Raman Spectroscopic Imaging of Human Bladder Resectates towards Intraoperative Cancer Assessment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Raman Spectroscopy
2.3. Data Processing
3. Results
3.1. Photomicrographs of Bladder Specimens
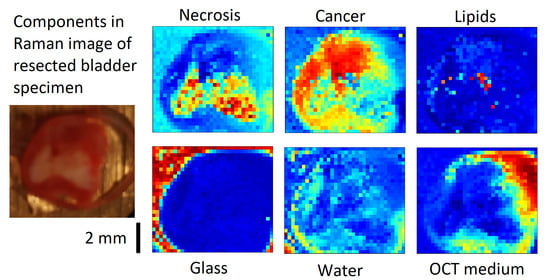
3.2. VCA of Cancer Sample 2
3.3. Overview of VCA Result of All Samples
3.4. Raman Spectra of Microplastics and Pigments in Bladder Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of bladder cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Lenis, A.T.; Lec, P.M.; Chamie, K. Bladder cancer: A review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.G.; Oh, W.K.; Galsky, M.D. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA A Cancer J. Clin. 2020, 70, 404–423. [Google Scholar] [CrossRef]
- Railkar, R.; Agarwal, P.K. Photodynamic therapy in the treatment of bladder cancer: Past challenges and current innovations. Eur. Urol. Focus 2018, 4, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Loshchenov, M.; Seregin, A.; Kalyagina, N.; Dadashev, E.; Borodkin, A.; Babaev, A.; Loran, O.; Loschenov, V. Fluorescence visualization of the borders of bladder tumors after tur with quantitative determination of diagnostic contrast. Transl. Biophotonics 2020, 2, e201900026. [Google Scholar] [CrossRef] [Green Version]
- Crow, P.; Molckovsky, A.; Stone, N.; Uff, J.; Wilson, B.; WongKeeSong, L.M. Assessment of fiberoptic near-infrared raman spectroscopy for diagnosis of bladder and prostate cancer. Urology 2005, 65, 1126–1130. [Google Scholar] [CrossRef]
- Draga, R.O.P.; Grimbergen, M.C.M.; Vijverberg, P.L.M.; Swol, C.F.P.v.; Jonges, T.G.N.; Kummer, J.A.; Ruud Bosch, J.L.H. In vivo bladder cancer diagnosis by high-volume raman spectroscopy. Anal. Chem. 2010, 82, 5993–5999. [Google Scholar] [CrossRef]
- Barman, I.; Dingari, N.C.; Singh, G.P.; Kumar, R.; Lang, S.; Nabi, G. Selective sampling using confocal raman spectroscopy provides enhanced specificity for urinary bladder cancer diagnosis. Anal. Bioanal. Chem. 2012, 404, 3091–3099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Li, X.; Broderick, N.; Liu, Y.; Zhou, Y.; Han, J.; Xu, W. Identification and characterization of bladder cancer by low-resolution fiber-optic raman spectroscopy. J. Biophotonics 2018, 11, e201800016. [Google Scholar] [CrossRef] [PubMed]
- Placzek, F.; Bautista, E.C.; Kretschmer, S.; Wurster, L.M.; Knorr, F.; González-Cerdas, G.; Erkkilä, M.T.; Stein, P.; Ataman, Ç.; Hermann, G.G.; et al. Morpho-molecular ex vivo detection and grading of non-muscle-invasive bladder cancer using forward imaging probe based multimodal optical coherence tomography and raman spectroscopy. Analyst 2020, 145, 1445–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stomp-Agenant, M.; van Dijk, T.; Onur, A.R.; Grimbergen, M.; van Melick, H.; Jonges, T.; Bosch, R.; van Swol, C. In vivo raman spectroscopy for bladder cancer detection using a superficial raman probe compared to a nonsuperficial raman probe. J. Biophotonics 2022, 15, e202100354. [Google Scholar] [CrossRef] [PubMed]
