganese oxide on the surfaces of Mn8/PG catalysts were MnO2 (i.e., 37.6°, 42.8°, 57.0°) [PDF#30-0820] and Mn2O3 [PDF#10-0069]. It is easier to form amorphous Mn2O3 than MnO2; thus, it is difficult to observe Mn2O3 by XRD. The intensity of the MnO2 diffraction peak in the catalyst was weakened after Mn8/PG catalysts were SO2-pretreated, and the intensity of the diffraction peak of the MnO2 catalyst was restored after the water-washed treatment. The abovementioned results were obtained because sulfur compounds, which were formed on the surface of the catalyst, covered MnO2 after the treatment in the SO2 atmosphere, and the characteristic diffraction peak intensity of MnO2 was weakened. The water-washed regeneration can remove sulfur compounds and restore the intensity of the diffraction peaks of MnO2.
2.2. Scanning Electron Microscopy
According to the abovementioned analysis, it is believed that the presence of sulfur compounds likely causes a significant change in the morphology of the catalyst; thus, SEM analysis was performed to investigate morphological changes of the three catalysts.
Figure 2a shows that the Mn
8/PG catalyst has a one-dimensional rod-shaped structure with individual rods being uniformly dispersed. These properties are considered to be essential to obtain superior catalyst activity. For the Mn
8/PG-S catalyst (
Figure 2b), a clear agglomeration of catalysts was observed. A paste-like compound wrapped the catalyst’s surface. For the Mn
8/PG-S-W catalyst (
Figure 2c), the individual rods were highly dispersed, and the paste-like compound on the rods was clearly removed. The appearance of the Mn
8/PG-S-W catalyst was similar to that of the Mn
8/PG catalyst. Combined with the BET analysis, it is suggested that the paste-like compound, which is formed during the sulfur treatment, causes a decrease in specific surface area and catalytic deactivation.
2.3. In Situ DRIFT
To further study the internal functional groups of the catalyst after the SO
2-pretreated poisoning and water-washed regeneration, the three catalysts were evaluated by FT-IR spectroscopy, as shown in
Figure 3. Two peaks can be seen in the wavelength range of 1300–800 cm
−1. All the samples show the same broad peak at 1020 cm
−1, which is attributed to the anti-symmetrical vibration of quartz Si–O–Si, which is contributed by the PG support. The peak vibration at 876 cm
−1 is attributed to the S–O stretching vibration that was observed on Mn
8/PG-S and Mn
8/PG-S-W samples [
19]. There was no change detected in the 1020 cm
−1 peak intensity for the three catalysts, which indicates that Mn
8/PG-S poisoning and Mn
8/PG-S-W regeneration did not affect the carrier, with SiO
2 as the main component of the catalyst. However, the peak intensity of S–O at 876 cm
−1 considerably changed for the three samples. For the Mn8/PG-S catalyst, there was a clear peak at that frequency, which indicates that sulfur compounds formed on the surface of the catalysts. After water-washed regeneration, the peak at that frequency became weak, which indicates that some of the sulfur compounds were washed away, and some metal sulfate salts remained on the catalyst surface.
On the basis of the abovementioned tests and characterization analyses, it can be suggested that the deactivation of the catalyst after the SO
2 pretreatment is due to the formation of soluble sulfur compounds rather than MnSO
4. Those sulfur compounds can be easily removed by water washing to completely restore the SCR activity of the catalyst. According to reports by Seong et al. [
20], at a higher SO
42− concentration, the isolated sulfate will be converted into a polynuclear sulfate type, which is larger and will wrap around the active component. It is reasonable to suggest that the soluble sulfur species is actually an SO
42− polymer.
2.4. Atomic Absorption Spectroscopy and X-ray Fluorescence Spectroscopy
Because of the compositional characteristics of the palygorskite carrier (magnesium aluminum silicate), the sample contains a large amount of O, Si, Mg, and Al elements. By comparing the Mn content of the three catalysts, we determined that the Mn content does not considerably change (as shown in
Table 2) and that the overall growth trend is attributed to the water-washing process, which washes away a part of the carrier.
