High Resolution Cell Positioning Based on a Flow Reduction Mechanism for Enhancing Deformability Mapping
Abstract
:1. Introduction
2. Flow Reduction Mechanism
2.1. Under Incompressibility of the Microfluidic Chip
2.2. Under Compressibility of the Microfluidic Chip
2.3. Analysis of the Flow Reduction Mechanism
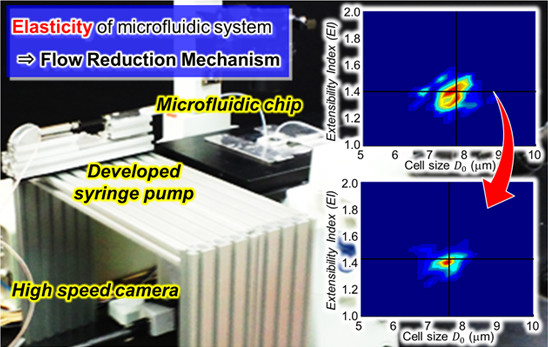
3. Experiments
3.1. Experimental System
3.2. Fabrication Process of the Microfluidic Chip
- Spin-coated SU-8 (KAYAKU, Co., Ltd. Tokyo, Japana) is patterned by laser lithography.
- The fabricated pattern is transcribed to PDMS (SILPOT 184 W/C, DOW CORNING TORAY, Co., Ltd. Tokyo, Japan). The thickness of the molded PDMS is 5 mm.
- The molded and punched PDMS is bonded with the glass substrate by heating after O2 plasma treatment. The size of the inlet and outlet hole is 1.5 mm.
3.3. Sample Preparation
- The microchannel is filled with standard saline solution.
- The blood is diluted by saline with a density of 2%.
- The blood-saline mixture is injected into the microchannel from the sample inlet.
3.4. Without Visual Feedback Control
3.5. With Visual Feedback Control
4. Application Example
4.1. Cell Extensibility
4.2. Extensibility Map
5. Discussions
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, Y.; Wan, K.T.; Roberts, K.P.; Bischof, J.C.; Nelson, B.J. Mechanical property characterization of mouse zona pellucida. IEEE Trans. NanoBiosci. 2003, 2, 279–286. [Google Scholar]
- Bremmell, K.E.; Evans, A.; Prestidge, C.A. Deformation and nano-rheology of red blood cells: An AFM investigation. Colloids Surf. B Biointerfaces 2006, 50, 43–48. [Google Scholar]
- Sakuma, S.; Arai, F. Cellular force measurement using a nanometric-probe-integrated microfluidic chip with a displacement reduction mechanism. J. Robot. Mechatron. 2013, 25, 277–284. [Google Scholar]
- Shelby, J.P.; White, J.; Ganesan, K.; Rathod, P.K.; Chiu, D.T. A microfluidic model for single-cell capillary obstruction by plasmodium-falciparuminfected erythrocytes. Proc. Natl. Acad. Sci. USA 2003, 100, 14618–14622. [Google Scholar]
- Hirose, Y.; Tadakuma, K.; Higashimori, M.; Arai, T.; Kaneko, M.; Iitsuka, R.; Yamanishi, Y.; Arai, F. A new stiffness evaluation toward high speed cell sorter, Proceedings of the IEEE Internatinal Conference on Robotics and Automation, Anchorage, AK, USA, 3–7 May 2010; pp. 4113–4118.
- Adamo, A.; Sharei, A.; Adamo, L.; Lee, B.; Mao, S.; Jensen, K.F. Microfluidics-based assessment of cell deformability. Anal. Chem. 2012, 84, 6438–6443. [Google Scholar]
- Zheng, Y.; Shojaei-Baghini, E.; Azad, A.; Wang, C.; Sun, Y. High-throughput biophysical measurement of human red blood cells. Lab Chip 2012, 12, 2560–2567. [Google Scholar]
- Fukui, W.; Kaneko, M.; Sakuma, S.; Kawahara, T.; Arai, F. μ-Cell fatigue test, Proceedings of the IEEE Internatinal Conference on Robotics and Automation, Saint Paul, MN, USA, 14–18 May 2012; pp. 4600–4605.
