Physical Activity as the Best Supportive Care in Cancer: The Clinician’s and the Researcher’s Perspectives
Abstract
:Simple Summary
Abstract
1. Introduction
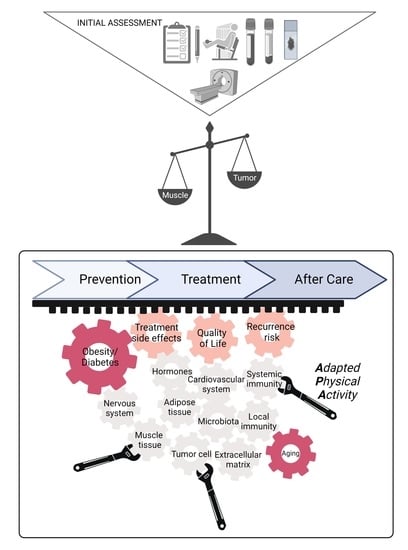
2. What Do We Know and Do in Routine Clinical Practice, in 2022?
2.1. Cancer Prevention
2.1.1. Increasing Influence of Overweight and Obesity
2.1.2. Direct Effect of Physical Exercise on Primary and Tertiary Cancer Prevention
2.2. Cancer Management and Treatments:
2.2.1. The Threat of Malnutrition and Sarcopenia
2.2.2. Pathophysiology of Cancer-Related Muscle Wasting and Dysfunction
2.2.3. Roles of Physical Exercise during Cancer Treatments
2.3. In Clinical Practice, What to Do?
2.4. Unmet Needs
3. Emerging Mechanistic Views
3.1. Systemic Effects
3.1.1. Muscle Secretome during Exercise
3.1.2. Muscle Crosstalk with Other Organs during Cancer
3.1.3. Effect of Exercise on the Hypothalamo-Hypophyseal-Adrenal Axis
3.1.4. Effect of Exercise on Treatment-Associated Toxicities
3.1.5. Effect of Exercise on the Systemic Immunity
3.1.6. Effect of Exercise on the Gut Microbiota
3.2. Tumor and the Microenvironment
3.2.1. Local Immunity
3.2.2. Fibroblasts and the ECM
3.2.3. Angiogenesis
3.2.4. Effect on the Tumor Cells
4. New Applications
4.1. Future of the Clinical Research
4.2. Future of the Preclinical Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coleman, M.; Forman, D.; Bryant, H.; Butler, J.; Rachet, B.; Maringe, C.; Nur, U.; Tracey, E.; Coory, M.; Hatcher, J.; et al. Cancer Survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An Analysis of Population-Based Cancer Registry Data. Lancet 2011, 377, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Anota, A.; Foucaut, A.-M.; Védie, A.-L.; Antoun, S.; Barnoud, D.; Bouleuc, C.; Chorin, F.; Cottet, V.; Fontaine, E.; et al. Nutrition and Physical Activity: French Intergroup Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC, SFP-APA, SFNCM, AFSOS). BMJ Support. Palliat. Care 2021, 11, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer Cachexia in Adult Patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Brown, J.C.; Gilmore, L.A. Physical Activity Reduces The Risk of Recurrence and Mortality in Cancer Patients. Exerc. Sport Sci. Rev. 2020, 48, 67–73. [Google Scholar] [CrossRef]
- Holmes, M.D.; Chen, W.Y.; Feskanich, D.; Kroenke, C.H.; Colditz, G.A. Physical Activity and Survival After Breast Cancer Diagnosis. JAMA 2005, 293, 2479–2486. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.; Sato, K.; Niedzwiecki, D.; Ye, X.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Impact of Physical Activity after Cancer Diagnosis on Survival in Patients with Recurrent Colon Cancer: Findings from CALGB 89803/Alliance. Clin. Color. Cancer 2013, 12, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Schwedhelm, C.; Boeing, H.; Hoffmann, G.; Aleksandrova, K.; Schwingshackl, L. Effect of Diet on Mortality and Cancer Recurrence among Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Nutr. Rev. 2016, 74, 737–748. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.C.; Lee, I.-M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; Berrington de Gonzalez, A.; Hartge, P.; et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef]
- Sedentary Behaviour Research Network. Letter to the Editor: Standardized Use of the Terms “Sedentary” and “Sedentary Behaviours.”. Appl. Physiol. Nutr. Metab. 2012, 37, 540–542. [Google Scholar] [CrossRef] [Green Version]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-Mass Index and Incidence of Cancer: A Systematic Review and Meta-Analysis of Prospective Observational Studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.S.; Peltonen, M.; Ahlin, S.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Jacobson, P.; Lönroth, H.; Maglio, C.; Näslund, I.; et al. Bariatric Surgery and Prevention of Type 2 Diabetes in Swedish Obese Subjects. N. Engl. J. Med. 2012, 367, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.S.; Sjöholm, K.; Jacobson, P.; Andersson-Assarsson, J.C.; Svensson, P.-A.; Taube, M.; Carlsson, B.; Peltonen, M. Life Expectancy after Bariatric Surgery in the Swedish Obese Subjects Study. N. Engl. J. Med. 2020, 383, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Van Kruijsdijk, R.C.M.; van der Wall, E.; Visseren, F.L.J. Obesity and Cancer: The Role of Dysfunctional Adipose Tissue. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2569–2578. [Google Scholar] [CrossRef] [Green Version]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and Inflammation: The Linking Mechanism and the Complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Papanicolaou, D.A. Interleukin-6: The Endocrine Cytokine. J. Clin. Endocrinol. Metab. 2000, 85, 1331–1333. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Karastergiou, K.; Mohamed-Ali, V. The Autocrine and Paracrine Roles of Adipokines. Mol. Cell. Endocrinol. 2010, 318, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halberg, N.; Wernstedt-Asterholm, I.; Scherer, P.E. The Adipocyte as an Endocrine Cell. Endocrinol. Metab. Clin. N. Am. 2008, 37, 753–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henegar, C.; Tordjman, J.; Achard, V.; Lacasa, D.; Cremer, I.; Guerre-Millo, M.; Poitou, C.; Basdevant, A.; Stich, V.; Viguerie, N.; et al. Adipose Tissue Transcriptomic Signature Highlights the Pathological Relevance of Extracellular Matrix in Human Obesity. Genome Biol. 2008, 9, R14. [Google Scholar] [CrossRef] [PubMed]
- Vila, I.K.; Badin, P.-M.; Marques, M.-A.; Monbrun, L.; Lefort, C.; Mir, L.; Louche, K.; Bourlier, V.; Roussel, B.; Gui, P.; et al. Immune Cell Toll-like Receptor 4 Mediates the Development of Obesity- and Endotoxemia-Associated Adipose Tissue Fibrosis. Cell Rep. 2014, 7, 1116–1129. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and Adipose Tissue Dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Divoux, A.; Tordjman, J.; Lacasa, D.; Veyrie, N.; Hugol, D.; Aissat, A.; Basdevant, A.; Guerre-Millo, M.; Poitou, C.; Zucker, J.-D.; et al. Fibrosis in Human Adipose Tissue: Composition, Distribution, and Link With Lipid Metabolism and Fat Mass Loss. Diabetes 2010, 59, 2817–2825. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, K.C.; Song, H.; Rosenzweig, N.; Jansen, D.A. Extracellular Matrix Substrata Alter Adipocyte Yield and Lipogenesis in Primary Cultures of Stromal-Vascular Cells from Human Adipose. Biotechnol. Lett. 2003, 25, 1967–1972. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Ginty, C.A. Fibronectin Modulation of Cell Shape and Lipogenic Gene Expression in 3t3-Adipocytes. Cell 1983, 35, 657–666. [Google Scholar] [CrossRef]
- Sasso, M.; Liu, Y.; Aron-Wisnewsky, J.; Bouillot, J.-L.; Abdennour, M.; Clet, M.; Sandrin, L.; le Naour, G.; Bedossa, P.; Tordjman, J.; et al. AdipoScan: A Novel Transient Elastography-Based Tool Used to Non-Invasively Assess Subcutaneous Adipose Tissue Shear Wave Speed in Obesity. Ultrasound Med. Biol. 2016, 42, 2401–2413. [Google Scholar] [CrossRef]
- Abdennour, M.; Reggio, S.; Le Naour, G.; Liu, Y.; Poitou, C.; Aron-Wisnewsky, J.; Charlotte, F.; Bouillot, J.-L.; Torcivia, A.; Sasso, M.; et al. Association of Adipose Tissue and Liver Fibrosis With Tissue Stiffness in Morbid Obesity: Links With Diabetes and BMI Loss After Gastric Bypass. J. Clin. Endocrinol. Metab. 2014, 99, 898–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrinelli, V.; Heuvingh, J.; du Roure, O.; Rouault, C.; Devulder, A.; Klein, C.; Lacasa, M.; Clément, E.; Lacasa, D.; Clément, K. Human Adipocyte Function Is Impacted by Mechanical Cues. J. Pathol. 2014, 233, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Bel Lassen, P.; Charlotte, F.; Liu, Y.; Bedossa, P.; Le Naour, G.; Tordjman, J.; Poitou, C.; Bouillot, J.-L.; Genser, L.; Zucker, J.-D.; et al. The FAT Score, a Fibrosis Score of Adipose Tissue: Predicting Weight-Loss Outcome After Gastric Bypass. J. Clin. Endocrinol. Metab. 2017, 102, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, G.-J.; Wang, Z.J.; Chu, Z.; Toffolo, G.; Manesso, E.; Sauer, P.J.J.; Sunehag, A.L. Strength Exercise Improves Muscle Mass and Hepatic Insulin Sensitivity in Obese Youth. Med. Sci. Sport. Exerc. 2010, 42, 1973–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rezende, L.F.M.; de Sá, T.H.; Markozannes, G.; Rey-López, J.P.; Lee, I.-M.; Tsilidis, K.K.; Ioannidis, J.P.A.; Eluf-Neto, J. Physical Activity and Cancer: An Umbrella Review of the Literature Including 22 Major Anatomical Sites and 770 000 Cancer Cases. Br. J. Sport. Med. 2018, 52, 826–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autenrieth, C.S.; Baumert, J.; Baumeister, S.E.; Fischer, B.; Peters, A.; Döring, A.; Thorand, B. Association between Domains of Physical Activity and All-Cause, Cardiovascular and Cancer Mortality. Eur. J. Epidemiol. 2011, 26, 91–99. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Association of Survival With Adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors After Colon Cancer Diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncol. 2018, 4, 783–790. [Google Scholar] [CrossRef]
- Nguyen, T.Y.V.; Batterham, M.J.; Edwards, C. Comparison of Resting Energy Expenditure Between Cancer Subjects and Healthy Controls: A Meta-Analysis. Nutr. Cancer 2016, 68, 374–387. [Google Scholar] [CrossRef] [Green Version]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of Malnutrition in Patients at First Medical Oncology Visit: The PreMiO Study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef] [Green Version]
- Lacau St Guily, J.; Bouvard, É.; Raynard, B.; Goldwasser, F.; Maget, B.; Prevost, A.; Seguy, D.; Romano, O.; Narciso, B.; Couet, C.; et al. NutriCancer: A French Observational Multicentre Cross-Sectional Study of Malnutrition in Elderly Patients with Cancer. J. Geriatr. Oncol. 2018, 9, 74–80. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilmi, M.; Jouinot, A.; Burns, R.; Pigneur, F.; Mounier, R.; Gondin, J.; Neuzillet, C.; Goldwasser, F. Body Composition and Sarcopenia: The next-Generation of Personalized Oncology and Pharmacology? Pharmacol. Ther. 2019, 196, 135–159. [Google Scholar] [CrossRef]
- Martin, L.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C.; Strasser, F.; Thoresen, L.; Jagoe, R.T.; Chasen, M.; et al. Diagnostic Criteria for the Classification of Cancer-Associated Weight Loss. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Védie, A.-L.; Joly, F.; Neuzillet, C. Nutrition En Oncologie Digestive: Synthèse Des Nouvelles Recommandations Du Thésaurus National de Cancérologie Digestive 2020. Hépato-Gastro Oncol. Dig. 2021, 28, 334–344. [Google Scholar] [CrossRef]
- Berardi, E.; Madaro, L.; Lozanoska-Ochser, B.; Adamo, S.; Thorrez, L.; Bouche, M.; Coletti, D. A Pound of Flesh: What Cachexia Is and What It Is Not. Diagnostics 2021, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer Cachexia in the Age of Obesity: Skeletal Muscle Depletion Is a Powerful Prognostic Factor, Independent of Body Mass Index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Nwachukwu, C.R.; Wu, Y.; Toesca, D.A.S.; Eyben, R.V.; Pollom, E.; Chang, D.T. Sarcopenia in Overweight or Obese Patient Is an Adverse Prognostic Factor in Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, e76. [Google Scholar] [CrossRef] [Green Version]
- Van Vledder, M.G.; Levolger, S.; Ayez, N.; Verhoef, C.; Tran, T.C.K.; IJzermans, J.N.M. Body Composition and Outcome in Patients Undergoing Resection of Colorectal Liver Metastases19. Br. J. Surg. 2012, 99, 550–557. [Google Scholar] [CrossRef]