Combined with the atomic absorption spectroscopy analysis, shown in
Table 3, we determined that there are small amounts of Mn and Mg in the water-washed solution of the Mn
8/PG catalyst because of the incompletely decomposed precursor Mn(NO
3)
2 and soluble magnesium salt on the carrier. A small amount of Mn was observed in the water-washed solutions of the Mn
8/PG-S catalyst that was washed once and thrice; this is attributed to the fact that SO
3 reacts with active manganese and eventually produces MnSO
4, which is not strongly bonded to the carrier. The Mn element that is present during the SO
2-pretreatment process is converted into the content of lost manganese. It can be determined that the amount of manganese removed during the SO
2 pretreatment process is considerably small (approximately 1% of manganese), which suggests that Mn is not lost during the SO
2 pretreatment process. The SO
2 pretreatment process does not wash away manganese present on the catalyst, which is an important prerequisite for the recovery of the catalyst activity. To further determine the loss of Mn during the SO
2 pretreatment process, we excluded other factors that may affect the experimental results. Thus, a simulation experiment with pure MnO
2 was performed. MnO
2 was used as a SO
2 pretreatment with the same process as for Mn
8/PG. It was determined that MnO
2 had the same trend of activity as that of the Mn
8/PG-S catalyst. The analysis of the water-washed solution of SO
2-pretreated MnO
2 showed that the loss of manganese was in the amount of 0.002%, which can be neglected. The elimination of deactivation of the catalyst via sulfation is not the main cause during the SO
2 pretreatment process.
To investigate the acidity and alkalinity of the three catalysts, the pH of the water-washed solution of the three catalysts was determined. The pH of the water-washed solution of the Mn8/PG catalyst was 7.56, which is neutral. The pH of the water-washed solution of the Mn8/PG-S catalyst was 5.3, which was weakly acidic. The pH of the water-washed solution of the Mn8/PG-S-W catalyst was 7.23. Clearly, compared to the fresh and water-washed samples, the Mn8/PG-S catalyst was more covered by soluble sulfur compounds. It can be concluded that the removal of acidic sulfur compounds during the SO2 pretreatment process is the main cause of the recovery of the deactivated catalyst.
2.5. Effect of SO2 Pretreatment and Water Washing on the Activity of Mn8/PG Catalysts
There are two reasons for the deactivation of metal oxides by SO2: the deposition of ammonium sulfate salt on the catalyst and the sulfation of active sites. To eliminate the effect of ammonium sulfate on the activity of the catalyst, Mn8/PG was SO2-pretreated under ammonia-free conditions to investigate the effect of metal sulfates on catalytic activity.
Figure 4 shows the NO conversion of different Mn
8/PG catalysts in the studied temperature range. The NO conversion of Mn
8/PG-S was significantly inhibited compared with Mn
8/PG, indicating that the activity of catalysts was remarkably affected by SO
2 pretreatment. The activity of the catalyst was considerably restored after Mn
8/PG-S catalysts were water-washed, and the NO conversion was even slightly higher than that of the Mn
8/PG catalyst. The results show that SO
2 pretreatment causes irreversible deactivation in the low-temperature range of the catalyst, which is consistent with the results of the research on the reversible and irreversible deactivation of the Cu-CHA NH
3-SCR catalyst by Hammershøia et al. [
21]. The NH
3-SCR activity of the Mn
8/PG-S catalyst considerably increased from 250 °C to 300 °C. This occurs because the oxidation efficiency of manganese increases with an increase in temperature, which directly leads to an increase in the SCR reaction rate per unit of time.
The NO conversion of the Mn8/PG-S catalyst is considerably inhibited compared with that of the Mn8/PG catalyst in the low-temperature range, indicating that the formation of ammonium sulfate on the catalyst surface is not the main cause of catalyst deactivation. The catalyst was possibly deactivated before the formation of ammonium sulfate. We tentatively concluded that the stratification of ammonium sulfate was not the main reason for the deactivation of the Mn8/PG catalyst.
2.6. X-ray Photoelectron Spectroscopy (XPS)
The S atom concentration of the Mn
8/PG-S catalyst is 3.18%, whereas the S atom concentration of the Mn
8/PG-S-W catalyst is 0.67% (
Table 4). Combined with the result shown in
Figure 5, it was determined that the denitration activity of the Mn
8/PG-S catalyst was restored to that of the fresh catalyst by washing with water. This observation suggests that the removal of S atoms during the water-washing process is essential for recovering the catalyst’s denitration activity.