- Sakuma, S.; Kuroda, K.; Tsai, C.H.D.; Fukui, W.; Arai, F.; Kaneko, M. Red blood cell fatigue evaluation based on the close-encountering point between extensibility and recoverability. Lab Chip 2014, 14, 1135–1141. [Google Scholar]
- Pamme, N.; Wilhelm, C. Magnetism and microfluidics. Lab Chip 2006, 6, 24–38. [Google Scholar]
- Maruo, S.; Inoue, H. Optically driven micropump produced by three-dimensional two-photon microfabrication. Appl. Phys. Lett. 2006, 89, 144101. [Google Scholar]
- Dao, M.; Lim, C.T.; Suresh, S. Mechanics of the human red blood cell deformed by optical tweezers. J. Mech. Phys. Solids. 2003, 51, 2259–2280. [Google Scholar]
- Lintel, H.T.G.V.; Pol, F.C.M.V.D.; Bouwstra, S. A piezoelectric micropump based on micromachining of silicon. Sens. Actuators. 1988, 15, 153–167. [Google Scholar]
- Krüger, J.; Singh, K.; O’Neill, A.; Jackson, C.; Morrison, A.; O’Brien, P. Development of a microfluidic device for fluorescence activated cell sorting. J. Micromech. Microeng. 2002, 12, 486–494. [Google Scholar]
- Chung, B.G.; Flanagan, L.A.; Rhee, S.W.; Schwartz, P.H.; Lee, A.P.; Monuki, E.S.; Jeon, N.L. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 2005, 5, 401–406. [Google Scholar]
- Mahalanabis, M.; Muayad, H.A.; Kulinski, M.D.; Altman, D.; Klapperich, C.M. Cell lysis and DNA extraction of gram-positive and gram-negative bacteria from whole blood in a disposable microfluidic chip. Lab Chip 2009, 9, 2811–2817. [Google Scholar]
- Ichikawa, A.; Tanikawa, T.; Akagi, S.; Ohba, K. Automatic cell cutting by high-precision microfluidic control. J. Robot. Mechatron. 2011, 23, 13–18. [Google Scholar]
- Gomez-Sjoberg, R.; Leyrat, A.A.; Pirone, D.M.; Chen, C.S.; Quake, S.R. Versatile, fully automated, microfluidic cell culture system. Anal. Chem. 2007, 79, 8557–8563. [Google Scholar]
- Tai, C.H.; Hsiung, S.K.; Chen, C.Y.; Tsai, M.L.; Lee, G.B. Biomed. Microdevices 2007, 9, 533–543.
- Tsukada, K.; Sekizuka, E.; Oshio, C.; Minamitani, H. Direct measurement of erythrocyte deformability in diabetes mellitus with a transparent microchannel capillary model and high-speed video camera system. Microvasc. Res. 2001, 61, 231–239. [Google Scholar]
- Lee, S.S.; Yim, Y.; Ahn, K.H.; Lee, S.J. Extensional flow-based assessment of red blood cell deformability using hyperbolic converging microchannel. Biomed. Microdevices 2009, 11, 37–43. [Google Scholar]
- Braunmuller, S.; Schmid, L.; Sackmann, E.; Franke, T. Hydrodynamic deformation reveals two coupled modes/time scales of red blood cell relaxation. Soft Matter 2012, 8, 11240–11248. [Google Scholar]








© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakuma, S.; Kuroda, K.; Arai, F.; Taniguchi, T.; Ohtani, T.; Sakata, Y.; Kaneko, M. High Resolution Cell Positioning Based on a Flow Reduction Mechanism for Enhancing Deformability Mapping. Micromachines 2014, 5, 1188-1201. https://doi.org/10.3390/mi5041188
Sakuma S, Kuroda K, Arai F, Taniguchi T, Ohtani T, Sakata Y, Kaneko M. High Resolution Cell Positioning Based on a Flow Reduction Mechanism for Enhancing Deformability Mapping. Micromachines. 2014; 5(4):1188-1201. https://doi.org/10.3390/mi5041188
Chicago/Turabian StyleSakuma, Shinya, Keisuke Kuroda, Fumihito Arai, Tatsunori Taniguchi, Tomohito Ohtani, Yasushi Sakata, and Makoto Kaneko. 2014. "High Resolution Cell Positioning Based on a Flow Reduction Mechanism for Enhancing Deformability Mapping" Micromachines 5, no. 4: 1188-1201. https://doi.org/10.3390/mi5041188