Catalytic Degradation of Chitosan by Supported Heteropoly Acids in Heterogeneous Systems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiment
2.2. Response Surface Methodology for Optimizing Experimental Design
2.2.1. Design and Results of Response Interview Experiments
2.2.2. Modeling and Variance Analysis
2.2.3. Response Surface and Contour Analysis
2.2.4. Best Technology and Reproducibility Experiments
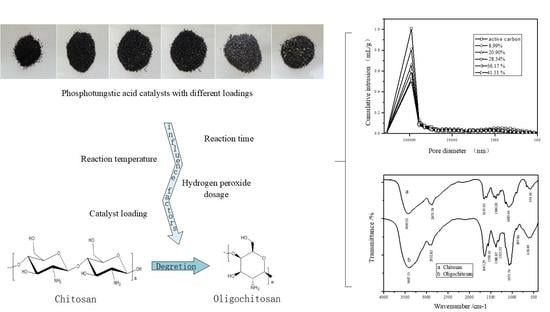
2.3. FTIR Spectral Analyses
2.3.1. Infrared Spectrum of Supported PTA
2.3.2. Infrared Spectra of Raw Materials/LWCS
2.4. Pore Structure Analysis of Supported PTA Catalyst
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Activated Carbon-Supported PTA Catalyst
3.3. Preparation of Low Molecular Weight Chitosan
3.4. Determination of Average Molecular Weight of Chitosan
3.5. Single-Factor Experiment
3.6. Response Surface Experiment
3.7. Fourier Transform Infrared Spectrometer (FT-IR)
3.8. Mercury Intrusion Porosimetry (MIP)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, I.Y.; Seo, S.J.; Moon, H.S. Chitosan and its derivatives for tissue engineering applications. J. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. J. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. J. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Yang, Z.; Fang, Y.; Ji, H. Controlled release and enhanced antibacterial activity of salicylic acid by hydrogen bonding with chitosan. J. Chin. J. Chem. Eng. 2016, 24, 421–426. [Google Scholar] [CrossRef]
- Shen, K.T.; Chen, M.H.; Chan, H.Y. Inhibitory effects of chitooligosaccharides on tumor growth and metastasis. J. Food Chem. Toxicol. 2009, 47, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Han, B.; Shao, K. Analgesis and wound healing effect of chitosan and carboxymethyl chitosan on scalded rats. J. Ocean Univ. China 2014, 13, 837–841. [Google Scholar] [CrossRef]
- Yuan, X.B.; Zheng, J.P.; Jiao, S.M.; Cheng, G.; Feng, C. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. J. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.Q.; Xiao, L.; Du, Y.M. Prediction and control of extent of deploymerization of chitosan by hydroperoxide. J. Wu Han Univ. J. (Nat. Sci. Ed.) 2000, 46, 195–198. [Google Scholar]
- Liu, Y.J.; Lou, C.Y.; Hou, Z.M.; Feng, Y.F. Process of Chitosan Oxide Degradation by Hydrogen Peroxide in Acetic Acid Homogeneous Phase. J. Funct. Polym. 2007, 19, 426–430. [Google Scholar]
- Zhao, Z.K.; Li, Z.S.; Wang, G.R.; Qiao, W.H.; Cheng, L.B. Heteropoly Acids Catalysts and Their Application in the Synthesis of Fine Chemicals. J. Prog. Chem. 2004, 16, 620–630. [Google Scholar]
- Wang, G.L.; Li, S.B.; Liu, J.L. Recent progress on heteropoly acid and its spurrort catalyst. J. Pet. Refin. Eng. 2002, 32, 46–51. [Google Scholar]
- Huang, Q.Z.; Wang, S.M.; Huang, J.F. Study on the heterogeneous degradation of chitosan with hydrogen peroxide under the catalysis of phosphotungstic acid. J. Carbohydr. Polym. 2007, 68, 761–765. [Google Scholar] [CrossRef]
- Xian, L.M.; Guo, H.C.; Jin, H. Discussion on Determination of Relative Number Average Molecular Weight of Chitosan-Oligosaccharide by Terminal Group Analysis Method. J. Ocean Univ. Qingdao 2005, 1, 142–144. [Google Scholar]