Figure 5a shows the XPS spectrum of the S 2p orbital of the Mn
8/PG-S catalyst. The S element mainly exists in the forms of S
4+ and S
6+ in the Mn
8/PG-S catalyst. The peak at 168.9 eV is attributed to SO
32−, which contributes to the sulfurous-acid generation by the SO
2 and H
2O adsorption on the surface of the catalyst. The peak at 169.7 eV is attributed to SO
42−, which contributes to the sulfuric-acid generation by SO
3 and H
2O on the surface of the catalysts and surface-generated MnSO
4 [
22,
23]. The S peak can be divided into S
6+ and S
4+. According to the calculated area of the two peaks, it was determined that the proportions of S
6+ and S
4+ are 49% and 51%, respectively. Compared to the catalytic reaction without a sulfur atmosphere, more S
4+ was oxidized to S
6+ due to the presence of MnO
2 (Mn
4+) and SO
2 when the catalytic reaction proceeded in the sulfur-rich atmosphere [
24]. Thus, sulfur was primarily present as a sulfuric acid and MnSO
4 on the surface of the catalyst. After water-washed regeneration, most S was washed away. The XPS spectrum of the S 2p orbital of the washed regenerated catalyst cannot be observed because of the extremely low content of S.
The XPS spectra of the Mn 2p orbitals for the three catalysts are shown in
Figure 5b. The Mn 2p 3/2 peak of the Mn
8/PG catalyst can be divided into two peaks: MnO
2 (643.1 eV) and Mn
2O
3 (641.6 eV) [
25]. Based on the calculation of the peak area, the proportions of MnO
2 and Mn
2O
3 are 68.2% and 38.8%, respectively. The peak of Mn 2p3/2 can be divided into three peaks, which correspond to MnO
2 (643 eV), Mn
2O
3 (641.8 eV), and manganese sulfate (644.9 eV), owing to the sulfur-containing atmosphere [
26]. The proportions of the abovementioned compounds were 25.2%, 38.6%, and 36.2%, respectively. The presence of MnO
2 and manganese sulfate in the Mn
8/PG-S catalyst indicate that the surface high-valence Mn is reduced to Mn
2+ by S, and then S and Mn combine to form stable MnSO
4 on the surface of Mn
8/PG-S catalysts. After splitting and fitting the orbital peak of the Mn
8/PG-S-W catalyst Mn 2p, the peak of Mn
2+ (MnSO
4) still exists in the Mn
8/PG-S-W catalyst. However, the activity of the catalyst was significantly restored, which suggests that the main cause of catalyst deactivation may not be MnSO
4.
2.7. H2-Temperature-Programmed Reduction (H2-TPR)
The redox capacity of manganese-based catalysts is a key factor in the catalytic reduction of NH
3-SCR [
27]. The reduction temperature can be used to evaluate the redox ability of catalysts. The low reduction temperature leads to the stronger redox ability of the catalysts [
28]. The redox properties of the three catalysts were investigated by performing the H
2-TPR analysis in
Figure 6 and measuring the produced H
2 concentration during the temperature increase from 50 °C to 800 °C. The profile of H
2 consumption on each catalyst, along with the temperature rising in the H
2-TPR experiment, is shown in
Figure 6.
For fresh samples, there are two prominent reduction peaks (307 °C and 456 °C), as shown in the H
2-TPR analysis. The two peaks correspond to the reduction of Mn
4+ and Mn
3+, respectively [
29,
30]. In the case of the Mn
8/PG-S catalyst, the reduction peak at 412 °C (Mn
4+) was clearly weakened, and a new reduction peak at 647 °C (Mn
2+) appeared. During the SO
2 pretreatment process of the Mn
8/PG catalyst, Mn
4+ and Mn
3+ were reduced to Mn
2+ by SO
2, S
4+ was oxidized to S
6+, and S
6+ was present on the surface of catalysts in the form of SO
42−. A shift in the reduction peak of the Mn
8/PG catalyst was caused by the sulfur compounds that are formed on the catalyst and that suppress the oxidizing activity of MnO
x, which is in agreement with the XPS result. In the case of the Mn
8/PG-S-W catalyst, the reduction peaks appeared at 390 °C and 501 °C. The intensity of the reduction peak of the Mn
8/PG-S-W catalyst Mn
4+ could not be completely restored to that of Mn
8/PG catalyst because a part of Mn
8/PG catalyst Mn
4+ was converted to Mn
2+, which could not be reoxidized to Mn
4+ by the water-washing process. The water-washing process removes the sulfur species from the sulfated catalyst, thereby restoring the redox capacity of the catalyst. This conclusion further proves that sulfur species are an important cause for catalyst deactivation.