- Villaseñor, A.; Ballard-Barbash, R.; Baumgartner, K.; Baumgartner, R.; Bernstein, L.; McTiernan, A.; Neuhouser, M.L. Prevalence and Prognostic Effect of Sarcopenia in Breast Cancer Survivors: The HEAL Study. J. Cancer Surviv. 2012, 6, 398–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzetti, F. Forcing the Vicious Circle: Sarcopenia Increases Toxicity, Decreases Response to Chemotherapy and Worsens with Chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Extermann, M. Measurement and Impact of Comorbidity in Older Cancer Patients. Crit. Rev. Oncol. Hematol. 2000, 35, 181–200. [Google Scholar] [CrossRef]
- Pedersen, B.K. Exercise-Induced Myokines and Their Role in Chronic Diseases. Brain Behav. Immun. 2011, 25, 811–816. [Google Scholar] [CrossRef]
- Katagiri, R.; Goto, A.; Nakagawa, T.; Nishiumi, S.; Kobayashi, T.; Hidaka, A.; Budhathoki, S.; Yamaji, T.; Sawada, N.; Shimazu, T.; et al. Increased Levels of Branched-Chain Amino Acid Associated With Increased Risk of Pancreatic Cancer in a Prospective Case–Control Study of a Large Cohort. Gastroenterology 2018, 155, 1474–1482.e1. [Google Scholar] [CrossRef]
- Mayers, J.R.; Wu, C.; Clish, C.B.; Kraft, P.; Torrence, M.E.; Fiske, B.P.; Yuan, C.; Bao, Y.; Townsend, M.K.; Tworoger, S.S.; et al. Elevation of Circulating Branched-Chain Amino Acids Is an Early Event in Human Pancreatic Adenocarcinoma Development. Nat. Med. 2014, 20, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.F.; Jones, L.W.; Andersen, J.L.; Daugaard, G.; Rorth, M.; Hojman, P. Muscle Dysfunction in Cancer Patients. Ann. Oncol. 2014, 25, 947–958. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal Muscle Mass and Distribution in 468 Men and Women Aged 18–88 Yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, R.N.; Counts, B.R.; Carson, J.A. Understanding Sex Differences in the Regulation of Cancer-Induced Muscle Wasting. Curr. Opin. Support. Palliat. Care 2018, 12, 394–403. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic Stress Promotes Tumor Growth and Angiogenesis in a Mouse Model of Ovarian Carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- White, J.P.; Puppa, M.J.; Narsale, A.; Carson, J.A. Characterization of the Male ApcMin/+ Mouse as a Hypogonadism Model Related to Cancer Cachexia. Biol. Open 2013, 2, 1346–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, M.D.; Wu, F.C. Androgen Effects on Skeletal Muscle: Implications for the Development and Management of Frailty. Asian J. 2014, 16, 203–212. [Google Scholar] [CrossRef]
- Dobs, A.S.; Boccia, R.V.; Croot, C.C.; Gabrail, N.Y.; Dalton, J.T.; Hancock, M.L.; Johnston, M.A.; Steiner, M.S. Effects of Enobosarm on Muscle Wasting and Physical Function in Patients with Cancer: A Double-Blind, Randomised Controlled Phase 2 Trial. Lancet Oncol. 2013, 14, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Bozzetti, F.; Mariani, L.; Lo Vullo, S.; Amerio, M.L.; Biffi, R.; Caccialanza, R.; Capuano, G.; Correja, I.; Cozzaglio, L.; Di Leo, A.; et al. The Nutritional Risk in Oncology: A Study of 1,453 Cancer Outpatients. Support. Care Cancer 2012, 20, 1919–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temparis, S.; Asensi, M.; Taillandier, D.; Aurousseau, E.; Larbaud, D.; Obled, A.; Béchet, D.; Ferrara, M.; Estrela, J.M.; Attaix, D. Increased ATP-Ubiquitin-Dependent Proteolysis in Skeletal Muscles of Tumor-Bearing Rats1. Cancer Res. 1994, 54, 5568–5573. [Google Scholar]
- Weber, M.; Sennlaub, F.; Souied, E.; Cohen, S.-Y.; Béhar-Cohen, F.; Milano, G.; Tadayoni, R. [Review and expert opinion in age related macular degeneration. Focus on the pathophysiology, angiogenesis and pharmacological and clinical data]. J. Fr. Ophtalmol. 2014, 37, 566–579. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tseng, L.-M.; Lee, H.-C. Role of Mitochondrial Dysfunction in Cancer Progression. Exp. Biol. Med. 2016, 241, 1281–1295. [Google Scholar] [CrossRef] [Green Version]
- Mallard, J.; Hucteau, E.; Charles, A.-L.; Bender, L.; Baeza, C.; Pélissie, M.; Trensz, P.; Pflumio, C.; Kalish-Weindling, M.; Gény, B.; et al. Chemotherapy Impairs Skeletal Muscle Mitochondrial Homeostasis in Early Breast Cancer Patients. J. Cachexia Sarcopenia Muscle 2022, 13, 1896–1907. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.; Matthews, C.; Ligibel, J.; Gerber, L.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sport. Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- Cheville, A.L.; Kollasch, J.; Vandenberg, J.; Shen, T.; Grothey, A.; Gamble, G.; Basford, J.R. A Home-Based Exercise Program to Improve Function, Fatigue, and Sleep Quality in Patients With Stage IV Lung and Colorectal Cancer: A Randomized Controlled Trial. J. Pain Symptom Manag. 2013, 45, 811–821. [Google Scholar] [CrossRef]
- Cormie, P.; Newton, R.U.; Spry, N.; Joseph, D.; Taaffe, D.R.; Galvão, D.A. Safety and Efficacy of Resistance Exercise in Prostate Cancer Patients with Bone Metastases. Prostate Cancer Prostatic Dis. 2013, 16, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Galvão, D.A.; Taaffe, D.R.; Spry, N.; Cormie, P.; Joseph, D.; Chambers, S.K.; Chee, R.; Peddle-Mcintyre, C.J.; Hart, N.H.; Baumann, F.T.; et al. Exercise Preserves Physical Function in Prostate Cancer Patients with Bone Metastases. Med. Sci. Sport. Exerc. 2018, 50, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henke, C.C.; Cabri, J.; Fricke, L.; Pankow, W.; Kandilakis, G.; Feyer, P.C.; de Wit, M. Strength and Endurance Training in the Treatment of Lung Cancer Patients in Stages IIIA/IIIB/IV. Support. Care Cancer 2014, 22, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Oldervoll, L.M.; Loge, J.H.; Lydersen, S.; Paltiel, H.; Asp, M.B.; Nygaard, U.V.; Oredalen, E.; Frantzen, T.L.; Lesteberg, I.; Amundsen, L.; et al. Physical Exercise for Cancer Patients with Advanced Disease: A Randomized Controlled Trial. Oncologist 2011, 16, 1649–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyszora, A.; Budzyński, J.; Wójcik, A.; Prokop, A.; Krajnik, M. Physiotherapy Programme Reduces Fatigue in Patients with Advanced Cancer Receiving Palliative Care: Randomized Controlled Trial. Support. Care Cancer 2017, 25, 2899–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rief, H.; Akbar, M.; Keller, M.; Omlor, G.; Welzel, T.; Bruckner, T.; Rieken, S.; Häfner, M.F.; Schlampp, I.; Gioules, A.; et al. Quality of Life and Fatigue of Patients with Spinal Bone Metastases under Combined Treatment with Resistance Training and Radiation Therapy—A Randomized Pilot Trial. Radiat. Oncol. 2014, 9, 151. [Google Scholar] [CrossRef]
- Zhou, W.; Wan, Y.-H.; Chen, Q.; Qiu, Y.-R.; Luo, X.-M. Effects of Tai Chi Exercise on Cancer-Related Fatigue in Patients With Nasopharyngeal Carcinoma Undergoing Chemoradiotherapy: A Randomized Controlled Trial. J. Pain Symptom Manag. 2018, 55, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Tsianakas, V.; Harris, J.; Ream, E.; Hemelrijck, M.V.; Purushotham, A.; Mucci, L.; Green, J.S.A.; Fewster, J.; Armes, J. CanWalk: A Feasibility Study with Embedded Randomised Controlled Trial Pilot of a Walking Intervention for People with Recurrent or Metastatic Cancer. BMJ Open 2017, 7, e013719. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-Week, Multimodal Exercise Counteracts a Progress of Chemotherapy-Induced Peripheral Neuropathy and Improves Balance and Strength in Metastasized Colorectal Cancer Patients: A Randomized Controlled Trial. Support. Care Cancer 2018, 26, 615–624. [Google Scholar] [CrossRef]
- Buss, T.; de Walden-Gałuszko, K.; Modlińska, A.; Osowicka, M.; Lichodziejewska-Niemierko, M.; Janiszewska, J. Kinesitherapy Alleviates Fatigue in Terminal Hospice Cancer Patients—An Experimental, Controlled Study. Support. Care Cancer 2010, 18, 743–749. [Google Scholar] [CrossRef]
- Zhao, S.G.; Alexander, N.B.; Djuric, Z.; Zhou, J.; Tao, Y.; Schipper, M.; Feng, F.Y.; Eisbruch, A.; Worden, F.P.; Strath, S.J.; et al. Maintaining Physical Activity during Head and Neck Cancer Treatment: Results of a Pilot Controlled Trial. Head Neck 2016, 38 (Suppl. 1), E1086–E1096. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cartmel, B.; Gottlieb, L.; Ercolano, E.A.; Li, F.; Harrigan, M.; McCorkle, R.; Ligibel, J.A.; von Gruenigen, V.E.; Gogoi, R.; et al. Randomized Trial of Exercise on Quality of Life in Women With Ovarian Cancer: Women’s Activity and Lifestyle Study in Connecticut (WALC). J. Natl. Cancer Inst. 2017, 109, djx072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuzillet, C.; Bouché, O.; Tournigand, C.; Chibaudel, B.; Bouguion, L.; Bengrine-Lefevre, L.; Lopez-Trabada Ataz, D.; Mabro, M.; Metges, J.-P.; Péré-Vergé, D.; et al. Adapted Physical Activity in Patients (Pts) with Advanced Pancreatic Cancer (APACaP): Results from a Prospective National Randomized GERCOR Trial. J. Clin. Oncol. 2022, 40 (Suppl. 16), 4007. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Chu, C.-H.; Wang, C.-C.; Song, T.-F.; Wei, G.-X. Effect of Acute Exercise and Cardiovascular Fitness on Cognitive Function: An Event-Related Cortical Desynchronization Study. Psychophysiology 2015, 52, 342–351. [Google Scholar] [CrossRef]
- Souza, R.W.A.; Piedade, W.P.; Soares, L.C.; Souza, P.A.T.; Aguiar, A.F.; Vechetti-Júnior, I.J.; Campos, D.H.S.; Fernandes, A.A.H.; Okoshi, K.; Carvalho, R.F.; et al. Aerobic Exercise Training Prevents Heart Failure-Induced Skeletal Muscle Atrophy by Anti-Catabolic, but Not Anabolic Actions. PLoS ONE 2014, 9, e110020. [Google Scholar] [CrossRef] [Green Version]
- Brandt, N.; Dethlefsen, M.M.; Bangsbo, J.; Pilegaard, H. PGC-1α and Exercise Intensity Dependent Adaptations in Mouse Skeletal Muscle. PLoS ONE 2017, 12, e0185993. [Google Scholar] [CrossRef] [Green Version]
- Pigna, E.; Berardi, E.; Aulino, P.; Rizzuto, E.; Zampieri, S.; Carraro, U.; Kern, H.; Merigliano, S.; Gruppo, M.; Mericskay, M.; et al. Aerobic Exercise and Pharmacological Treatments Counteract Cachexia by Modulating Autophagy in Colon Cancer. Sci. Rep. 2016, 6, 26991. [Google Scholar] [CrossRef] [Green Version]
- Konopka, A.R.; Harber, M.P. Skeletal Muscle Hypertrophy after Aerobic Exercise Training. Exerc. Sport Sci. Rev. 2014, 42, 53–61. [Google Scholar] [CrossRef]
- Visser, M.; Pluijm, S.M.F.; Stel, V.S.; Bosscher, R.J.; Deeg, D.J.H. Physical Activity as a Determinant of Change in Mobility Performance: The Longitudinal Aging Study Amsterdam. J. Am. Geriatr. Soc. 2002, 50, 1774–1781. [Google Scholar] [CrossRef]
- Pudkasam, S.; Pitcher, M.; Fisher, M.; O’Connor, A.; Chinlumprasert, N.; Stojanovska, L.; Polman, R.; Apostolopoulos, V. The PAPHIO Study Protocol: A Randomised Controlled Trial with a 2 × 2 Crossover Design of Physical Activity Adherence, Psychological Health and Immunological Outcomes in Breast Cancer Survivors. BMC Public Health 2020, 20, 696. [Google Scholar] [CrossRef]
- Hoving, J.; Broekhuizen, M.; Frings-Dresen, M. Return to Work of Breast Cancer Survivors: A Systematic Review of Intervention Studies. BMC Cancer 2009, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Süß, P.; Schulte, D.M.; Letsch, A.; Jensen, W. Supportive Care in Oncology—From Physical Activity to Nutrition. Nutrients 2022, 14, 1149. [Google Scholar] [CrossRef] [PubMed]
