Bee Products and Colorectal Cancer—Active Components and Mechanism of Action
Abstract
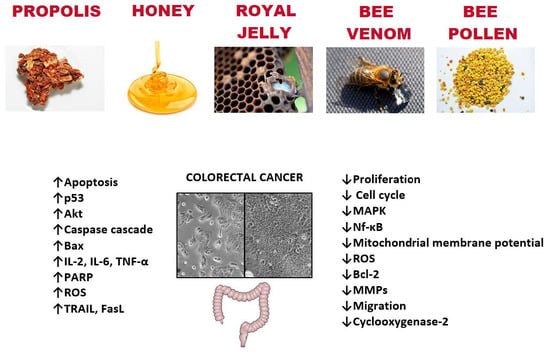
:1. Introduction
2. Bee Products—Anti-Colon Cancer Potential
2.1. Colon Cancer—Epidemiology and Risk Factors
2.2. Propolis
Anti-Colon Cancer Potential of Bee Propolis
| Compounds | Type of Cancer | Type of Study | Activity | References |
|---|---|---|---|---|
| Pinocembrin galagin luteolin | Colon cancer | In vitro/HTC-116 | ↑ cytotoxic activity | |
| ↑ apoptosis | Vukovic et al. 2018 [73] | |||
| ↓ superoxide anion radical ↓ nitrites | ||||
| CAPE | Colon cancer/ Gastric adenocarcinoma | In vitro/HTC-116, HT-29, AGS, SW480, CT26 In vivo/male Wistar rats | ↑ cytotoxic activity ↑ genotoxic activity ↑ caspase-3/7 ↓ ROS ↑ G1 phase ↓ cyclin D1, c-myc ↓ beta-catenin/T-cell factor ↓ formation of (ACF) and tumors | Gajek et al., 2020 Xiang et al., 2006 Fraser et al., 2016 Liao et al., 2003 Borrelli et. al., 2002 [74,75,76,77,78] |
| CAPE-pNO2 | Colon cancer | In vitro/HT-29 In vivo/Male BALB/c nude mice | ↑ p53 ↑ caspase-3 ↑ Bax ↑ P38 ↑ CytoC ↑ P21Cip1 ↑ P27Kip1 ↓ CDK2, c-Myc ↑ G0/G1 phase ↑ inhibition of tumor growth ↓ VEGF | Tang et al., 2017 [79] |
| Galangin | Colon cancer | In vitro/HCT-15, HT-29 | ↑ cytotoxic activity ↑ caspase 3, 9 ↑ DNA condensation ↓ mitochondrial membrane potential | Ha et al., 2013 [80] |
| Artepilin C Baccharin Drupanin | Colon cancer | In vitro/DLD-1 | ↑ TRAIL, FasL ↑ miR-143 ↓ MAPK/Erk5 ↓ c-Myc | Kumazaki et al., 2014 [81] |
| Artepilin C | Colon cancer, Liver hepatoblastoma | In vitro/ Caco-2 HepG2 | ↑ G0/G1 phase ↓ cyclin D/cyclin-dependent kinase 4 ↑ Cip1/p21, Kip1/p27 | Shimizu et al., 2005 [82] |
| Mucronulatol | Colon carcinoma | In vitro/HCT8 | ↑ sub-G1 phase ↑ Cip1/p21, Kip1/p27 ↓ cyclin E, CDK4 | Diaz-Carballo et al., 2008 [83] |
| Plukenetione A | Colon cancer wild-type, -FU-resistan, SN38-resistant Oleocecal carcinoma wild-type, SN38-resistant, Raltitrexed-resistant | In vitro/HT29 WT, HT29 24R, HT29 SN3, HCT8 WT, HCT8 SN38, HCT8 ICID | ↑ G0/G1 phase ↑ DNA fragmentation ↓ expression of topoisomerase II-beta, ↓ EGF receptor | Diaz-Carballo et al., 2008 [84] |
| Chrysin | Colon cancer | In vitro/ SW48, SW480, SW620, HT-29, HCT-116 | ↓ viability ↑ LC3-II autophagy marker ↑ ROS ↓ protein kinase B(Akt) ↓ rapamycin (mTOR) | Lin et al., 2018 [85] |
2.3. Bee Honey
Anti-Colon Cancer Activity of Bee Honey
| Compounds | Type of Cancer | Type of Study | Activity | References |
|---|---|---|---|---|
| Eugenol | Ehrlich ascites carcinoma | In vivo/ BALB/c mice In vitro/HTC-15, HT-29 | ↑ %Tumor growth inhibition ↑ Sub G1 phase ↑ ROS ↑ DNA fragmentation ↓ MMPs ↑ p53 ↑ PARP ↑ caspase 3 | Jaganathan, 2010 Jaganathan et al., 2011 [107,110] |
| 5-Hydroxymethyl-2-furfural | Colon cancer Breast cancer | In vitro/ HT29, MDA in silico | Block Aquaporin-1 ↓ migration | Chow et al., 2020 [111] |
| Caffeic acid | Colon cancer | In vitro/HCT 15 | ↓ proliferation ↑ sub G1 phase ↓ colony formation ↑ROS ↓ mitochondrial membrane potential | Jaganathan, 2012 [112] |
| p-coumaric acid | Colon cancer | In vitro/HCT 15, HT-29 | ↓ proliferation ↑ sub G1 phase ↓ colony formation ↑ ROS ↓ mitochondrial membrane potential | Jaganathan, 2013 [113] |
| Polysaccharides isolated from Alhagi honey | --- | In vivo/ICR mice treatment cyclophosphamide (chemotherapeutic in colon cancer) | ↑ Peyer’s patch count ↑ IL-2, IL-6, TNF-α ↑ SOD ↑ β-defensin ↓ MDA, DAO ↑ p-ERK expression ↓ p-JNK, p-p38 | Cai et al., 2021 [114] |
| 3′-Hydroksypterostilben | ---- | In vivo/ICR mice/azoxymethane (AOM)/dextran sodium sulfate (DSS) model | ↓ number of tumors in AOM/DSS-treated mice ↓ nitric oxide synthase ↓ cyclooxygenase-2, ↓ IL-6 | Lai et al., 2017 [115] |
2.4. Bee Pollen