2.8. Activity Test on Catalysts Treated by HCl
The results of atomic absorption spectroscopy showed that a small amount of Mg
2+ exists in the water-washed solution of different catalysts. To eliminate the potential influence of sulfurization of elements from the carrier, we pretreated the PG carrier with 1.2 mol/L HCl to make sure the metal oxides in the carrier were completely removed. Then, manganese oxide was reloaded by the wetness co-impregnation method to prepare different Mn
8/PG-HCl catalysts, and the results are shown in
Figure 7. Because PG contains large amounts of alkali earth metal oxides (e.g., MgO and Al
2O
3) that can contribute to a lower SCR activity, PG may be sulfurized by SO
2 to block the surface of the carrier. After pretreatment with an HCl solution, PG was considerably inactive throughout the low-temperature range, which indicates that alkali earth metal oxides, which provided activity in the carrier, were considerably removed. When MnO
x was reloaded, the activity of the Mn
8/PG-HCl catalyst was almost the same as that of Mn
8/PG, which indicates that Mn, as an active component, provided the main denitrification activity in the lower temperature range. Mn
8/PG-S-HCl and Mn
8/PG-S-W-HCl catalysts showed a trend that was similar to that which occurred after the Mn
8/PG treatment, which indicates deactivation due to the Mn
8/PG sulfurization of alkali earth metal from the carrier. However, it is debatable whether the deactivation of the catalyst was caused by the formation of sulfur compounds from the active component.
2.9. SCR Activity Test of MnSO4-Doped PG
To evaluate the potential deactivation of the catalyst by the formation of MnSO
4, the PG carrier was doped with MnSO
4 and then tested for SCR.
Figure 8 shows the NO conversion of PG; MnSO
4 (1%)/PG and MnSO
4 (8%)/PG were compared. The experimental results show that PG has a certain catalytic activity, which is attributed to the existence of oxygen-containing functional groups, which are an active component and can promote the redox process [
17]. After the loading of 1% MnSO
4 (simulates SO
2-pretreated poisoning with S content during the elemental analysis), a considerable increase in the SCR activity was observed. However, for MnSO
4 (8%)/PG (assuming all active components of MnO
x are completely deactivated), a clear decrease in the SCR activity appeared. This result shows that Mn
2+ has a certain activity, and a small amount of MnSO
4 is easily distributed on the catalyst, which is favorable for catalytic activity. When the content of MnSO
4 is 8%, the MnSO
4 bulk phase is large. Thus, an excessively high MnSO
4 content is not advantageous for the uniform dispersion on the catalyst, aggregation tends to easily occur, and the activity of PG decreases. This result further confirms that the small amount of MnSO
4 that is generated during the SO
2 pretreatment process is not the main reason for the deactivation of the catalyst.
2.10. SO2 Adsorption and TPD
Considering that the oxidation of SO
2 is frequently induced by the adsorption of SO
2 on catalysts, the performance of SO
2 adsorption on Mn
8/PG, Mn
8/PG-S, and Mn
8/PG-S-W catalysts was measured. Each sample was exposed to an SO
2-containing flowing gas for 100 min until equilibrium. The TPD of SO
2 for each sample from 50 °C to 1000 °C was also performed. The profiles of SO
2 adsorption equilibria and SO
2 TPD for the three catalysts are compared in
Figure 9.
As shown in
Figure 9, SO
2 adsorption equilibrium profiles obtained at 50 °C for 100 min vary significantly. For the Mn
8/PG-S catalyst, SO
2 penetrates immediately and reaches the equilibrium. However, 6 and 12 min diffusion time was observed for the Mn
8/PG-S-W and Mn
8/PG catalysts, respectively. This implies that the Mn
8/PG-S catalyst has almost no SO
2 adsorption capacity, and the water-washing process can restore a part of its SO
2 adsorption capacity. By calculating the corresponding area of each curve, the SO
2 adsorption capacity of each catalyst can be compared more directly. The SO
2 adsorption capacities of the Mn
8/PG, Mn
8/PG-S and Mn
8/PG-S-W catalysts are 1.34 × 10
−4, 4.14 × 10
−5, and 6.7 × 10
−5 mol·g
−1, respectively. Adsorbed SO
2 is oxidized to SO
3 by MnO
x during the adsorption process.