- Van Waart, H.; Stuiver, M.M.; van Harten, W.H.; Geleijn, E.; Kieffer, J.M.; Buffart, L.M.; de Maaker-Berkhof, M.; Boven, E.; Schrama, J.; Geenen, M.M.; et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise during Adjuvant Chemotherapy on Physical Fitness, Fatigue and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J. Clin. Oncol. 2015, 33, 1918–1927. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sport. Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvão, D.A.; Chinapaw, M.J.; et al. Effects and Moderators of Exercise on Quality of Life and Physical Function in Patients with Cancer: An Individual Patient Data Meta-Analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society Nutrition and Physical Activity Guideline for Cancer Survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cañamero, S.; Cobo-Cuenca, A.I.; Carmona-Torres, J.M.; Pozuelo-Carrascosa, D.P.; Santacruz-Salas, E.; Rabanales-Sotos, J.A.; Cuesta-Mateos, T.; Laredo-Aguilera, J.A. Impact of Physical Exercise in Advanced-Stage Cancer Patients: Systematic Review and Meta-Analysis. Cancer Med. 2022, 11, 3714–3727. [Google Scholar] [CrossRef]
- Pudkasam, S.; Feehan, J.; Talevski, J.; Vingrys, K.; Polman, R.; Chinlumprasert, N.; Stojanovska, L.; Apostolopoulos, V. Motivational Strategies to Improve Adherence to Physical Activity in Breast Cancer Survivors: A Systematic Review and Meta-Analysis. Maturitas 2021, 152, 32–47. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose Tissue, Obesity and Adipokines: Role in Cancer Promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Warner, A.B.; Jones, L.W.; Dewhirst, M.W. Exercise as Adjunct Therapy in Cancer. Semin. Radiat. Oncol. 2019, 29, 16–24. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef] [PubMed]
- Delezie, J.; Handschin, C. Endocrine Crosstalk Between Skeletal Muscle and the Brain. Front. Neurol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [Green Version]
- Wackerhage, H.; Ratkevicius, A. Signal Transduction Pathways That Regulate Muscle Growth. Essays Biochem. 2008, 44, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Rose-John, S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschke, S.; Eckel, J. Adipo-Myokines: Two Sides of the Same Coin—Mediators of Inflammation and Mediators of Exercise. Mediat. Inflamm. 2013, 2013, e320724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, S.; Tamura, Y.; Kakehi, S.; Sanada, H.; Kawamori, R.; Watada, H. Exercise-Induced Increase in IL-6 Level Enhances GLUT4 Expression and Insulin Sensitivity in Mouse Skeletal Muscle. Biochem. Biophys. Res. Commun. 2016, 473, 947–952. [Google Scholar] [CrossRef]
- Iizuka, K.; Machida, T.; Hirafuji, M. Skeletal Muscle Is an Endocrine Organ. J. Pharmacol. Sci. 2014, 125, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Hittel, D.S.; Berggren, J.R.; Shearer, J.; Boyle, K.; Houmard, J.A. Increased Secretion and Expression of Myostatin in Skeletal Muscle From Extremely Obese Women. Diabetes 2009, 58, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Cadavid, N.F.; Taylor, W.E.; Yarasheski, K.; Sinha-Hikim, I.; Ma, K.; Ezzat, S.; Shen, R.; Lalani, R.; Asa, S.; Mamita, M.; et al. Organization of the Human Myostatin Gene and Expression in Healthy Men and HIV-Infected Men with Muscle Wasting. Proc. Natl. Acad. Sci. USA 1998, 95, 14938–14943. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.L.; Hittel, D.S.; McPherron, A.C. Expression and Function of Myostatin in Obesity, Diabetes, and Exercise Adaptation. Med. Sci. Sport. Exerc. 2011, 43, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, e6644631. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Kishioka, Y.; Wakamatsu, J.; Hattori, A.; Hennebry, A.; Berry, C.J.; Sharma, M.; Kambadur, R.; Nishimura, T. Decorin Binds Myostatin and Modulates Its Activity to Muscle Cells. Biochem. Biophys. Res. Commun. 2006, 340, 675–680. [Google Scholar] [CrossRef]
- Kishioka, Y.; Thomas, M.; Wakamatsu, J.; Hattori, A.; Sharma, M.; Kambadur, R.; Nishimura, T. Decorin Enhances the Proliferation and Differentiation of Myogenic Cells through Suppressing Myostatin Activity. J. Cell. Physiol. 2008, 215, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Tortoriello, D.V.; Sidis, Y.; Holtzman, D.A.; Holmes, W.E.; Schneyer, A.L. Human Follistatin-Related Protein: A Structural Homologue of Follistatin with Nuclear Localization. Endocrinology 2001, 142, 3426–3434. [Google Scholar] [CrossRef]
- Gilson, H.; Schakman, O.; Kalista, S.; Lause, P.; Tsuchida, K.; Thissen, J.-P. Follistatin Induces Muscle Hypertrophy through Satellite Cell Proliferation and Inhibition of Both Myostatin and Activin. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E157–E164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Li, Y.; Lu, A.; Gharaibeh, B.; Ma, J.; Kobayashi, T.; Quintero, A.J.; Huard, J. Follistatin Improves Skeletal Muscle Healing after Injury and Disease through an Interaction with Muscle Regeneration, Angiogenesis, and Fibrosis. Am. J. Pathol. 2011, 179, 915–930. [Google Scholar] [CrossRef]
- Fukumoto, M.; Takeuchi, T.; Koubayashi, E.; Harada, S.; Ota, K.; Kojima, Y.; Higuchi, K. Induction of Brain-Derived Neurotrophic Factor in Enteric Glial Cells Stimulated by Interleukin-1β via a c-Jun N-Terminal Kinase Pathway. J. Clin. Biochem. Nutr. 2020, 66, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, C.; Antunes, B.M.; Giacon, T.R.; Vanderlei, L.C.M.; Campos, E.Z.; Peres, F.P.; Clark, N.W.; Panissa, V.L.G.; Lira, F.S. Influence of Acute and Chronic High-Intensity Intermittent Aerobic Plus Strength Exercise on BDNF, Lipid and Autonomic Parameters. J. Sport. Sci. Med. 2019, 18, 359–368. [Google Scholar]
- McKay, B.R.; Nederveen, J.P.; Fortino, S.A.; Snijders, T.; Joanisse, S.; Kumbhare, D.A.; Parise, G. Brain-Derived Neurotrophic Factor Is Associated with Human Muscle Satellite Cell Differentiation in Response to Muscle-Damaging Exercise. Appl. Physiol. Nutr. Metab. 2020, 45, 581–590. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.W.; Taudorf, S.; et al. Brain-Derived Neurotrophic Factor (BDNF) and Type 2 Diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Goulding, E.H.; Zang, K.; Cepoi, D.; Cone, R.D.; Jones, K.R.; Tecott, L.H.; Reichardt, L.F. Brain-Derived Neurotrophic Factor Regulates Energy Balance Downstream of Melanocortin-4 Receptor. Nat. Neurosci. 2003, 6, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Ando, D.; Takamatsu, K.; Goto, K. Resistance Exercise Induces a Greater Irisin Response than Endurance Exercise. Metabolism 2015, 64, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Siopi, A.; Mougios, V.; Park, K.H.; Mantzoros, C.S. Irisin in Response to Exercise in Humans With and Without Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E453–E457. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in Metabolic Diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin Prevents and Restores Bone Loss and Muscle Atrophy in Hind-Limb Suspended Mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [Green Version]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and Role of Irisin in Glucose Homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like Is a Hormone That Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [Green Version]
- Ushach, I.; Arrevillaga-Boni, G.; Heller, G.N.; Pone, E.; Hernandez-Ruiz, M.; Catalan-Dibene, J.; Hevezi, P.; Zlotnik, A. Meteorin-like/Meteorin-β Is a Novel Immunoregulatory Cytokine Associated with Inflammation. J. Immunol. 2018, 201, 3669–3676. [Google Scholar] [CrossRef] [Green Version]
- Senesi, P.; Luzi, L.; Terruzzi, I. Adipokines, Myokines, and Cardiokines: The Role of Nutritional Interventions. Int. J. Mol. Sci. 2020, 21, 8372. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The Role of Leucine and Its Metabolites in Protein and Energy Metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Papadopetraki, A.; Maridaki, M.; Zagouri, F.; Dimopoulos, M.-A.; Koutsilieris, M.; Philippou, A. Physical Exercise Restrains Cancer Progression through Muscle-Derived Factors. Cancers 2022, 14, 1892. [Google Scholar] [CrossRef]
- Brooks, G.A.; Fahey, T.D.; White, T.P. Exercise Physiology: Human Bioenergetics and Its Applications, 2nd ed.; Mayfield Publishing Company: Mountain View, CA, USA, 1996. [Google Scholar]
- Brooks, G.A. The Precious Few Grams of Glucose During Exercise. Int. J. Mol. Sci. 2020, 21, 5733. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Shonibare, Z.; Monavarian, M.; Arend, R.C.; Lee, N.Y.; Inman, G.J.; Mythreye, K. TGFβ Signaling Networks in Ovarian Cancer Progression and Plasticity. Clin. Exp. Metastasis 2021, 38, 139–161. [Google Scholar] [CrossRef]
- Dardare, J.; Witz, A.; Merlin, J.-L.; Gilson, P.; Harlé, A. SMAD4 and the TGFβ Pathway in Patients with Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 3534. [Google Scholar] [CrossRef]
- Luo, J.; Chen, X.-Q.; Li, P. The Role of TGF-β and Its Receptors in Gastrointestinal Cancers. Transl. Oncol. 2019, 12, 475–484. [Google Scholar] [CrossRef]
- Kretzschmar, M. Transforming Growth Factor-Beta and Breast Cancer: Transforming Growth Factor-Beta/SMAD Signaling Defects and Cancer. Breast Cancer Res. 2000, 2, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Balsano, R.; Kruize, Z.; Lunardi, M.; Comandatore, A.; Barone, M.; Cavazzoni, A.; Re Cecconi, A.D.; Morelli, L.; Wilmink, H.; Tiseo, M.; et al. Transforming Growth Factor-Beta Signaling in Cancer-Induced Cachexia: From Molecular Pathways to the Clinics. Cells 2022, 11, 2671. [Google Scholar] [CrossRef]
- Lima, J.D.C.C.; Simoes, E.; de Castro, G.; Morais, M.R.P.T.; de Matos-Neto, E.M.; Alves, M.J.; Pinto, N.I.; Figueredo, R.G.; Zorn, T.M.T.; Felipe-Silva, A.S.; et al. Tumour-Derived Transforming Growth Factor-β Signalling Contributes to Fibrosis in Patients with Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2019, 10, 1045–1059. [Google Scholar] [CrossRef] [Green Version]
- Guttridge, D.C. A TGF-β Pathway Associated with Cancer Cachexia. Nat. Med. 2015, 21, 1248–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waning, D.L.; Mohammad, K.S.; Reiken, S.; Xie, W.; Andersson, D.C.; John, S.; Chiechi, A.; Wright, L.E.; Umanskaya, A.; Niewolna, M.; et al. Excess TGF-β Mediates Muscle Weakness Associated with Bone Metastases in Mice. Nat. Med. 2015, 21, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.; Hill, C.S. Alterations in Components of the TGF-Beta Superfamily Signaling Pathways in Human Cancer. Cytokine Growth Factor Rev. 2006, 17, 41–58. [Google Scholar] [CrossRef]
- Bierie, B.; Moses, H.L. Tumour Microenvironment: TGFbeta: The Molecular Jekyll and Hyde of Cancer. Nat. Rev. Cancer 2006, 6, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, Y.; Yin, Q.; Liu, S.; Wang, Q.; Tang, Y.; Cao, C. Clinical and Prognostic Significance of Serum Transforming Growth Factor-Beta1 Levels in Patients with Pancreatic Ductal Adenocarcinoma. Braz. J. Med. Biol. Res. 2016, 49, e5485. [Google Scholar] [CrossRef] [Green Version]
- Sheen-Chen, S.-M.; Chen, H.-S.; Sheen, C.-W.; Eng, H.-L.; Chen, W.-J. Serum Levels of Transforming Growth Factor Beta1 in Patients with Breast Cancer. Arch. Surg. 2001, 136, 937–940. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-H.; Shao, Y.-Y.; Chan, S.-Y.; Huang, C.-Y.; Hsu, C.-H.; Cheng, A.-L. High Serum Transforming Growth Factor-Β1 Levels Predict Outcome in Hepatocellular Carcinoma Patients Treated with Sorafenib. Clin. Cancer Res. 2015, 21, 3678–3684. [Google Scholar] [CrossRef] [Green Version]
- Sartori, R.; Gregorevic, P.; Sandri, M. TGFβ and BMP Signaling in Skeletal Muscle: Potential Significance for Muscle-Related Disease. Trends Endocrinol. Metab. 2014, 25, 464–471. [Google Scholar] [CrossRef]