2.5. Royal Jelly
2.6. Bee Venom
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Seedi, H.R.; Khalifa, S.A.M.; Abd El-Wahed, A.A.; Gao, R.; Guo, Z.; Tahir, H.E.; Zhao, C.; Du, M.; Farag, M.A.; Musharraf, S.G.; et al. Honeybee products: An updated review of neurological actions. Trends Food Sci. Technol. 2020, 101, 17–27. [Google Scholar] [CrossRef]
- Karou, D.; Nadembega, W.M.C.; Ouattara, L.; Ilboudo, D.P.; Canini, A.; Nikiéma, J.B.; Simpore, J.; Colizzi, V.; Traore, A.S. African ethnopharmacology and new drug discovery. Med. Aromat. Plant Sci. Biotechnol. 2007, 1, 61–69. [Google Scholar]
- Afrin, S.; Haneefa, S.M.; Fernandez-Cabezudo, M.J.; Giampieri, F.; Al-Ramadi, B.K.; Battino, M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: An evidence-based review. Nutr. Res. Rev. 2020, 33, 50–76. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey bee products: Preclinical and clinical studies of their anti-inflammatory and immunomodulatory properties. Front Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Naggar, Y.A.; Algethami, A.F.; Shou, Q.; Alsharif, S.M.; et al. Bee pollen: Clinical trials and patent applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical prospects of bee products: Special focus on anticancer, antibacterial, antiviral, and antiparasitic properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef]
- Münstedt, K.; Männle, H. Bee products and their role in cancer prevention and treatment. Complement. Ther. Med. 2020, 51, 102390. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee collected pollen and bee bread: Bioactive constituents and health benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Prakash, S.; Lorenzo, J.M.; Chandran, D.; Dhumal, S.; Dey, A.; Senapathy, M.; Rais, N.; Singh, S.; Kalkreuter, P.; et al. Apitherapy and Periodontal Disease: Insights into In Vitro, In Vivo, and Clinical Studies. Antioxidants 2022, 11, 823. [Google Scholar] [CrossRef]
- Jull, A.B.; Cullum, N.; Dumville, J.C.; Westby, M.J.; Deshpande, S.; Walker, N. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2015, 2015, CD005083. [Google Scholar] [CrossRef] [Green Version]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Mittelman, S.D. The Role of Diet in Cancer Prevention and Chemotherapy Efficacy. Annu. Rev. Nutr. 2020, 40, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Wild, C.P. Global Cancer Patterns: Causes and Prevention. Lancet 2014, 383, 549–557. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Münstedt, K.; Männle, H. Using bee products for the prevention and treatment of oral mucositis induced by cancer treatment. Molecules 2019, 24, 3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keene, M.R.; Heslop, I.M.; Sabesan, S.S.; Glass, B.D. Complementary and alternative medicine use in cancer: A systematic review. Complement. Ther. Clin. Pract. 2019, 35, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Levine, J.S.; Ahnen, D.J. Clinical practice. Adenomatous polyps of the colon. N. Engl. J. Med. 2006, 355, 2551–2557. [Google Scholar] [CrossRef] [Green Version]
- Risio, M. The natural history of adenomas. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Remo, A.; Fassan, M.; Vanoli, A.; Bonetti, L.R.; Barresi, V.; Tatangelo, F.; Gafà, R.; Giordano, G.; Pancione, M.; Grillo, F.; et al. Morphology and molecular features of rare colorectal carcinoma histotypes. Cancers 2019, 11, 1036. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277. [Google Scholar] [CrossRef] [PubMed]
- Sninsky, J.A.; Shore, B.M.; Lupu, G.V.; Crockett, S.D. Risk Factors for Colorectal Polyps and Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Ortega, L.S.; Bradbury, K.E.; Cross, A.J.; Morris, J.S.; Gunter, M.J.; Murphy, N.A. Prospective Investigation of Body Size, Body Fat Composition and Colorectal Cancer Risk in the UK Biobank. Sci. Rep. 2017, 7, 17807. [Google Scholar] [CrossRef] [Green Version]