Figure 6 shows that there is a difference between the SO
2 adsorption equilibrium concentration and the inlet concentration, and the difference between the SO
2 equilibrium concentration and the inlet concentration (400 ppm) is believed to be attributed to SO
2 oxidizing to SO
3. The SO
2 oxidation rates of the Mn
8/PG, Mn
8/PG-S, and Mn
8/PG-S-W catalysts are 6.25%, 1.25%, and 2%, respectively. SO
2 is continuously adsorbed and oxidized by the active component. A part of SO
2 forms MnSO
4 with the active component, and the other part forms a removable sulfur compound. These removable sulfur compounds are responsible for the decreased SO
2 adsorption and oxidation capacity. Removable sulfur compounds that cover the active component can be removed by the water-washing process, thereby recovering the adsorption capacity of the catalyst. Because the water-washing process does not wash away MnSO
4, the oxidizing capability is not significantly recovered. Most of the soluble sulfur compounds can be removed by the water-washing process; thus, the adsorption capacity of the catalyst is recovered. Because MnSO
4 is still present after the water-washing process (the presence of SO
42− may inhibit the adsorption of SO
2), the adsorption and oxidation capacities of the catalyst are not fully recovered.
For direct comparison with SO
2 adsorption, the SO
2-TPD profiles of each catalyst are shown in
Figure 9. The figure shows that there is no SO
2 desorption peak for the Mn
8/PG catalyst. A considerable SO
2 desorption peak was obtained on the Mn
8/PG-S catalyst in the temperature range of 600 °C–800 °C. This may be attributed to the joint desorption action of a soluble sulfur compound and MnSO
4. The desorption peak of the water-washed regenerated catalyst was observed at 700 °C–800 °C, and the peak was weaker than that of the sulfur Mn
8/PG-S sample. This desorption peak may be attributed to the decomposition of MnSO
4, which remained after water washing. By comparing the SO
2 desorption peak areas of the Mn
8/PG-S and Mn
8/PG-S-W catalysts, it can be observed that the soluble sulfur compound is the main product of SO
2, which is also the main reason for the deactivation of the Mn
8/PG-S catalyst.
2.11. NH3 Adsorption and TPD
It is widely believed that the adsorption and activation of NH
3 on the catalyst surface is the first important step in the SCR reaction, according to either E–R or L–H mechanism [
31]. In general, an increase in the acidity of the catalyst surface is favorable for the adsorption of NH
3 and SCR activity. The NH
3 adsorption ability of each sample was measured, followed by the TPD experiments. During the adsorption, the catalyst was exposed to a gas mixture containing 600 ppm of NH
3 balanced by N
2 at 50 °C for more than 100 min until equilibrium. Then, after a sweeping with N
2, TPD profiles were also recorded, as shown in
Figure 10.
From the comparison of the NH3 adsorption equilibrium curves for the three catalysts, it was observed that the diffusion time of the Mn8/PG-S sample was 4 min faster than that of the fresh sample. The area enclosed by the NH3 curve of the Mn8/PG-S catalyst was significantly smaller than that of the fresh sample during the process of adsorption equilibrium, which indicates that the NH3 adsorption on the Mn8/PG-S catalyst was considerably inhibited. However, the diffusion time and the area enclosed by the NH3 curve of the Mn8/PG-S-W catalyst were found to be slightly greater than those of fresh samples, which indicates that the NH3 adsorption of the catalyst was efficiently recovered during the removal of sulfate species from the poisoned catalyst.
Figure 10 shows the NH
3 TPD curve after 125 min of adsorption. There are several clear desorption peaks of NH
3 in the range of 50–900 °C: a weak acidic site desorption peak at 132–380 °C, a moderate acidic site desorption peak at 380–640 °C, and a strong acidic site desorption peak at 640–775 °C. The desorption peak of the Mn
8/PG-S catalyst is lower than that of the Mn
8/PG catalyst due to the reduction of stored NH
3 during the adsorption. However, the temperature corresponding to the desorption peak did not change, which indicates that the binding position of NH
3 on the catalyst did not change. The amount of desorption of NH
3 on the water-washed regeneration catalyst was slightly higher than that of the Mn
8/PG catalyst, which indicates that the water-washing process can remove the SO
42− polymer, which inhibits the adsorption of NH
3.
The NH3 adsorption-desorption curve confirms that the formation of ammonium sulfates is not the main cause of the decrease in catalytic activity. The deactivation of the catalyst occurred before the possible formation of ammonium sulfates. Based on the result that the NH3 adsorption of the catalyst was considerably inhibited by the SO2 pretreatment of the catalyst, it can be speculated that the formation of an SO42− polymer caused the pore blockage of the catalyst and the occupation of active sites for NH3 adsorption.