- Mendias, C.L.; Gumucio, J.P.; Davis, M.E.; Bromley, C.W.; Davis, C.S.; Brooks, S.V. Transforming Growth Factor-Beta Induces Skeletal Muscle Atrophy and Fibrosis through the Induction of Atrogin-1 and Scleraxis. Muscle Nerve 2012, 45, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Castells, J.; Allibert, V.; Emerit, A.; Zolotoff, C.; Cardot-Ruffino, V.; Gallot, Y.S.; Vernus, B.; Chauvet, V.; Bartholin, L.; et al. Hypothalamic–Pituitary–Adrenal Axis Activation and Glucocorticoid-Responsive Gene Expression in Skeletal Muscle and Liver of Apc Mice. J. Cachexia Sarcopenia Muscle 2022, 13, 1686–1703. [Google Scholar] [CrossRef]
- Carlson, M.E.; Hsu, M.; Conboy, I.M. Imbalance between PSmad3 and Notch Induces CDK Inhibitors in Old Muscle Stem Cells. Nature 2008, 454, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Williamson, D.; Gallagher, P.; Harber, M.; Hollon, C.; Trappe, S. Mitogen-Activated Protein Kinase (MAPK) Pathway Activation: Effects of Age and Acute Exercise on Human Skeletal Muscle. J. Physiol. 2003, 547, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.C.; Fedorov, Y.V.; Rosenthal, R.S.; Olwin, B.B. ERK1/2 Is Required for Myoblast Proliferation but Is Dispensable for Muscle Gene Expression and Cell Fusion. J. Cell. Physiol. 2001, 186, 104–115. [Google Scholar] [CrossRef]
- Allen, R.E.; Boxhorn, L.K. Regulation of Skeletal Muscle Satellite Cell Proliferation and Differentiation by Transforming Growth Factor-Beta, Insulin-like Growth Factor I, and Fibroblast Growth Factor. J. Cell. Physiol. 1989, 138, 311–315. [Google Scholar] [CrossRef]
- Allen, R.E.; Boxhorn, L.K. Inhibition of Skeletal Muscle Satellite Cell Differentiation by Transforming Growth Factor-Beta. J. Cell. Physiol. 1987, 133, 567–572. [Google Scholar] [CrossRef]
- Li, Y.; Foster, W.; Deasy, B.M.; Chan, Y.; Prisk, V.; Tang, Y.; Cummins, J.; Huard, J. Transforming Growth Factor-Β1 Induces the Differentiation of Myogenic Cells into Fibrotic Cells in Injured Skeletal Muscle: A Key Event in Muscle Fibrogenesis. Am. J. Pathol. 2004, 164, 1007–1019. [Google Scholar] [CrossRef]
- Hackney, A.C.; Viru, A. Twenty-Four-Hour Cortisol Response to Multiple Daily Exercise Sessions of Moderate and High Intensity. Clin. Physiol. 1999, 19, 178–182. [Google Scholar] [CrossRef]
- Zen, M.; Canova, M.; Campana, C.; Bettio, S.; Nalotto, L.; Rampudda, M.; Ramonda, R.; Iaccarino, L.; Doria, A. The Kaleidoscope of Glucorticoid Effects on Immune System. Autoimmun. Rev. 2011, 10, 305–310. [Google Scholar] [CrossRef]
- Vega, S.R.; Knicker, A.; Hollmann, W.; Bloch, W.; Strüder, H.K. Effect of Resistance Exercise on Serum Levels of Growth Factors in Humans. Horm. Metab. Res. 2010, 42, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; Berrington de Gonzalez, A.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure Time Physical Activity and Mortality: A Detailed Pooled Analysis of the Dose-Response Relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coletti, D. Chemotherapy-Induced Muscle Wasting: An Update. Eur. J. Transl. Myol. 2018, 28, 7587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, E.; Bresciani, E.; Rizzi, L.; Cappellari, O.; De Luca, A.; Torsello, A.; Liantonio, A. Cisplatin-Induced Skeletal Muscle Dysfunction: Mechanisms and Counteracting Therapeutic Strategies. Int. J. Mol. Sci. 2020, 21, 1242. [Google Scholar] [CrossRef]
- Hiensch, A.E.; Bolam, K.A.; Mijwel, S.; Jeneson, J.A.L.; Huitema, A.D.R.; Kranenburg, O.; van der Wall, E.; Rundqvist, H.; Wengstrom, Y.; May, A.M. Doxorubicin-Induced Skeletal Muscle Atrophy: Elucidating the Underlying Molecular Pathways. Acta Physiol. 2020, 229, e13400. [Google Scholar] [CrossRef] [Green Version]
- Gilliam, L.A.A.; St. Clair, D.K. Chemotherapy-Induced Weakness and Fatigue in Skeletal Muscle: The Role of Oxidative Stress. Antioxid. Redox Signal. 2011, 15, 2543–2563. [Google Scholar] [CrossRef] [Green Version]
- Pin, F.; Couch, M.E.; Bonetto, A. Preservation of Muscle Mass as a Strategy to Reduce the Toxic Effects of Cancer Chemotherapy on Body Composition. Curr. Opin. Support. Palliat. Care 2018, 12, 420–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berg, M.M.G.A.; Kok, D.E.; Posthuma, L.; Kamps, L.; Kelfkens, C.S.; Buist, N.; Geenen, M.; Haringhuizen, A.; Heijns, J.B.; van Lieshout, R.H.M.A.; et al. Body Composition Is Associated with Risk of Toxicity-Induced Modifications of Treatment in Women with Stage I–IIIB Breast Cancer Receiving Chemotherapy. Breast Cancer Res. Treat. 2019, 173, 475–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Huang, Y.; Liu, Y.; Chen, Y. Irisin Enhances Doxorubicin-Induced Cell Apoptosis in Pancreatic Cancer by Inhibiting the PI3K/AKT/NF-ΚB Pathway. Med. Sci. Monit. 2019, 25, 6085–6096. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the Exercise-Inducible Myokine Irisin on Malignant and Non-Malignant Breast Epithelial Cell Behavior in Vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Campelj, D.G.; Goodman, C.A.; Rybalka, E. Chemotherapy-Induced Myopathy: The Dark Side of the Cachexia Sphere. Cancers 2021, 13, 3615. [Google Scholar] [CrossRef]
- Sauter, K.A.D.; Wood, L.J.; Wong, J.; Iordanov, M.; Magun, B.E. Doxorubicin and Daunorubicin Induce Processing and Release of Interleukin-1β through Activation of the NLRP3 Inflammasome. Cancer Biol. 2011, 11, 1008–1016. [Google Scholar] [CrossRef] [Green Version]
- Braun, T.P.; Szumowski, M.; Levasseur, P.R.; Grossberg, A.J.; Zhu, X.; Agarwal, A.; Marks, D.L. Muscle Atrophy in Response to Cytotoxic Chemotherapy Is Dependent on Intact Glucocorticoid Signaling in Skeletal Muscle. PLoS ONE 2014, 9, e106489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, T.P.; Grossberg, A.J.; Krasnow, S.M.; Levasseur, P.R.; Szumowski, M.; Zhu, X.X.; Maxson, J.E.; Knoll, J.G.; Barnes, A.P.; Marks, D.L. Cancer- and Endotoxin-Induced Cachexia Require Intact Glucocorticoid Signaling in Skeletal Muscle. FASEB J. 2013, 27, 3572–3582. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.P.; Zhu, X.; Szumowski, M.; Scott, G.D.; Grossberg, A.J.; Levasseur, P.R.; Graham, K.; Khan, S.; Damaraju, S.; Colmers, W.F.; et al. Central Nervous System Inflammation Induces Muscle Atrophy via Activation of the Hypothalamic–Pituitary–Adrenal Axis. J. Exp. Med. 2011, 208, 2449–2463. [Google Scholar] [CrossRef]
- Gilson, H.; Schakman, O.; Combaret, L.; Lause, P.; Grobet, L.; Attaix, D.; Ketelslegers, J.M.; Thissen, J.P. Myostatin Gene Deletion Prevents Glucocorticoid-Induced Muscle Atrophy. Endocrinology 2007, 148, 452–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliam, L.A.A.; Moylan, J.S.; Patterson, E.W.; Smith, J.D.; Wilson, A.S.; Rabbani, Z.; Reid, M.B. Doxorubicin Acts via Mitochondrial ROS to Stimulate Catabolism in C2C12 Myotubes. Am. J. Physiol. Cell Physiol. 2012, 302, C195–C202. [Google Scholar] [CrossRef] [Green Version]
- Fanzani, A.; Zanola, A.; Rovetta, F.; Rossi, S.; Aleo, M.F. Cisplatin Triggers Atrophy of Skeletal C2C12 Myotubes via Impairment of Akt Signalling Pathway and Subsequent Increment Activity of Proteasome and Autophagy Systems. Toxicol. Appl. Pharmacol. 2011, 250, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, H.W.; Powers, S.K. Mitochondrial Dysfunction Is a Common Denominator Linking Skeletal Muscle Wasting Due to Disease, Aging, and Prolonged Inactivity. Antioxidants 2021, 10, 588. [Google Scholar] [CrossRef]
- Sorensen, J.C.; Cheregi, B.D.; Timpani, C.A.; Nurgali, K.; Hayes, A.; Rybalka, E. Mitochondria: Inadvertent Targets in Chemotherapy-Induced Skeletal Muscle Toxicity and Wasting? Cancer Chemother. Pharm. 2016, 78, 673–683. [Google Scholar] [CrossRef]
- Garcia, J.M.; Cata, J.P.; Dougherty, P.M.; Smith, R.G. Ghrelin Prevents Cisplatin-Induced Mechanical Hyperalgesia and Cachexia. Endocrinology 2008, 149, 455–460. [Google Scholar] [CrossRef]
- Bresciani, E.; Rizzi, L.; Molteni, L.; Ravelli, M.; Liantonio, A.; Ben Haj Salah, K.; Fehrentz, J.-A.; Martinez, J.; Omeljaniuk, R.J.; Biagini, G.; et al. JMV2894, a Novel Growth Hormone Secretagogue, Accelerates Body Mass Recovery in an Experimental Model of Cachexia. Endocrine 2017, 58, 106–114. [Google Scholar] [CrossRef]
- Dickey, D.T.; Muldoon, L.L.; Doolittle, N.D.; Peterson, D.R.; Kraemer, D.F.; Neuwelt, E.A. Effect of N-Acetylcysteine Route of Administration on Chemoprotection against Cisplatin-Induced Toxicity in Rat Models. Cancer Chemother. Pharm. 2008, 62, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, H.; Sagara, A.; Arakawa, K.; Sugiyama, R.; Hirosaki, A.; Takase, K.; Jo, A.; Sato, K.; Chiba, Y.; Yamazaki, M.; et al. Mechanisms of Cisplatin-Induced Muscle Atrophy. Toxicol. Appl. Pharmacol. 2014, 278, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Garcia, J.M.; Scherer, T.; Chen, J.; Guillory, B.; Nassif, A.; Papusha, V.; Smiechowska, J.; Asnicar, M.; Buettner, C.; Smith, R.G. Inhibition of Cisplatin-Induced Lipid Catabolism and Weight Loss by Ghrelin in Male Mice. Endocrinology 2013, 154, 3118–3129. [Google Scholar] [CrossRef] [Green Version]
- Torsello, A.; Luoni, M.; Schweiger, F.; Grilli, R.; Guidi, M.; Bresciani, E.; Deghenghi, R.; Müller, E.E.; Locatelli, V. Novel Hexarelin Analogs Stimulate Feeding in the Rat through a Mechanism Not Involving Growth Hormone Release. Eur. J. Pharmacol. 1998, 360, 123–129. [Google Scholar] [CrossRef]
- Borner, T.; Loi, L.; Pietra, C.; Giuliano, C.; Lutz, T.A.; Riediger, T. The Ghrelin Receptor Agonist HM01 Mimics the Neuronal Effects of Ghrelin in the Arcuate Nucleus and Attenuates Anorexia-Cachexia Syndrome in Tumor-Bearing Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R89–R96. [Google Scholar] [CrossRef] [Green Version]
- Villars, F.O.; Pietra, C.; Giuliano, C.; Lutz, T.A.; Riediger, T. Oral Treatment with the Ghrelin Receptor Agonist HM01 Attenuates Cachexia in Mice Bearing Colon-26 (C26) Tumors. Int. J. Mol. Sci. 2017, 18, 986. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-T.; Liao, J.-M.; Ko, J.-L.; Lee, Y.-L.; Chang, H.-Y.; Wu, C.-H.; Ou, C.-C. D-Methionine Ameliorates Cisplatin-Induced Muscle Atrophy via Inhibition of Muscle Degradation Pathway. Integr. Cancer Ther. 2019, 18, 1534735419828832. [Google Scholar] [CrossRef] [Green Version]
- De Luca, A.; Pierno, S.; Camerino, D.C. Taurine: The Appeal of a Safe Amino Acid for Skeletal Muscle Disorders. J. Transl. Med. 2015, 13, 243. [Google Scholar] [CrossRef] [Green Version]
- De Luca, A.; Pierno, S.; Tricarico, D.; Desaphy, J.-F.; Liantonio, A.; Barbieri, M.; Camerino, C.; Montanari, L.; Camerino, D.C. Taurine and Skeletal Muscle Ion Channels. In Taurine 4: Taurine and Excitable Tissues; Advances in Experimental Medicine and Biology; Della Corte, L., Huxtable, R.J., Sgaragli, G., Tipton, K.F., Eds.; Springer: Boston, MA, USA, 2002; pp. 45–56. [Google Scholar] [CrossRef]
- Hojman, P.; Fjelbye, J.; Zerahn, B.; Christensen, J.F.; Dethlefsen, C.; Lonkvist, C.K.; Brandt, C.; Gissel, H.; Pedersen, B.K.; Gehl, J. Voluntary Exercise Prevents Cisplatin-Induced Muscle Wasting during Chemotherapy in Mice. PLoS ONE 2014, 9, e109030. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.H.; Seo, D.Y.; Lee, S.H.; Shin, C.; Jamrasi, P.; Han, J.; Song, W. Effects of Exercise on AKT/PGC1-α/FOXO3a Pathway and Muscle Atrophy in Cisplatin-Administered Rat Skeletal Muscle. Korean J. Physiol. Pharm. 2021, 25, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.I.; Rubini, E.d.C.; Meirelles, F.d.O.; da Silva, E.B. Aerobic Exercise and Cardiac Function of Murines Exposed to Doxorubicin: A Meta-Analysis. Arq. Bras. Cardiol. 2020, 115, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Servaes, P.; Verhagen, C.; Bleijenberg, G. Fatigue in Cancer Patients during and after Treatment: Prevalence, Correlates and Interventions. Eur. J. Cancer 2002, 38, 27–43. [Google Scholar] [CrossRef]