- Taieb, A.; Berkovic, G.; Haifler, M.; Cheshnovsky, O.; Shaked, N.T. Classification of tissue biopsies by raman spectroscopy guided by quantitative phase imaging and its application to bladder cancer. J. Biophotonics 2022, 15, e202200009. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.; Belay, B.; Bergner, N.; Romeike, B.F.; Reichart, R.; Kalff, R.; Popp, J. Advances in optical biopsy--correlation of malignancy and cell density of primary brain tumors using raman microspectroscopic imaging. Analyst 2012, 137, 5533–5537. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.; Neudert, L.; Simat, T.; Salzer, R. Near infrared raman spectra of human brain lipids. Spectrochim. Acta A 2005, 61, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.; Codrich, D.; Pelizzo, G.; Sergo, V. Raman and ftir microscopic imaging of colon tissue: A comparative study. J. Biophoton. 2008, 1, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Bergner, N.; Krafft, C.; Geiger, K.D.; Kirsch, M.; Schackert, G.; Popp, J. Unsupervised unmixing of raman microspectroscopic images for morphochemical analysis of non-dried brain tumor specimens. Anal. Bioanal. Chem. 2012, 403, 719–725. [Google Scholar] [CrossRef]
- Krafft, C.; Schmitt, M.; Schie, I.W.; Cialla-May, D.; Matthäus, C.; Bocklitz, T.; Popp, J. Label-free molecular imaging of biological cells and tissues by linear and nonlinear raman spectroscopic approaches. Angew. Chem. Int. Ed. 2017, 56, 4392–4430. [Google Scholar] [CrossRef]
- Böke, J.S.; Popp, J.; Krafft, C. Optical photothermal infrared spectroscopy with simultaneously acquired raman spectroscopy for two-dimensional microplastic identification. Sci. Rep. 2022, 12, 18785. [Google Scholar] [CrossRef]
- Darvin, M.E.; Schleusener, J.; Parenz, F.; Seidel, O.; Krafft, C.; Popp, J.; Lademann, J. Confocal raman microscopy combined with optical clearing for identification of inks in multicolored tattooed skin in vivo. Analyst 2018, 143, 4990–4999. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.; Popp, J. Opportunities of optical and spectral technologies in intraoperative histopathology. Optica 2023, 10, 214–231. [Google Scholar] [CrossRef]
- Bergner, N.; Medyukhina, A.; Geiger, K.D.; Kirsch, M.; Schackert, G.; Krafft, C.; Popp, J. Hyperspectral unmixing of raman micro-images for assessment of morphological and chemical parameters in non-dried brain tumor specimens. Anal. Bioanal. Chem. 2013, 405, 8719–8728. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Ridgway, N.D. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.J.E.; Dudgeon, A.P.; Ferguson, D.J.; Shore, A.C.; Stone, N. Utilization of raman spectroscopy to identify breast cancer from the water content in surgical samples containing blue dye. Transl. Biophotonics 2021, 3, e202000023. [Google Scholar] [CrossRef]
- Aaboubout, Y.; ten Hove, I.; Smits, R.W.H.; Hardillo, J.A.; Puppels, G.J.; Koljenovic, S. Specimen-driven intraoperative assessment of resection margins should be standard of care for oral cancer patients. Oral Dis. 2021, 27, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Sun, W.; Jin, C.; Bai, Y.; Ma, R.; Deng, Y.; Gao, Y.; Pan, G.; Yang, Z.; Yan, L. Blood uptake and urine excretion of nano- and micro-plastics after a single exposure. Sci. Total Environ. 2022, 848, 157639. [Google Scholar] [CrossRef]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef]