2.12. NO Adsorption and TPD
Currently, some researchers believe that the SCR reaction follows the L–H mechanism, and the NO adsorption behavior in the L–H mechanism considerably affects the SCR reaction [
32,
33]. To investigate the effect of soluble sulfur compounds (SO
42− polymer) on the adsorption and desorption of NO on the catalyst, we performed the adsorption and desorption analyses of NO on the three catalysts. During the absorption period, the catalyst was placed in an atmosphere containing 600 ppm of NO with N
2 as the carrier gas at 50 °C, and the outlet NO concentration is shown in
Figure 11.
The adsorption equilibrium curves for the three catalysts at 50 °C before 80 min are shown in
Figure 11. The diffusion time of the Mn
8/PG-S catalyst by NO was 50 min faster than that of the Mn
8/PG catalyst. The enclosed area of the Mn
8/PG-S catalyst was significantly smaller than that of the Mn
8/PG catalyst during the adsorption period, which indicates that the capacity of stored NO was considerably affected by the generated sulfur compounds. The adsorption equilibrium time of the Mn
8/PG-S-W catalyst is approximately the same as that of the Mn
8/PG catalyst. However, the amount of adsorbed NO is slightly lower than that of the Mn
8/PG catalyst, which may be due to the inhibition of NO adsorption by the residual SO
42−.
There are two distinct desorption peaks for fresh Mn
8/PG. The first desorption peak appears at 250–320 °C. The desorption peak is attributed to the decomposition of the NO adsorption product, which is mainly involved in SCR. It may be formed by the decomposition of bridging nitrate salts [
34]. The second peak belongs to the high-temperature peak at approximately 400 °C. It may be formed by the oxidation of NO to form nitrite salts and nitrate salts, which are decomposed and desorbed at high temperatures [
35]. Because the decomposition temperature of this nitrate is considerably high, it cannot easily participate in the SCR reaction at low temperatures. When the catalyst is reacted in a sulfur-containing atmosphere, the low- and high-temperature peaks are both attenuated. The NO storage is reduced during the adsorption period due to the presence of sulfur compounds. The water-washed regeneration process can remove the SO
42− polymer from the catalyst surface, but MnSO
4 still exists. The presence of MnSO
4 inhibits the activation of NO; thus, the peak intensity does not recover at 400 °C.
2.13. Temperature-Programmed Surface Reaction (TPSR)
To explore the effect of sulfuric acid and sulfurous acid on the surface environment of the catalyst, a further investigation was conducted using the TPSR, as shown in
Figure 12.
As a result, the concentration of NO was quickly increased to an inlet concentration of 570 ppm at 4 min for the Mn8/PG catalyst without NH3 adsorption. When the temperature of the system increased above 230 °C, the NO concentration gradually decreased to a low concentration. After 80 min, the NO concentration did not reach the inlet concentration due to the consumption of the NO heat reaction under high temperature conditions.
For the Mn8/PG catalyst with NH3 adsorption, there is a clear inverted peak in the TPSR curve. The larger NO depletion peak at 20–80 min is attributed to the consumption of the NO surface reaction with adsorbed NH3. The TPSR curve of the Mn8/PG-S catalyst also shows a distinct consumption peak (1.94 × 10−4 mol·g−1), but the depletion peak attributed to the adsorbed NH3 reaction at the same time period was lower than the Mn8/PG consumption peak (2.26 × 10−4 mol·g−1). The presence of soluble sulfur compounds inhibits the adsorption of NH3 at certain specific acidic sites, which are the most critical active sites during low-temperature SCR reactions. The TPSR curve for the Mn8/PG-S-W catalyst shows the same consumption peak (2.69 × 10−4 mol·g−1) as that of the Mn8/PG catalyst in the same time period because the water-washing process can remove the SO42− polymer at the key active sites in the low-temperature SCR reaction. Meanwhile, it was observed that the amount of activated adsorption of NH3 by the Mn8/PG-S-W catalyst is slightly higher than that of the Mn8/PG catalyst, which may be an important reason why the activity of the Mn8/PG-S-W catalyst is slightly higher than that of the Mn8/PG catalyst.