- Kuhnt, S.; Ernst, J.; Singer, S.; Rüffer, J.U.; Kortmann, R.-D.; Stolzenburg, J.-U.; Schwarz, R. Fatigue in Cancer Survivors—Prevalence and Correlates. Onkologie 2009, 32, 312–317. [Google Scholar] [CrossRef]
- Patrick, D.L.; Ferketich, S.L.; Frame, P.S.; Harris, J.J.; Hendricks, C.B.; Levin, B.; Link, M.P.; Lustig, C.; McLaughlin, J.; Ried, L.D.; et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, 15–17 July 2002. J. Natl. Cancer Inst. 2003, 95, 1110–1117. [Google Scholar] [CrossRef] [Green Version]
- Henry, D.H.; Viswanathan, H.N.; Elkin, E.P.; Traina, S.; Wade, S.; Cella, D. Symptoms and Treatment Burden Associated with Cancer Treatment: Results from a Cross-Sectional National Survey in the U.S. Support. Care Cancer 2008, 16, 791–801. [Google Scholar] [CrossRef]
- Carpenter, J.S.; Elam, J.L.; Ridner, S.H.; Carney, P.H.; Cherry, G.J.; Cucullu, H.L. Sleep, Fatigue, and Depressive Symptoms in Breast Cancer Survivors and Matched Healthy Women Experiencing Hot Flashes. Oncol. Nurs. Forum 2004, 31, 591–5598. [Google Scholar] [CrossRef] [Green Version]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-Analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef]
- Országhová, Z.; Mego, M.; Chovanec, M. Long-Term Cognitive Dysfunction in Cancer Survivors. Front. Mol. Biosci. 2021, 8, 770413. [Google Scholar] [CrossRef]
- Ren, X.; Wang, X.; Sun, J.; Hui, Z.; Lei, S.; Wang, C.; Wang, M. Effects of Physical Exercise on Cognitive Function of Breast Cancer Survivors Receiving Chemotherapy: A Systematic Review of Randomized Controlled Trials. Breast 2022, 63, 113–122. [Google Scholar] [CrossRef]
- Lyon, D.E.; Cohen, R.; Chen, H.; Kelly, D.L.; McCain, N.L.; Starkweather, A.; Ahn, H.; Sturgill, J.; Jackson-Cook, C.K. Relationship of Systemic Cytokine Concentrations to Cognitive Function over Two Years in Women with Early Stage Breast Cancer. J. Neuroimmunol. 2016, 301, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Starkweather, A.; Kelly, D.L.; Thacker, L.; Wright, M.L.; Jackson-Cook, C.K.; Lyon, D.E. Relationships among Psychoneurological Symptoms and Levels of C-Reactive Protein over 2 Years in Women with Early-Stage Breast Cancer. Support. Care Cancer 2017, 25, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repka, C.P.; Hayward, R. Oxidative Stress and Fitness Changes in Cancer Patients after Exercise Training. Med. Sci. Sport. Exerc. 2016, 48, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Bachman, J.F.; Chakkalakal, J.V. Insights into Muscle Stem Cell Dynamics during Postnatal Development. FEBS J. 2022, 289, 2710–2722. [Google Scholar] [CrossRef]
- Bachman, J.F.; Blanc, R.S.; Paris, N.D.; Kallenbach, J.G.; Johnston, C.J.; Hernady, E.; Williams, J.P.; Chakkalakal, J.V. Radiation-Induced Damage to Prepubertal Pax7+ Skeletal Muscle Stem Cells Drives Lifelong Deficits in Myofiber Size and Nuclear Number. iScience 2020, 23, 101760. [Google Scholar] [CrossRef]
- Gianfaldoni, S.; Gianfaldoni, R.; Wollina, U.; Lotti, J.; Tchernev, G.; Lotti, T. An Overview on Radiotherapy: From Its History to Its Current Applications in Dermatology. Open Access Maced. J. Med. Sci. 2017, 5, 521–525. [Google Scholar] [CrossRef] [Green Version]
- Quirós, P.M.; Ramsay, A.J.; Sala, D.; Fernández-Vizarra, E.; Rodríguez, F.; Peinado, J.R.; Fernández-García, M.S.; Vega, J.A.; Enríquez, J.A.; Zorzano, A.; et al. Loss of Mitochondrial Protease OMA1 Alters Processing of the GTPase OPA1 and Causes Obesity and Defective Thermogenesis in Mice. EMBO J. 2012, 31, 2117–2133. [Google Scholar] [CrossRef]
- Yamamori, T.; Yasui, H.; Yamazumi, M.; Wada, Y.; Nakamura, Y.; Nakamura, H.; Inanami, O. Ionizing Radiation Induces Mitochondrial Reactive Oxygen Species Production Accompanied by Upregulation of Mitochondrial Electron Transport Chain Function and Mitochondrial Content under Control of the Cell Cycle Checkpoint. Free Radic. Biol. Med. 2012, 53, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Michelucci, A.; Boncompagni, S.; Pietrangelo, L.; García-Castañeda, M.; Takano, T.; Malik, S.; Dirksen, R.T.; Protasi, F. Transverse Tubule Remodeling Enhances Orai1-Dependent Ca2+ Entry in Skeletal Muscle. eLife 2019, 8, e47576. [Google Scholar] [CrossRef]
- Boncompagni, S.; Rossi, A.E.; Micaroni, M.; Beznoussenko, G.V.; Polishchuk, R.S.; Dirksen, R.T.; Protasi, F. Mitochondria Are Linked to Calcium Stores in Striated Muscle by Developmentally Regulated Tethering Structures. Mol. Biol. Cell 2009, 20, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.N.; Kallenbach, J.G.; Orciuoli, H.M.; Paris, N.D.; Bachman, J.F.; Johnston, C.J.; Hernady, E.; Williams, J.P.; Dirksen, R.T.; Chakkalakal, J.V. Endurance Exercise Attenuates Juvenile Irradiation-Induced Skeletal Muscle Functional Decline and Mitochondrial Stress. Skelet. Muscle 2022, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.Z.; Klitzman, B.; Dodge, R.; Dewhirst, M.W. Diminished Leukocyte-Endothelium Interaction in Tumor Microvessels1. Cancer Res. 1992, 52, 4265–4268. [Google Scholar] [PubMed]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Chapter Fifteen—Exercise and the Regulation of Immune Functions. In Progress in Molecular Biology and Translational Science; Molecular and Cellular Regulation of Adaptation to Exercise; Bouchard, C., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 135, pp. 355–380. [Google Scholar] [CrossRef]
- Campbell, J.P.; Riddell, N.E.; Burns, V.E.; Turner, M.; van Zanten, J.J.C.S.V.; Drayson, M.T.; Bosch, J.A. Acute Exercise Mobilises CD8+ T Lymphocytes Exhibiting an Effector-Memory Phenotype. Brain Behav. Immun. 2009, 23, 767–775. [Google Scholar] [CrossRef] [PubMed]
- LaVoy, E.C.; Hussain, M.; Reed, J.; Kunz, H.; Pistillo, M.; Bigley, A.B.; Simpson, R.J. T-Cell Redeployment and Intracellular Cytokine Expression Following Exercise: Effects of Exercise Intensity and Cytomegalovirus Infection. Physiol. Rep. 2017, 5, e13070. [Google Scholar] [CrossRef]
- Rooney, B.V.; Bigley, A.B.; LaVoy, E.C.; Laughlin, M.; Pedlar, C.; Simpson, R.J. Lymphocytes and Monocytes Egress Peripheral Blood within Minutes after Cessation of Steady State Exercise: A Detailed Temporal Analysis of Leukocyte Extravasation. Physiol. Behav. 2018, 194, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Benschop, R.J.; Nijkamp, F.P.; Ballieux, R.E.; Heijnen, C.J. The Effects of β-Adrenoceptor Stimulation on Adhesion of Human Natural Killer Cells to Cultured Endothelium. Br. J. Pharmacol. 1994, 113, 1311–1316. [Google Scholar] [CrossRef]
- Dimitrov, S.; Lange, T.; Born, J. Selective Mobilization of Cytotoxic Leukocytes by Epinephrine. J. Immunol. 2010, 184, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Krüger, K.; Alack, K.; Ringseis, R.; Mink, L.; Pfeifer, E.; Schinle, M.; Gindler, K.; Kimmelmann, L.; Walscheid, R.; Muders, K.; et al. Apoptosis of T-Cell Subsets after Acute High-Intensity Interval Exercise. Med. Sci. Sport. Exerc. 2016, 48, 2021–2029. [Google Scholar] [CrossRef]
- Turner, J.E.; Spielmann, G.; Wadley, A.J.; Aldred, S.; Simpson, R.J.; Campbell, J.P. Exercise-Induced B Cell Mobilisation: Preliminary Evidence for an Influx of Immature Cells into the Bloodstream. Physiol. Behav. 2016, 164, 376–382. [Google Scholar] [CrossRef]
- Hutnick, N.A.; Williams, N.I.; Kraemer, W.J.; Orsega-Smith, E.; Dixon, R.H.; Bleznak, A.D.; Mastro, A.M. Excercise and Lymphocyte Activation Following Chemotherapy for Breast Cancer. Med. Sci. Sport. Exerc. 2005, 37, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, D.R.; Murta, E.F.C.; Michelin, M.A. The Influence of Physical Activity on the Profile of Immune Response Cells and Cytokine Synthesis in Mice with Experimental Breast Tumors Induced by 7,12-Dimethylbenzanthracene. Eur. J. Cancer Prev. 2013, 22, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, H.; Tang, X.; Yang, Y.; Vieira, V.J.; Niu, Y.; Ma, Y. Effect of Exercise Training Intensity on Murine T-Regulatory Cells and Vaccination Response. Scand. J. Med. Sci. Sport. 2012, 22, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Rundqvist, H.; Veliça, P.; Barbieri, L.; Gameiro, P.A.; Bargiela, D.; Gojkovic, M.; Mijwel, S.; Reitzner, S.M.; Wulliman, D.; Ahlstedt, E.; et al. Cytotoxic T-Cells Mediate Exercise-Induced Reductions in Tumor Growth. eLife 2020, 9, e59996. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.A.; Keylock, K.T.; Lowder, T.; Vieira, V.J.; Zelkovich, W.; Dumich, S.; Colantuano, K.; Lyons, K.; Leifheit, K.; Cook, M.; et al. Cardiovascular Exercise Training Extends Influenza Vaccine Seroprotection in Sedentary Older Adults: The Immune Function Intervention Trial. J. Am. Geriatr. Soc. 2009, 57, 2183–2191. [Google Scholar] [CrossRef]
- Platten, M.; von Knebel Doeberitz, N.; Oezen, I.; Wick, W.; Ochs, K. Cancer Immunotherapy by Targeting IDO1/TDO and Their Downstream Effectors. Front. Immunol. 2015, 5, 673. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Zamarin, D.; Li, Y.; Gasmi, B.; Munn, D.H.; Allison, J.P.; Merghoub, T.; Wolchok, J.D. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015, 13, 412–424. [Google Scholar] [CrossRef] [Green Version]
- Meireson, A.; Devos, M.; Brochez, L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front. Immunol. 2020, 11, 531491. [Google Scholar] [CrossRef]
- Muller, A.J.; DuHadaway, J.B.; Donover, P.S.; Sutanto-Ward, E.; Prendergast, G.C. Inhibition of Indoleamine 2,3-Dioxygenase, an Immunoregulatory Target of the Cancer Suppression Gene Bin1, Potentiates Cancer Chemotherapy. Nat. Med. 2005, 11, 312–319. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An Endogenous Tumour-Promoting Ligand of the Human Aryl Hydrocarbon Receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolušić, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of Tumoral Immune Resistance by Inhibition of Tryptophan 2,3-Dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.; Chang, M.Y.; Parker, K.H.; Beury, D.W.; DuHadaway, J.B.; Flick, H.E.; Boulden, J.; Sutanto-Ward, E.; Soler, A.P.; Laury-Kleintop, L.D.; et al. IDO Is a Nodal Pathogenic Driver of Lung Cancer and Metastasis Development. Cancer Discov. 2012, 2, 722–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, D.J.; Rossi, M.; Romano, E.; Ghith, J.; Yuan, J.; Munn, D.H.; Young, J.W. Indoleamine 2,3-Dioxygenase–Expressing Mature Human Monocyte-Derived Dendritic Cells Expand Potent Autologous Regulatory T Cells. Blood 2009, 114, 555–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-Derived Catabolites Are Responsible for Inhibition of T and Natural Killer Cell Proliferation Induced by Indoleamine 2,3-Dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwu, P.; Du, M.X.; Lapointe, R.; Do, M.; Taylor, M.W.; Young, H.A. Indoleamine 2,3-Dioxygenase Production by Human Dendritic Cells Results in the Inhibition of T Cell Proliferation. J. Immunol. 2000, 164, 3596–3599. [Google Scholar] [CrossRef] [Green Version]
- Mondal, A.; Smith, C.; DuHadaway, J.B.; Sutanto-Ward, E.; Prendergast, G.C.; Bravo-Nuevo, A.; Muller, A.J. IDO1 Is an Integral Mediator of Inflammatory Neovascularization. EBioMedicine 2016, 14, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3 Dioxygenase and Metabolic Control of Immune Responses. Trends Immunol. 2013, 34, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, P.; Schmidt, M.E.; Prentzell, M.T.; Berdel, B.; Wiskemann, J.; Kellner, K.H.; Debus, J.; Ulrich, C.; Opitz, C.A.; Steindorf, K. Resistance Exercise Reduces Kynurenine Pathway Metabolites in Breast Cancer Patients Undergoing Radiotherapy. Front. Oncol. 2019, 9, 962. [Google Scholar] [CrossRef]