| Laboratory No. | Hydrogen Peroxide Dosage (A) | Catalyst Loading (B)/% | Reaction Time (C)/h | Reaction Temperature (D)/°C | Response Value (Y)% |
|---|---|---|---|---|---|
| 1 | −1 | 0 | 1 | 0 | 36.1 |
| 2 | 1 | −1 | 0 | 0 | 29.7 |
| 3 | 0 | 0 | 0 | 0 | 27.4 |
| 4 | −1 | 0 | 0 | 1 | 31.8 |
| 5 | 0 | 0 | −1 | −1 | 41.7 |
| 6 | 0 | −1 | 0 | −1 | 25.7 |
| 7 | 0 | 1 | −1 | 0 | 31.8 |
| 8 | 0 | 1 | 0 | 1 | 33.2 |
| 9 | 0 | 0 | −1 | 1 | 41.7 |
| 10 | 0 | 1 | 1 | 0 | 33.4 |
| 11 | 0 | −1 | 1 | 0 | 38.2 |
| 12 | 0 | −1 | 0 | 1 | 32.2 |
| 13 | 1 | 0 | −1 | 0 | 32.7 |
| 14 | −1 | 1 | 0 | 0 | 32.1 |
| 15 | 1 | 1 | 0 | 0 | 29.4 |
| 16 | 0 | 0 | 0 | 0 | 27.4 |
| 17 | 0 | 0 | 0 | 0 | 27.4 |
| 18 | 0 | 0 | 0 | 0 | 27.4 |
| 19 | 0 | 1 | 0 | −1 | 31.7 |
| 20 | −1 | 0 | 0 | −1 | 33.8 |
| 21 | 0 | 0 | 1 | −1 | 32.5 |
| 22 | 0 | 0 | 0 | 0 | 27.4 |
| 23 | 0 | −1 | −1 | 0 | 40.7 |
| 24 | 0 | 0 | 1 | 1 | 36.3 |
| 25 | 1 | 0 | 0 | −1 | 30 |
| 26 | 1 | 0 | 1 | 0 | 36.1 |
| 27 | −1 | −1 | 0 | 0 | 36 |
| 28 | 1 | 0 | 0 | 1 | 28.9 |
| 29 | −1 | 0 | −1 | 0 | 35.4 |
| Source | Squares | df | Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| Model | 408.27 | 14 | 29.16 | 2.95 | 0.0261 |
| A | 28.21 | 1 | 28.21 | 2.85 | 0.1134 |
| B | 9.90 | 1 | 9.90 | 1.0 | 0.3341 |
| C | 10.83 | 1 | 10.83 | 1.09 | 0.3132 |
| D | 6.31 | 1 | 6.31 | 0.64 | 0.4380 |
| AB | 3.24 | 1 | 3.24 | 0.33 | 0.5762 |
| AC | 1.82 | 1 | 1.82 | 0.18 | 0.6743 |
| AD | 0.20 | 1 | 0.20 | 0.020 | 0.8883 |
| BC | 4.20 | 1 | 4.20 | 0.42 | 0.5251 |
| BD | 6.25 | 1 | 6.25 | 0.63 | 0.4400 |
| CD | 3.61 | 1 | 3.61 | 0.36 | 0.5555 |
| A2 | 14.68 | 1 | 14.68 | 1.48 | 0.2434 |
| B2 | 20.25 | 1 | 20.25 | 2.05 | 0.1745 |
| C2 | 325.07 | 1 | 325.07 | 32.85 | <0.0001 |
| D2 | 38.67 | 1 | 38.67 | 3.91 | 0.0681 |
| Residual | 138.52 | 14 | 9.89 | ||
| Lack of Fit | 138.52 | 10 | 13.85 | ||
| Pure Error | 0.32 | 4 | 0.054 | ||
| Cor Total | 546.80 | 28 |
| Loadings | Porosity | Average Pore Size (nm) | Total Pore Volume (mL/g) | Total Hole Specific Surface Area (m2/g) | Apparent Density (g/mL) |
|---|---|---|---|---|---|
| activated carbon | 51.23% | 1275.6 | 0.7375 | 676.9 | 1.42 |
| 8.99% | 47.30% | 9893.3 | 0.5845 | 784.6 | 1.53 |
| 20.90% | 43.58% | 6336.7 | 0.4901 | 821.2 | 1.58 |
| 28.34% | 39.27% | 759.3 | 0.3889 | 890.1 | 1.66 |
| 36.17% | 27.71% | 4928.6 | 0.4314 | 734.6 | 1.70 |
| 41.51% | 40.96% | 8667.6 | 0.3870 | 643.9 | 1.79 |
| Level | Hydrogen Peroxide Dosage (A) | Catalyst Loading (B)/% | Reaction Time (C)/h | Reaction Temperature (D)/°C |
|---|---|---|---|---|
| −1 | 1 | 20% | 2 | 60 |
| 0 | 2 | 30% | 3 | 70 |
| 1 | 3 | 40% | 4 | 80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ma, Z.; Min, Y.; Wang, H.; Zhang, R.; Zhang, X.; Song, Y. Catalytic Degradation of Chitosan by Supported Heteropoly Acids in Heterogeneous Systems. Catalysts 2020, 10, 1078. https://doi.org/10.3390/catal10091078
Zhang H, Ma Z, Min Y, Wang H, Zhang R, Zhang X, Song Y. Catalytic Degradation of Chitosan by Supported Heteropoly Acids in Heterogeneous Systems. Catalysts. 2020; 10(9):1078. https://doi.org/10.3390/catal10091078
Chicago/Turabian StyleZhang, Hang, Zhipeng Ma, Yunpeng Min, Huiru Wang, Ru Zhang, Xuecheng Zhang, and Yimin Song. 2020. "Catalytic Degradation of Chitosan by Supported Heteropoly Acids in Heterogeneous Systems" Catalysts 10, no. 9: 1078. https://doi.org/10.3390/catal10091078