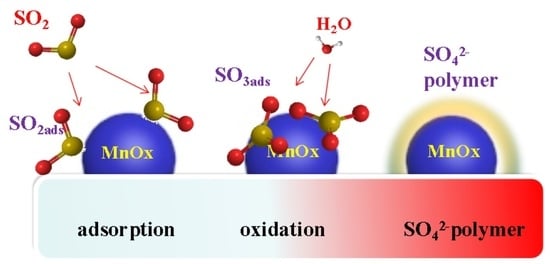
2.14. Proposed SO2-Pretreated Poisoning and Water-Washed Regeneration Mechanism Model
To better illustrate and summarize the abovementioned characterization analysis, in
Figure 13, we propose a mechanism model for SO
2-pretreated poisoning and water-washed regeneration.
The process of SO2-pretreated poisoning of the catalyst is as follows. First, SO2 adsorbs on the active component, MnOx, of the Mn8/PG catalyst. Then, SO2 is oxidized by the active component to SO3 and adsorbed on the surface of the active component. When the concentration of SO42− on the active site reaches a certain level, sulfate is converted into an SO42− polymer. Thus, the specific surface area of the catalyst is decreased, which corresponds to a reduction in the adsorption and activation of NH3 and NO.
The mechanism of water-washed regeneration is as follows. The water-washing process can easily wash away the SO42− polymer from the active component. Thus, the catalytic activity of the active components can be restored.
3. Experimental
3.1. Materials
The palygorskite, supplied by Xinzhou New Material Factory (Anhui, China), was ground and sieved through 0.42–0.84 mm mesh. The chemical composition of the palygorskite was 69.44 wt.% SiO2, 11.84 wt.% Al2O3, 5.14 wt.% Fe2O3, 12.16 wt.% MgO, 0.43 wt.% TiO2, 0.5 wt.% K2O, 0.21 wt.% CaO, and 0.28 wt.% others. The surface area, pore volume, and pore size of palygorskite were 228.5 m2·g−1, 0.41 cc·g−1, 2.8 nm, respectively. All used chemicals, including manganese nitrate, manganese sulfate and hydrochloric acid, were analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, SCR, China) Simulated flue gas provided by Nanjing Shangyuan Industrial Gas Plant, (Nanjing, China). MnO2 is produced by calcination of manganese nitrate.
3.2. Catalyst Preparation
Fresh Mn8/PG catalyst: The catalysts were prepared by the wetness co-impregnation loading of active species. Palygorskite, as a support, was pore-volume impregnated by a mixed aqueous solution of manganese nitrate as a precursor. The mass ratios of manganese to palygorskite were 8 wt%. The catalysts were initially dried at 50 °C for 6 h, followed by drying at 110 °C for 12 h. The dried catalysts were ground and sieved through 20–40 mm meshes and subsequently calcined in air at 300 °C for 3 h. Thus, the obtained catalysts were designated as Mn8/PG, where “8” refers to the mass percentage of each element.
SO2-pretreated catalyst: A total of 2-g fresh Mn8/PG catalyst was continuously ventilated under the following reaction conditions: 400 ppm SO2, 3 vol% O2, N2 as an equilibrium gas, total flow rate of 400 mL·min−1, and reaction temperature of 250 °C for 5 h until SO2 adsorption was saturated. The sample was labeled as Mn8/PG-S.
Water-washed catalyst: A total of 2-g Mn8/PG-S catalyst was added to an Erlenmeyer flask containing 50-mL deionized water; the mixture was placed in an SHA-B-type water bath, stirred at a constant temperature (25 °C) for 10 min, and then dried at 105 °C to obtain a water-washed catalyst. The sample was labeled as Mn8/PG-S-W.
Multi-cycles of SO2 pretreatment followed by water washing of the catalyst were also carried out to evaluate the potential weight loss of manganese species. Concrete steps are as follows: first, in the absence of NH3, SO2 was piped in, the catalyst was presulfurized, again according to the process of the catalyst presulfurization water experiment, and dried after a bath. The catalyst was then washed in SO2, secondary vulcanization processing took place, followed by water processing, the cycle. The samples were labeled as Mn8/PG-W-x, where “x” means the number of cycles.
MnSO4 (x)/PG catalyst: MnSO4 was impregnated with the equal volume method. Firstly, 10 g PG and a certain amount of MnSO4 were weighed, and a certain amount of deionized water was added to MnSO4 to be stirred and dissolved. Then, manganese sulfate solution was poured into PG, stirred for 15 min, and allowed to stand for 24 h to obtain the MnSO4 (X)/PG catalyst. The mass of manganese sulfate was calculated according to the load ratio, X.