- Emery, A.; Moore, S.; Turner, J.E.; Campbell, J.P. Reframing How Physical Activity Reduces The Incidence of Clinically-Diagnosed Cancers: Appraising Exercise-Induced Immuno-Modulation As An Integral Mechanism. Front. Oncol. 2022, 12, 788113. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Obesity, Diabetes, and Gut Microbiota: The Hygiene Hypothesis Expanded? Diabetes Care 2010, 33, 2277–2284. [Google Scholar] [CrossRef] [Green Version]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbajo-Pescador, S.; Porras, D.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Juarez-Fernández, M.; Cuevas, M.J.; Mauriz, J.L.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Beneficial Effects of Exercise on Gut Microbiota Functionality and Barrier Integrity, and Gut-Liver Crosstalk in an in Vivo Model of Early Obesity and Non-Alcoholic Fatty Liver Disease. Dis. Model. Mech. 2019, 12, dmm039206. [Google Scholar] [CrossRef] [Green Version]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Olcoz, J.L.; Jover, R.; Jorquera, F.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Functional Interactions between Gut Microbiota Transplantation, Quercetin, and High-Fat Diet Determine Non-Alcoholic Fatty Liver Disease Development in Germ-Free Mice. Mol. Nutr. Food Res. 2019, 63, 1800930. [Google Scholar] [CrossRef] [PubMed]
- Vajro, P.; Paolella, G.; Fasano, A. Microbiota and Gut-Liver Axis: A Mini-Review on Their Influences on Obesity and Obesity Related Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Bouter, K.E.; van Raalte, D.H.; Groen, A.K.; Nieuwdorp, M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017, 152, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise Training Modulates the Gut Microbiota Profile and Impairs Inflammatory Signaling Pathways in Obese Children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 Inflammasome Instigates Obesity-Induced Inflammation and Insulin Resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the Gut and Tumor Microbiota in Cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef]
- Montégut, L.; de Cabo, R.; Zitvogel, L.; Kroemer, G. Science-Driven Nutritional Interventions for the Prevention and Treatment of Cancer. Cancer Discov. 2022, 12, 2258–2279. [Google Scholar] [CrossRef]
- Ogino, S.; Meyerhardt, J.A.; Irahara, N.; Niedzwiecki, D.; Hollis, D.; Saltz, L.B.; Mayer, R.J.; Schaefer, P.; Whittom, R.; Hantel, A.; et al. KRAS Mutation in Stage III Colon Cancer and Clinical Outcome Following Intergroup Trial CALGB 89803. Clin. Cancer Res. 2009, 15, 7322–7329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz Rajoka, M.S.; Shi, J.; Mehwish, H.M.; Zhu, J.; Li, Q.; Shao, D.; Huang, Q.; Yang, H. Interaction between Diet Composition and Gut Microbiota and Its Impact on Gastrointestinal Tract Health. Food Sci. Hum. Wellness 2017, 6, 121–130. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W. Diet-Microbiota Interactions and Their Implications for Healthy Living. Nutrients 2013, 5, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Hussan, H.; Clinton, S.K.; Roberts, K.; Bailey, M.T. Fusobacterium’s Link to Colorectal Neoplasia Sequenced: A Systematic Review and Future Insights. World J. Gastroenterol. 2017, 23, 8626–8650. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, J.; Shang, X.; Sun, K.; Zhou, J.; Liu, J.; Chi, R.; Xu, T. Exercise Modifies the Disease-Relevant Gut Microbial Shifts in Post-Traumatic Osteoarthritis Rats. Bone Jt. Res. 2022, 11, 214–225. [Google Scholar] [CrossRef]
- Andreyev, J. Gastrointestinal Symptoms after Pelvic Radiotherapy: A New Understanding to Improve Management of Symptomatic Patients. Lancet Oncol. 2007, 8, 1007–1017. [Google Scholar] [CrossRef]
- Sehgal, K.; Khanna, S. Gut Microbiome and Checkpoint Inhibitor Colitis. Intest. Res. 2021, 19, 360–364. [Google Scholar] [CrossRef]
- Cho, J.; Kim, D.; Kang, H. Exercise Preconditioning Attenuates the Response to Experimental Colitis and Modifies Composition of Gut Microbiota in Wild-Type Mice. Life 2020, 10, 200. [Google Scholar] [CrossRef]
- Ribeiro Franco, P.I.; Rodrigues, A.P.; de Menezes, L.B.; Pacheco Miguel, M. Tumor Microenvironment Components: Allies of Cancer Progression. Pathol. Res. Pract. 2020, 216, 152729. [Google Scholar] [CrossRef]
- Fisher, D.T.; Chen, Q.; Skitzki, J.J.; Muhitch, J.B.; Zhou, L.; Appenheimer, M.M.; Vardam, T.D.; Weis, E.L.; Passanese, J.; Wang, W.-C.; et al. IL-6 Trans-Signaling Licenses Mouse and Human Tumor Microvascular Gateways for Trafficking of Cytotoxic T Cells. J. Clin. Investig. 2011, 121, 3846–3859. [Google Scholar] [CrossRef]
- Hong, J.; Tobin, N.P.; Rundqvist, H.; Li, T.; Lavergne, M.; García-Ibáñez, Y.; Qin, H.; Paulsson, J.; Zeitelhofer, M.; Adzemovic, M.Z.; et al. Role of Tumor Pericytes in the Recruitment of Myeloid-Derived Suppressor Cells. J. Natl. Cancer Inst. 2015, 107, djv209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigley, A.B.; Rezvani, K.; Chew, C.; Sekine, T.; Pistillo, M.; Crucian, B.; Bollard, C.M.; Simpson, R.J. Acute Exercise Preferentially Redeploys NK-Cells with a Highly-Differentiated Phenotype and Augments Cytotoxicity against Lymphoma and Multiple Myeloma Target Cells. Brain Behav. Immun. 2014, 39, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sio, A.; Chehal, M.K.; Tsai, K.; Fan, X.; Roberts, M.E.; Nelson, B.H.; Grembecka, J.; Cierpicki, T.; Krebs, D.L.; Harder, K.W. Dysregulated Hematopoiesis Caused by Mammary Cancer Is Associated with Epigenetic Changes and Hox Gene Expression in Hematopoietic Cells. Cancer Res. 2013, 73, 5892–5904. [Google Scholar] [CrossRef] [Green Version]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.-P.; Koelwyn, G.J.; Jones, L.W.; Demaria, S. Exercise Reduces Immune Suppression and Breast Cancer Progression in a Preclinical Model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Turbitt, W.J.; Xu, Y.; Sosnoski, D.M.; Collins, S.D.; Meng, H.; Mastro, A.M.; Rogers, C.J. Physical Activity Plus Energy Restriction Prevents 4T1.2 Mammary Tumor Progression, MDSC Accumulation, and an Immunosuppressive Tumor Microenvironment. Cancer Prev. Res. 2019, 12, 493–506. [Google Scholar] [CrossRef]
- Timmons, B.W.; Cieslak, T. Human Natural Killer Cell Subsets and Acute Exercise: A Brief Review. Exerc. Immunol. Rev. 2008, 14, 8–23. [Google Scholar]
- Kim, S.; Iizuka, K.; Aguila, H.L.; Weissman, I.L.; Yokoyama, W.M. In Vivo Natural Killer Cell Activities Revealed by Natural Killer Cell-Deficient Mice. Proc. Natl. Acad. Sci. USA 2000, 97, 2731–2736. [Google Scholar] [CrossRef] [Green Version]
- Gomes-Santos, I.L.; Amoozgar, Z.; Kumar, A.S.; Ho, W.W.; Roh, K.; Talele, N.P.; Curtis, H.; Kawaguchi, K.; Jain, R.K.; Fukumura, D. Exercise Training Improves Tumor Control by Increasing CD8+ T-Cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade. Cancer Immunol. Res. 2021, 9, 765–778. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Kurz, E.; Hirsch, C.A.; Dalton, T.; Shadaloey, S.A.; Khodadadi-Jamayran, A.; Miller, G.; Pareek, S.; Rajaei, H.; Mohindroo, C.; Baydogan, S.; et al. Exercise-Induced Engagement of the IL-15/IL-15Rα Axis Promotes Anti-Tumor Immunity in Pancreatic Cancer. Cancer Cell 2022, 40, 720–737.e5. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.; Saddawi-Konefka, R.; Vermi, W.; Koebel, C.M.; Arthur, C.; White, J.M.; Uppaluri, R.; Andrews, D.M.; Ngiow, S.F.; Teng, M.W.L.; et al. Cancer Immunoediting by the Innate Immune System in the Absence of Adaptive Immunity. J. Exp. Med. 2012, 209, 1869–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, D.R.; Aleixo, A.A.R.; Murta, E.F.C.; Michelin, M.A. Innate Immune Response Adaptation in Mice Subjected to Administration of DMBA and Physical Activity. Oncol. Lett. 2014, 7, 886–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, E.M.; Cohn, R.D. TGFβ Signaling: Its Role in Fibrosis Formation and Myopathies. Curr. Opin. Rheumatol. 2012, 24, 628–634. [Google Scholar] [CrossRef]
- Pickup, M.W.; Owens, P.; Moses, H.L. TGF-β, Bone Morphogenetic Protein, and Activin Signaling and the Tumor Microenvironment. Cold Spring Harb. Perspect. Biol. 2017, 9, a022285. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Alves, R.; Abdalla, D.R.; Iunes, D.H.; Mariano, K.O.P.; Borges, J.B.C.; Murta, E.F.C.; Michelin, M.A.; Carvalho, L.C. Influence of an Exergaming Training Program on Reducing the Expression of IL-10 and TGF-β in Cancer Patients. Games Health J. 2020, 9, 446–452. [Google Scholar] [CrossRef]
- Eka Widiastuti, I.A.; Arsyad, A.; Idris, I.; Patellongi, I.; Kadriyan, H.; Buanayuda, G.W.; Sari, D.P.; Rosyidi, R.M. Exercise Adaptations and TGF-Β1 Levels in Recreational Cyclists. Ann. Med. Surg. 2021, 70, 102872. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Cao, Y.; Moeller, B. Cycling Hypoxia and Free Radicals Regulate Angiogenesis and Radiotherapy Response. Nat. Rev. Cancer 2008, 8, 425–437. [Google Scholar] [CrossRef] [Green Version]
- Cooke, V.G.; LeBleu, V.S.; Keskin, D.; Khan, Z.; O’Connell, J.T.; Teng, Y.; Duncan, M.B.; Xie, L.; Maeda, G.; Vong, S.; et al. Pericyte Depletion Results in Hypoxia-Associated Epithelial-to-Mesenchymal Transition and Metastasis Mediated by Met Signaling Pathway. Cancer Cell 2012, 21, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Griffioen, A.W.; Damen, C.A.; Martinotti, S.; Blijham, G.H.; Groenewegen, G. Endothelial Intercellular Adhesion Molecule-1 Expression Is Suppressed in Human Malignancies: The Role of Angiogenic Factors1. Cancer Res. 1996, 56, 1111–1117. [Google Scholar]
- Vasudev, N.S.; Reynolds, A.R. Anti-Angiogenic Therapy for Cancer: Current Progress, Unresolved Questions and Future Directions. Angiogenesis 2014, 17, 471–494. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Jiang, W.; Sells, J.L.; Neil, E.S.; McGinley, J.N.; Thompson, H.J. Effect of Nonmotorized Wheel Running on Mammary Carcinogenesis: Circulating Biomarkers, Cellular Processes, and Molecular Mechanisms in Rats. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Sanft, T.B.; Cartmel, B.; Harrigan, M.; Li, F.; Loftfield, E.; Playdon, M.; Zhou, Y.; Gross, C.P.; Ligibel, J.A.; Schmitz, K.H.; et al. Impact of Weight Loss and Exercise on VEGF Levels in Breast Cancer Survivors. J. Clin. Oncol. 2016, 34 (Suppl. 15), 10103. [Google Scholar] [CrossRef]
- Vaupel, P. Hypoxia and Aggressive Tumor Phenotype: Implications for Therapy and Prognosis. Oncologist 2008, 13 (Suppl. 3), 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, C.C.; Kojima, H.; Lukashev, D.; Armstrong, J.; Farber, M.; Apasov, S.G.; Sitkovsky, M.V. Differential Effects of Physiologically Relevant Hypoxic Conditions on T Lymphocyte Development and Effector Functions. J. Immunol. 2001, 167, 6140–6149. [Google Scholar] [CrossRef] [Green Version]
- Cramer, T.; Yamanishi, Y.; Clausen, B.E.; Förster, I.; Pawlinski, R.; Mackman, N.; Haase, V.H.; Jaenisch, R.; Corr, M.; Nizet, V.; et al. HIF-1α Is Essential for Myeloid Cell-Mediated Inflammation. Cell 2003, 112, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.M.; Wilson, W.R. Exploiting Tumour Hypoxia in Cancer Treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Barsoum, I.B.; Smallwood, C.A.; Siemens, D.R.; Graham, C.H. A Mechanism of Hypoxia-Mediated Escape from Adaptive Immunity in Cancer Cells. Cancer Res. 2014, 74, 665–674. [Google Scholar] [CrossRef] [Green Version]
- McCullough, D.J.; Nguyen, L.M.-D.; Siemann, D.W.; Behnke, B.J. Effects of Exercise Training on Tumor Hypoxia and Vascular Function in the Rodent Preclinical Orthotopic Prostate Cancer Model. J. Appl. Physiol. 2013, 115, 1846–1854. [Google Scholar] [CrossRef] [Green Version]