- Cordero, E.; Rüger, J.; Marti, D.; Mondol, A.S.; Hasselager, T.; Mogensen, K.; Hermann, G.G.; Popp, J.; Schie, I.W. Bladder tissue characterization using probe-based raman spectroscopy: Evaluation of tissue heterogeneity and influence on the model prediction. J. Biophotonics 2020, 13, e201960025. [Google Scholar] [CrossRef]
- Sepehri, M.; Sejersen, T.; Qvortrup, K.; Lerche, C.M.; Serup, J. Tattoo pigments are observed in the kupffer cells of the liver indicating blood-borne distribution of tattoo ink. Dermatology 2017, 233, 86–93. [Google Scholar] [CrossRef]
- Foerster, M.; Schreiver, I.; Luch, A.; Schüz, J. Tattoo inks and cancer. Cancer Epidemiol. 2020, 65, 101655. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Peters, R.J.B.; Janssen, H.-G.; Nielen, M.W.F.; Ruggeri, F.S. Microplastics and nanoplastics in food, water, and beverages, part ii. Methods. TrAC Trends Anal. Chem. 2022, 157, 116819. [Google Scholar] [CrossRef]
- Krafft, C. Raman Spectroscopy of Proteins and Nucleic Acids: From Amino Acids and Nucleotides to Large Assemblies. Encycl. Anal. Chem. 2018, 1–15. [Google Scholar] [CrossRef]
- Shaik, T.A.; Alfonso-García, A.; Zhou, X.; Arnold, K.M.; Haudenschild, A.K.; Krafft, C.; Griffiths, L.G.; Popp, J.; Marcu, L. FLIm-Guided Raman Imaging to Study Cross-Linking and Calcification of Bovine Pericardium. Anal. Chem. 2020, 92, 10659–10667. [Google Scholar] [CrossRef]







| VC1 | VC2 | VC3 | VC4 | VC5 | VC6 | VC7 | |
|---|---|---|---|---|---|---|---|
| 1N | Pigment 1 | Lipid | Water | OCT | Pigment 1 | Glass | Tumor |
| 1T | PPS | Lipid/protein | Glass | OCT | Necrosis | Epithelium | Water |
| 2N | Unknown | OCT | Tumor | Water | Glass | ||
| 2T | Glass | Water | Tumor | OCT | Lipid | Necrosis | |
| 3N | Glass | Lipid | Water | Tumor | |||
| 3T | PS | Glass | OCT | Tumor | |||
| 4N | Lipid | Pigment 2 | Water | Glass | Control | OCT | |
| 4T | Glass | Unknown | OCT | Carbon | Lipid | Tumor | |
| 5N | Pigment 1 | Lipid | Glass | Water | Control | ||
| 5T | Glass | Water | OCT | Lipid | Necrosis | Tumor | |
| 6N | Lipid | Water | Control | Glass | Epithelium | ||
| 6T | Pigment 3 | PS | Glass | OCT | Tumor | Water | |
| 7N | Control | Glass | Water | Pigment | Epithelium | ||
| 7T | Pigment 1 | Glass | Tumor | Pigment 1 | Tumor | ||
| 8N | Pigment 1 | Control | Glass | Water | Epithelium | ||
| 8T | |||||||
| 9N | Lipid | Glass | Noise | Water | Pigment 1 | Control | |
| 9T | Noise | Tumor | Noise | Noise | Glass | Water | Lipid |
| 10N | Lipid/protein | Glass/OCT | OCT | Water | Glass | Control | |
| 10T | Glass | Lipid/protein | unknown | Necrosis | Carotene | Water | Carotene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krafft, C.; Popp, J.; Bronsert, P.; Miernik, A. Raman Spectroscopic Imaging of Human Bladder Resectates towards Intraoperative Cancer Assessment. Cancers 2023, 15, 2162. https://doi.org/10.3390/cancers15072162
Krafft C, Popp J, Bronsert P, Miernik A. Raman Spectroscopic Imaging of Human Bladder Resectates towards Intraoperative Cancer Assessment. Cancers. 2023; 15(7):2162. https://doi.org/10.3390/cancers15072162
Chicago/Turabian StyleKrafft, Christoph, Jürgen Popp, Peter Bronsert, and Arkadiusz Miernik. 2023. "Raman Spectroscopic Imaging of Human Bladder Resectates towards Intraoperative Cancer Assessment" Cancers 15, no. 7: 2162. https://doi.org/10.3390/cancers15072162