3.3. Catalytic Activity Test
Catalytic performance of the catalysts for NH
3-SCR was evaluated in a fixed-bed quartz tube (with an inner diameter of 15 mm and a height of 80 mm) continuous-flow reactor containing 3 mL of the catalysts. The evaluation device consists of three parts: simulated flue-gas region, fixed-bed reactor, and analysis region. The device composition is shown in
Figure 14. The typical feed-gas composition was as follows: 600 ppm of NO, 600 ppm of NH
3, 400 ppm of SO
2, 3% O
2, and the balance of N
2. The concentrations of NO and SO
2 in the inlet and outlet streams of the reactor were monitored online by an NO analyzer (Testo 350XL, Testo SE & Co. KGaA, Lenzkirch, Germany) and an SO
2 (MRU-OPTIMA7) analyzer (Testo SE & Co. KGaA, Germany), respectively. All data were collected after 40 min when the SCR reaction reached a steady state.
The denitrification performance of catalysts was based on the NO conversion (X
NO). The calculation formula is as follows:
where [NO
x]
in is the inlet concentration of NO and [NO
x]in is the outlet concentration of NO.
3.4. Catalyst Characterization
The surface area of the catalyst was measured using a Quantachrome Nove (2200 ev) analyzer (Anton Paar Co. Ltd, Graz, Austria). The microscopic surface composition of the catalysts was analyzed using a new Hitachi SU8020 high-resolution field-emission scanning electron microscopy (SEM) instrument (Carl Zeiss AG, Oberkochen, Germany). The powder XRD measurement was performed on a Rigaku D/MAx2500V system (PANalytical B.V., Almelo, Netherlands) with a Cu Kα radiation. The XPS analysis was performed on an ESCALAB 250-type electron energy spectrometer (Thermo Fisher Scientific, Waltham, America) with Al Kα, hν = 1486.6 eV and a power of 150 W. The X-ray fluorescence (XRF) spectroscopic analysis was performed on an XRF-1800 X-ray fluorescence spectrometer (Shimadzu, Kyoto, Janpan), Rh target, a maximum power of 4 kW, with a minimum area of 0.5 mm.
The NH3 isothermal adsorption and temperature-programmed desorption measurement of ammonia (NH3-TPD) catalysts was performed in fixed quartz reaction tubes. A total of 0.5-g catalysts was loaded into a quartz tube, pretreated at 110 °C for 3 h in N2 flow (100 mL·min−1), and cooled to 50 °C before the measurement. The adsorption of NH3 was performed at 50 °C. After adsorption saturation, the catalysts were heated at a rate of 5 °C·min−1, from 50 °C to 900 °C under a constant N2 flow of 200 mL·min−1. The desorbed amount of NH3 was monitored online by mass spectrometry (Hiden QIC-200, Beijing Enghead Analysis Technology Co., Ltd, Beijing, China). SO2 and NO isothermal adsorption and TPD experiments were consistent with NH3-TPD experiments.
Hydrogen temperature-programmed reduction (H2-TPR) experiments were performed using a homemade H2-TPR device (Tianjin Xianquan Instrument Co. Ltd, Tianjin, China). A total of 50.0-mg catalyst with a particle diameter of 0.38–0.83 mm was placed into the device, a 5% H2–Ar gas mixture was passed, and the gas-flow rate was set at 20 mL·min−1. The baseline was purged at 50 °C until the baseline stabilized and switched to the analytical state. Then, the temperature was increased from 50 °C to 850 °C at a ramping rate of 10 °C·min−1. Hydrogen concentration was measured using a thermal conductivity cell (TCD detector, Tianjin Xianquan Instrument Co. Ltd, Tianjin, China) to produce a TPR spectrum. The detection temperature was 80 °C, and the bridge flow was 60 mA.
Temperature-programmed surface reactions (TPSR) were carried out to study the reactivity of catalyst-pre-adsorbed ammonia with gaseous NO. The catalysts (0.5 g) were adsorbed with NH3 at 50 °C, then reacted with simulated flue gas containing 600 ppm NO/Ar and 3% O2 from 50 °C to 300 °C at a rate of 5 °C·min−1, and the total gas-flow rate was 350 mL·min−1. Finally, the gas concentrations of NO were measured by a flue-gas analyzer (Testo350-XL, Testo SE & Co. KGaA, Germany).