- Schadler, K.L.; Thomas, N.J.; Galie, P.A.; Bhang, D.H.; Roby, K.C.; Addai, P.; Till, J.E.; Sturgeon, K.; Zaslavsky, A.; Chen, C.S.; et al. Tumor Vessel Normalization after Aerobic Exercise Enhances Chemotherapeutic Efficacy. Oncotarget 2016, 7, 65429–65440. [Google Scholar] [CrossRef] [Green Version]
- Betof, A.S.; Lascola, C.D.; Weitzel, D.; Landon, C.; Scarbrough, P.M.; Devi, G.R.; Palmer, G.; Jones, L.W.; Dewhirst, M.W. Modulation of Murine Breast Tumor Vascularity, Hypoxia, and Chemotherapeutic Response by Exercise. J. Natl. Cancer Inst. 2015, 107, djv040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.W.; Viglianti, B.L.; Tashjian, J.A.; Kothadia, S.M.; Keir, S.T.; Freedland, S.J.; Potter, M.Q.; Jung Moon, E.; Schroeder, T.; Herndon, J.E.; et al. Effect of Aerobic Exercise on Tumor Physiology in an Animal Model of Human Breast Cancer. J. Appl. Physiol. 2010, 108, 343–348. [Google Scholar] [CrossRef]
- Florez Bedoya, C.A.; Cardoso, A.C.F.; Parker, N.; Ngo-Huang, A.; Petzel, M.Q.; Kim, M.P.; Fogelman, D.; Romero, S.G.; Wang, H.; Park, M.; et al. Exercise during Preoperative Therapy Increases Tumor Vascularity in Pancreatic Tumor Patients. Sci. Rep. 2019, 9, 13966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.W.; Antonelli, J.; Masko, E.M.; Broadwater, G.; Lascola, C.D.; Fels, D.; Dewhirst, M.W.; Dyck, J.R.B.; Nagendran, J.; Flores, C.T.; et al. Exercise Modulation of the Host-Tumor Interaction in an Orthotopic Model of Murine Prostate Cancer. J. Appl. Physiol. 2012, 113, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumacher, O.; Galvão, D.A.; Taaffe, D.R.; Chee, R.; Spry, N.; Newton, R.U. Exercise Modulation of Tumour Perfusion and Hypoxia to Improve Radiotherapy Response in Prostate Cancer. Prostate Cancer Prostatic Dis. 2021, 24, 1–14. [Google Scholar] [CrossRef]
- Brown, M.; Rébillard, A.; Hart, N.H.; O’Connor, D.; Prue, G.; O’Sullivan, J.M.; Jain, S. Modulating Tumour Hypoxia in Prostate Cancer Through Exercise: The Impact of Redox Signalling on Radiosensitivity. Sport. Med. Open 2022, 8, 48. [Google Scholar] [CrossRef]
- Xian, D.; Song, J.; Yang, L.; Xiong, X.; Lai, R.; Zhong, J. Emerging Roles of Redox-Mediated Angiogenesis and Oxidative Stress in Dermatoses. Oxidative Med. Cell. Longev. 2019, 2019, e2304018. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.W.; Fels, D.R.; West, M.; Allen, J.D.; Broadwater, G.; Barry, W.T.; Wilke, L.G.; Masko, E.; Douglas, P.S.; Dash, R.C.; et al. Modulation of Circulating Angiogenic Factors and Tumor Biology by Aerobic Training in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Cancer Prev. Res. 2013, 6, 925–937. [Google Scholar] [CrossRef] [Green Version]
- Van Doorslaer de Ten Ryen, S.; Deldicque, L. The Regulation of the Metastatic Cascade by Physical Activity: A Narrative Review. Cancers 2020, 12, 153. [Google Scholar] [CrossRef] [Green Version]
- Piguet, A.-C.; Saran, U.; Simillion, C.; Keller, I.; Terracciano, L.; Reeves, H.L.; Dufour, J.-F. Regular Exercise Decreases Liver Tumors Development in Hepatocyte-Specific PTEN-Deficient Mice Independently of Steatosis. J. Hepatol. 2015, 62, 1296–1303. [Google Scholar] [CrossRef] [Green Version]
- Saran, U.; Foti, M.; Dufour, J.-F. Cellular and Molecular Effects of the MTOR Inhibitor Everolimus. Clin. Sci. 2015, 129, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Saran, U.; Guarino, M.; Rodríguez, S.; Simillion, C.; Montani, M.; Foti, M.; Humar, B.; St-Pierre, M.V.; Dufour, J.-F. Anti-Tumoral Effects of Exercise on Hepatocellular Carcinoma Growth. Hepatol. Commun. 2018, 2, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, G.-E.; El Khoury, D. Exercise-Induced Irisin, the Fat Browning Myokine, as a Potential Anticancer Agent. J. Obes. 2019, 2019, e6561726. [Google Scholar] [CrossRef] [PubMed]
- Provatopoulou, X.; Georgiou, G.P.; Kalogera, E.; Kalles, V.; Matiatou, M.A.; Papapanagiotou, I.; Sagkriotis, A.; Zografos, G.C.; Gounaris, A. Serum Irisin Levels Are Lower in Patients with Breast Cancer: Association with Disease Diagnosis and Tumor Characteristics. BMC Cancer 2015, 15, 898. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin Suppresses the Migration, Proliferation, and Invasion of Lung Cancer Cells via Inhibition of Epithelial-to-Mesenchymal Transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, M.; Zhang, N.; Pan, H.; Lin, G.; Li, N.; Wang, L.; Yang, H.; Yan, K.; Gong, F. Serum and Adipose Tissue MRNA Levels of ATF3 and FNDC5/Irisin in Colorectal Cancer Patients With or Without Obesity. Front. Physiol. 2018, 9, 1125. [Google Scholar] [CrossRef]
- Liu, J.; Song, N.; Huang, Y.; Chen, Y. Irisin Inhibits Pancreatic Cancer Cell Growth via the AMPK-MTOR Pathway. Sci. Rep. 2018, 8, 15247. [Google Scholar] [CrossRef] [Green Version]
- Vulczak, A.; Souza, A.d.O.; Ferrari, G.D.; Azzolini, A.E.C.S.; Pereira-da-Silva, G.; Alberici, L.C. Moderate Exercise Modulates Tumor Metabolism of Triple-Negative Breast Cancer. Cells 2020, 9, 628. [Google Scholar] [CrossRef] [Green Version]
- Baghy, K.; Reszegi, A.; Tátrai, P.; Kovalszky, I. Decorin in the Tumor Microenvironment. In Tumor Microenvironment: Extracellular Matrix Components—Part B; Advances in Experimental Medicine and Biology; Birbrair, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–38. [Google Scholar] [CrossRef]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin as a Multivalent Therapeutic Agent against Cancer. Adv. Drug Deliv. Rev. 2016, 97, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ge, Y.; Cheng, Q.; Zhang, Q.; Fang, L.; Zheng, J. Decorin Is a Pivotal Effector in the Extracellular Matrix and Tumour Microenvironment. Oncotarget 2018, 9, 5480–5491. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, T.; Yoshio, S.; Sakamoto, Y.; Hashida, R.; Koya, S.; Hirota, K.; Nakano, D.; Yamamura, S.; Niizeki, T.; Matsuse, H.; et al. Impact of Decorin on the Physical Function and Prognosis of Patients with Hepatocellular Carcinoma. J. Clin. Med. 2020, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Calses, P.C.; Crawford, J.J.; Lill, J.R.; Dey, A. Hippo Pathway in Cancer: Aberrant Regulation and Therapeutic Opportunities. Trends Cancer 2019, 5, 297–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, S.; Wang, H.; Shi, Z.; Dong, A.; Zhang, W.; Song, X.; He, F.; Wang, Y.; Zhang, Z.; Wang, W.; et al. A Peptide Mimicking VGLL4 Function Acts as a YAP Antagonist Therapy against Gastric Cancer. Cancer Cell 2014, 25, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Fitamant, J.; Kottakis, F.; Benhamouche, S.; Tian, H.S.; Chuvin, N.; Parachoniak, C.A.; Nagle, J.M.; Perera, R.M.; Lapouge, M.; Deshpande, V.; et al. YAP Inhibition Restores Hepatocyte Differentiation in Advanced HCC, Leading to Tumor Regression. Cell Rep. 2015, 10, 1692–1707. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.-X.; Zhang, Y.; Park, H.W.; Jewell, J.L.; Chen, Q.; Deng, Y.; Pan, D.; Taylor, S.S.; Lai, Z.-C.; Guan, K.-L. Protein Kinase A Activates the Hippo Pathway to Modulate Cell Proliferation and Differentiation. Genes Dev. 2013, 27, 1223–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Judson, R.N.; Tremblay, A.M.; Knopp, P.; White, R.B.; Urcia, R.; De Bari, C.; Zammit, P.S.; Camargo, F.D.; Wackerhage, H. The Hippo Pathway Member Yap Plays a Key Role in Influencing Fate Decisions in Muscle Satellite Cells. J. Cell Sci. 2012, 125, 6009–6019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, K.I.; Judson, R.; Medlow, P.; Reid, K.; Kurth, T.B.; Burniston, J.G.; Ratkevicius, A.; Bari, C.D.; Wackerhage, H. Yap Is a Novel Regulator of C2C12 Myogenesis. Biochem. Biophys. Res. Commun. 2010, 393, 619–624. [Google Scholar] [CrossRef]
- Tremblay, A.M.; Missiaglia, E.; Galli, G.G.; Hettmer, S.; Urcia, R.; Carrara, M.; Judson, R.N.; Thway, K.; Nadal, G.; Selfe, J.L.; et al. The Hippo Transducer YAP1 Transforms Activated Satellite Cells and Is a Potent Effector of Embryonal Rhabdomyosarcoma Formation. Cancer Cell 2014, 26, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Qi, X.; McAnally, J.; Schwartz, R.J.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Regulation of Insulin-Like Growth Factor Signaling by Yap Governs Cardiomyocyte Proliferation and Embryonic Heart Size. Sci. Signal. 2011, 4, ra70. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, B.M.; Hamilton, D.L.; Tremblay, A.M.; Wackerhage, H. The Hippo Signal Transduction Network for Exercise Physiologists. J. Appl. Physiol. 2016, 120, 1105–1117. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Hansen, L.S.; Lillelund, C.; Andersen, C.; Gehl, J.; Christensen, J.F.; Pedersen, B.K.; Hojman, P. Exercise-Induced Catecholamines Activate the Hippo Tumor Suppressor Pathway to Reduce Risks of Breast Cancer Development. Cancer Res. 2017, 77, 4894–4904. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.W.; Lim, C.J.; Guo, K.; Ng, C.P.; Lee, I.; Hunziker, W.; Zeng, Q.; Hong, W. A Role for TAZ in Migration, Invasion, and Tumorigenesis of Breast Cancer Cells. Cancer Res. 2008, 68, 2592–2598. [Google Scholar] [CrossRef] [Green Version]
- Cordenonsi, M.; Zanconato, F.; Azzolin, L.; Forcato, M.; Rosato, A.; Frasson, C.; Inui, M.; Montagner, M.; Parenti, A.R.; Poletti, A.; et al. The Hippo Transducer TAZ Confers Cancer Stem Cell-Related Traits on Breast Cancer Cells. Cell 2011, 147, 759–772. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, B.; Liu, H.; Yang, K.; Thapa, S.; Zhang, H.; Li, L.; Yu, S. Development and Validation of a Clinically Applicable Score to Classify Cachexia Stages in Advanced Cancer Patients. J. Cachexia Sarcopenia Muscle 2018, 9, 306–314. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, Y.; Kobayashi, T.; Chayahara, N.; Imamura, Y.; Toyoda, M.; Kiyota, N.; Mukohara, T.; Nishiumi, S.; Azuma, T.; Yoshida, M.; et al. Metabolomics Evaluation of Serum Markers for Cachexia and Their Intra-Day Variation in Patients with Advanced Pancreatic Cancer. PLoS ONE 2014, 9, e113259. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.L.; Lee, D.E.; Rosa-Caldwell, M.E.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Huseman, K.; Sataranatarajan, K.; Van Remmen, H.; Washington, T.A.; et al. Protein Imbalance in the Development of Skeletal Muscle Wasting in Tumour-Bearing Mice. J. Cachexia Sarcopenia Muscle 2018, 9, 987–1002. [Google Scholar] [CrossRef] [Green Version]
- Prado, C.M.M. Body Composition in Chemotherapy: The Promising Role of CT Scans. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 525–533. [Google Scholar] [CrossRef]
- Vazeille, C.; Jouinot, A.; Durand, J.-P.; Neveux, N.; Boudou-Rouquette, P.; Huillard, O.; Alexandre, J.; Cynober, L.; Goldwasser, F. Relation between Hypermetabolism, Cachexia, and Survival in Cancer Patients: A Prospective Study in 390 Cancer Patients before Initiation of Anticancer Therapy. Am. J. Clin. Nutr. 2017, 105, 1139–1147. [Google Scholar] [CrossRef] [Green Version]
- Dolly, A.; Dumas, J.-F.; Servais, S. Cancer Cachexia and Skeletal Muscle Atrophy in Clinical Studies: What Do We Really Know? J. Cachexia Sarcopenia Muscle 2020, 11, 1413–1428. [Google Scholar] [CrossRef]
- Singh, B.; Zopf, E.M.; Howden, E.J. Effect and Feasibility of Wearable Physical Activity Trackers and Pedometers for Increasing Physical Activity and Improving Health Outcomes in Cancer Survivors: A Systematic Review and Meta-Analysis. J. Sport Health Sci. 2022, 11, 184–193. [Google Scholar] [CrossRef]
- Brickwood, K.-J.; Watson, G.; O’Brien, J.; Williams, A.D. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2019, 7, e11819. [Google Scholar] [CrossRef]
- Sezgin, M.G.; Bektas, H. Effect of Peer Mentoring on Physical Activity in Patients with Cancer: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Clin. Nurs. 2022, in press. [Google Scholar] [CrossRef]
- Ngo-Huang, A.; Parker, N.H.; Bruera, E.; Lee, R.E.; Simpson, R.; O’Connor, D.P.; Petzel, M.Q.B.; Fontillas, R.C.; Schadler, K.; Xiao, L.; et al. Home-Based Exercise Prehabilitation During Preoperative Treatment for Pancreatic Cancer Is Associated With Improvement in Physical Function and Quality of Life. Integr. Cancer Ther. 2019, 18, 1534735419894061. [Google Scholar] [CrossRef]
- Gustafson, M.P.; Wheatley-Guy, C.M.; Rosenthal, A.C.; Gastineau, D.A.; Katsanis, E.; Johnson, B.D.; Simpson, R.J. Exercise and the Immune System: Taking Steps to Improve Responses to Cancer Immunotherapy. J. Immunother. Cancer 2021, 9, e001872. [Google Scholar] [CrossRef]
- Martín Ruiz, A.; Fiuza Luces, M.d.C.; Rincón Castanedo, C.; Fernández Moreno, D.; González Gálvez, B.; Martínez Martínez, E.; Martín Acosta, P.; Coronado, M.J.; Franco Luzón, L.; Lucía Mulas, A.; et al. Benefits of Exercise and Immunotherapy in a Murine Model of Human Non-Small-Cell Lung Carcinoma. Exerc. Immunother. 2020, 26, 100–115. [Google Scholar]
- Bay, M.L.; Unterrainer, N.; Stagaard, R.; Pedersen, K.S.; Schauer, T.; Staffeldt, M.M.; Christensen, J.F.; Hojman, P.; Pedersen, B.K.; Gehl, J. Voluntary Wheel Running Can Lead to Modulation of Immune Checkpoint Molecule Expression. Acta Oncol. 2020, 59, 1447–1454. [Google Scholar] [CrossRef]
- Lacey, J.; Lomax, A.J.; McNeil, C.; Marthick, M.; Levy, D.; Kao, S.; Nielsen, T.; Dhillon, H.M. A Supportive Care Intervention for People with Metastatic Melanoma Being Treated with Immunotherapy: A Pilot Study Assessing Feasibility, Perceived Benefit, and Acceptability. Support. Care Cancer 2019, 27, 1497–1507. [Google Scholar] [CrossRef]
- Gouez, M.; Pérol, O.; Pérol, M.; Caux, C.; Ménétrier-Caux, C.; Villard, M.; Walzer, T.; Delrieu, L.; Saintigny, P.; Marijnen, P.; et al. Effect of Acute Aerobic Exercise before Immunotherapy and Chemotherapy Infusion in Patients with Metastatic Non-Small-Cell Lung Cancer: Protocol for the ERICA Feasibility Trial. BMJ Open 2022, 12, e056819. [Google Scholar] [CrossRef]
- Shim, Y.J.; Kim, H.J.; Oh, S.C.; Lee, S.I.; Choi, S.W. Exercise during Adjuvant Treatment for Colorectal Cancer: Treatment Completion, Treatment-Related Toxicities, Body Composition, and Serum Level of Adipokines. Cancer Manag. Res. 2019, 11, 5403–5412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleckner, I.R.; Dunne, R.F.; Asare, M.; Cole, C.; Fleming, F.; Fung, C.; Lin, P.-J.; Mustian, K.M. Exercise for Toxicity Management in Cancer—A Narrative Review. Oncol. Hematol. Rev. 2018, 14, 28–37. [Google Scholar] [CrossRef]
- DiFrancesco, T.; Khanna, A.; Stubblefield, M.D. Clinical Evaluation and Management of Cancer Survivors with Radiation Fibrosis Syndrome. Semin. Oncol. Nurs. 2020, 36, 150982. [Google Scholar] [CrossRef]
- Alves, M.J.; Figuerêdo, R.G.; Azevedo, F.F.; Cavallaro, D.A.; Neto, N.I.P.; Lima, J.D.C.; Matos-Neto, E.; Radloff, K.; Riccardi, D.M.; Camargo, R.G.; et al. Adipose Tissue Fibrosis in Human Cancer Cachexia: The Role of TGFβ Pathway. BMC Cancer 2017, 17, 190. [Google Scholar] [CrossRef] [Green Version]
- Narsale, A.A.; Carson, J.A. Role of IL-6 In Cachexia—Therapeutic Implications. Curr. Opin. Support. Palliat. Care 2014, 8, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Barbe, M.F.; Hilliard, B.A.; Amin, M.; Harris, M.Y.; Hobson, L.J.; Cruz, G.E.; Popoff, S.N. Blocking CTGF/CCN2 Reduces Established Skeletal Muscle Fibrosis in a Rat Model of Overuse Injury. FASEB J. 2020, 34, 6554–6569. [Google Scholar] [CrossRef] [Green Version]
- Moustafa, M.; Chiblak, S.; Lipson, K.; Wei, Q.; Brons, S.; Haberer, T.; Weichert, W.; Debus, J.; Abdollahi, A. Combined Inhibition of CTGF-Signaling and Radiation Therapy in Pancreatic Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, S175. [Google Scholar] [CrossRef]
- Branch, J.D. Effect of Creatine Supplementation on Body Composition and Performance: A Meta-Analysis. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 198–226. [Google Scholar] [CrossRef] [PubMed]
- Chilibeck, P.D.; Kaviani, M.; Candow, D.G.; Zello, G.A. Effect of Creatine Supplementation during Resistance Training on Lean Tissue Mass and Muscular Strength in Older Adults: A Meta-Analysis. Open Access J. Sport. Med. 2017, 8, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Penna, F.; Busquets, S.; Toledo, M.; Pin, F.; Massa, D.; López-Soriano, F.J.; Costelli, P.; Argilés, J.M. Erythropoietin Administration Partially Prevents Adipose Tissue Loss in Experimental Cancer Cachexia Models. J. Lipid Res. 2013, 54, 3045–3051. [Google Scholar] [CrossRef]






| Term | Definition |
|---|---|
| Adapted physical activity (APA) | The exercise intervention structured and supervised by a professional must be individualized for each patient, according to their preferences, disease, treatments, symptoms, in order to be feasible and safe for the patient. |
| Resistance training | Exercises training muscles against an external force, usually shorter than endurance training. |
| Endurance training | Repeated isotonic exercises that last in time to improve aerobic capacity [10]. |
| MET | Metabolic equivalent used to quantify the induced energy expenditure during exercise, means of a standardized program derived from the Compendium of Physical Activities, unit: minutes/week [10]. |
| Light physical activity | Less than 3 METs: activities resulting in little or no increase in breathing or heart rate |
| Vigorous physical activity | More than 6 METs: moderate to large increases in breathing and heart rate [10]. |
| Sedentary behavior | “Any waking behavior characterized by an energy expenditure less than or equal to 1.5 METs, while in a sitting, reclining or lying posture” [11]. |
| Author | Cancer’s Type | Age | Number of Patients in Exercise Group | Number of Patients in Control Group | Description of Intervention | Significant Improvement with Exercise | Non-Significant Improvement with Exercise | Dropout Rate |
|---|---|---|---|---|---|---|---|---|
| Cheville et al., 2013 [70] | Lung and colorectal cancer; Stage IV | 63.8 ± 12.5 | 33 | 33 | Eight-wk homebased resistance exercise and walking exercise | Ambulatory Post-Acute Care Daily Mobility Short Form (p = 0.02) Fatigue (p = 0.03) Sleep (p = 0.05) | Ambulatory Post-Acute Care Daily Activities Short Form HRQoL | 15% |
| Cormie et al., 2013 [71] | Prostate cancer; secondary bone metastases | 73.1 ± 7.5 | 10 | 10 | Twelve-wk sup low-level aerobic exercise and resistance exercise, targeting major muscle groups | Physical function (p = 0.016) 400-m Walk (p = 0.010) Body lean mass (p = 0.026) Lean mass (p = 0.003) | Fatigue HRQoL | 25% |
| Galvao et al., 2018 [72] | Prostate cancer; bone metastases | 69.7 ± 7.6 | 28 | 29 | Twelve-wk sup, combined aerobic exercise, resistance exercis, and flexibility exercise | Physical Function (p = 0.03) Leg Extension (p = 0.03) | Four hundred m Walk Test Up and Go Test Lean Mass Body Fat Mass Fatigue | 14% |
| Henke et al., 2013 [73] | Lung cancer Stage IIIA/IIIB/IV | NA | 25 | 21 | Three chemotherapy cycles long Combined aerobic exercise and resistance exercise | Staircase Walking (p = 0.05) Physical Functioning (p = 0.02) Cognitive Functioning (p = 0.05) | HRQoL Emotional Functioning Symptom | 34% |
| Neuzillet et al., 2022 [83] | Advanced pancreatic cancer | 64 | 157 | 156 | Sixteen-week APA program | Global Health Status, Physical Functioning, Cognitive Functioning, Social Functioning, Appetite Loss | Insomnia, Financial Difficulties, Constipation | 17% |
| Oldervoll et al., 2011 [74] | Incurable, metastatic cancer and life expectancy of 3–24 months: gastrointestinal, breast, lung, urological | 62.6 ± 11.3 | 121 | 110 | Eight-wk sup, combined exercise and aerobic exercise | Shuttle Walk test (p = 0.008) Handgrip Strength (p = 0.01) | Total Fatigue Physical Fatigue Mental Fatigue Sit-to-Stand | 29.4% |
| Pyszora et al., 2017 [75] | Advanced cancer patients, admitted to palliative care: urogenital, lung, hematological, digestive cancer | 72.4 ± 9.5 | 30 | 30 | Two-wk physiotherapeutic exercise | Fatigue Severity (p < 0.01) | Depression Anxiety | NA |
| Rief et al., 2014 [76] | Cancer patients with metastatic progress: lung, prostate, breast, renal, melanoma | 61.3 ± 10.1 | 30 | 30 | Two-wk sup isometric resistance exercise followed by 12-wk home-based training | Thirty s Sit-to-Stand (p < 0.001), HRQoL (p = 0.01) Fatigue (p = 0.01) Pain (p = 0.003) | Functional Interference Emotional Interference Cognitive Interference Overall Survival Progression-Free Survival | 20% |
| Tsianakas et al., 2017 [78] | Recurrent advancing or metastatic cancer: prostate, gynecological, hematologycal, breast, colorectal | 65 ± 11.7 | 21 | 21 | Twelve-wk walking intervention | none | HRQoL Global Fatigue Score | 35% |
| Zhou et al., 2017 [77] | Advanced nasopharyngeal cancer stage III/IV | NA | 57 | 57 | Tai Chi exercise (24-form Yang style) 5 h per week | Fatigue (p < 0.05) General Fatigue (p < 0.05) Physical Fatigue (p < 0.05) Emotional Fatigue (p < 0.05) | Mental Fatigue | 27% |
| Zimmer et al., 2018 [79] | Metastasized colorectal cancer | 68.5 | 17 | 13 | Eight-wk sup exercise, combining endurance, resistance exercise, and balance exercise | Muscle Strength (p = 0.002) | Physical Well-Being Functional Well-Being Social Well-Being Emotional Well-Being HRQoL | 20% |
| Buss et al., 2009 [80] | Advanced cancer patients; short lifetime expectancy | NA | 38 | 19 | Four-wk sup, individualized kinesiotherapy | Fatigue (p < 0.001) Diminution Intensification Physical Symptoms (p < 0.05) | QoL | 24.5% |
| Zhao et al., 2015 [81] | Head and neck squamous cell cancer, Stage III and IV | 57 ± 7 | 11 | 9 | Fourteen-wk resistance exercise and walking exercise | Vitality/Fatigue (p < 0.05) Mental Well-Being (p < 0.05) Strength Knee Extension (p < 0.05) Mental Well-Being (p < 0.05) | HRQoL BMI Lean Body Mass Physical Activity | 15% |
| Zhou et al., 2017 [82] | Ovarian cancer stage III and IV | 57.3 | 74 | 70 | One hundred and fifty minutes per week of moderate-intensity exercise | Attention Control (p = 0.02), Social (p = 0.02), General Health (p = 0.004) | Physical Functioning | 13.4% |
| APA Characteristics | Specificity to Include | Tools |
|---|---|---|
| Progressive | Tolerance | A facilitator, regular assessments |
| No harmful effects | phase of learning, defining a training load | |
| Regular | Autonomy | Supervision |
| Commitment | motivational interview, playful exercises | |
| Patient-specific | Type of cancer | medical history |
| Stage of the disease | initial assessment | |
| Treatment | medical history | |
| Side effects of the treatment | initial assessment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torregrosa, C.; Chorin, F.; Beltran, E.E.M.; Neuzillet, C.; Cardot-Ruffino, V. Physical Activity as the Best Supportive Care in Cancer: The Clinician’s and the Researcher’s Perspectives. Cancers 2022, 14, 5402. https://doi.org/10.3390/cancers14215402
Torregrosa C, Chorin F, Beltran EEM, Neuzillet C, Cardot-Ruffino V. Physical Activity as the Best Supportive Care in Cancer: The Clinician’s and the Researcher’s Perspectives. Cancers. 2022; 14(21):5402. https://doi.org/10.3390/cancers14215402
Chicago/Turabian StyleTorregrosa, Cécile, Frédéric Chorin, Eva Ester Molina Beltran, Cindy Neuzillet, and Victoire Cardot-Ruffino. 2022. "Physical Activity as the Best Supportive Care in Cancer: The Clinician’s and the Researcher’s Perspectives" Cancers 14, no. 21: 5402. https://doi.org/10.3390/cancers14215402