- Xue, K.; Li, F.F.; Chen, Y.W.; Zhou, Y.H.; He, J. Body mass index and the risk of cancer in women compared with men: A meta-analysis of prospective cohort studies. Eur. J. Cancer Prev. 2017, 26, 94–105. [Google Scholar] [CrossRef]
- Zeng, H.; Lazarova, D.L. Obesity-related colon cancer: Dietary factors and their mechanisms of anticancer action. Clin. Exp. Pharmacol. Physiol. 2012, 39, 161–167. [Google Scholar] [CrossRef]
- Adams, T.D.; Gress, R.E.; Smith, S.C.; Chad Halverson, R.; Simper, S.C.; Rosamond, W.D.; Lamonte, M.J.; Stroup, A.M.; Hunt, S.C. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 2007, 357, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer-Viewpoint of the IARC Working Group. International Agency for Research on Cancer Handbook Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [Green Version]
- Bailly, L.; Fabre, R.; Pradier, C.; Iannelli, A. Colorectal Cancer Risk Following Bariatric Surgery in a Nationwide Study of French Individuals With Obesity. JAMA Surg. 2020, 155, 395. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N.; Wolk, A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1679. [Google Scholar] [CrossRef] [PubMed]
- Kanadiya, M.K.; Gohel, T.D.; Sanaka, M.R.; Thota, P.N.; Shubrook, J.H. Relationshipbetween type-2 diabetes and use of metformin with risk of colorectal adenoma in an American population receiving colonoscopy. J. Diabetes Complicat. 2013, 27, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Eddi, R.; Karki, A.; Shah, A.; DeBari, V.A.; DePasquale, J.R. Association of type 2 diabetes and colon adenomas. J. Gastrointest. Cancer 2012, 43, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Pretlow, T.P.; Schoen, R.E. Aberrant crypt foci: What we knowand what we need to know. Clin. Gastroenterol. Hepatol. 2007, 5, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, M.; Meier, C.; Krahenbuhl, S.; Jick, S.S.; Meier, C.R. Metformin, sulfonylureas, or other antidiabetes drugs and the risk of lactic acidosis or hypoglycemia: A nested case-control analysis. Diabetes Care 2008, 31, 2086–2091. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [Green Version]
- Durko, L.; Malecka-Panas, E. Lifestyle modifications and colorectal cancer. Curr. Color. Cancer Rep. 2014, 10, 45–54. [Google Scholar] [CrossRef] [Green Version]
- McNabb, S.; Harrison, T.A.; Albanes, D.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Cambell, P.T.; Cao, Y.; Chang-Claude, J.; Chan, A.; et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. Int. J. Cancer 2020, 146, 861–873. [Google Scholar] [CrossRef]
- Botteri, E.; Borroni, E.; Sloan, E.K.; Bagnardi, V.; Bosetti, C.; Peveri, G.; Santucci, C.; Specchia, C.; van den Brandt, P.; Gallus, S.; et al. Smoking and Colorectal Cancer Risk, Overall and by Molecular Subtypes: A Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 1940–1949. [Google Scholar] [CrossRef]
- Citronberg, J.; Bostick, R.; Ahearn, T.; Turgeon, D.K.; Ruffin, M.T.; Djuric, Z.; Sen, A.; Brenner, D.E.; Zick, S.M. Effects of ginger supplementation on cell-cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer: Results from a pilot, randomized, and controlled trial. Cancer Prev. Res. (Phila). 2013, 6, 271–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahir, A.A.; Sani, N.F.; Murad, N.A.; Makpol, S.; Ngah, W.Z.; Yusof, Y.A. Combined ginger extract and Gelam Honey Modulate Ras/ERKand P13K/AKT pathway genes in colon cancer HT29 cells. Nutr. J. 2005, 14, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groot, A.C. Propolis: A review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis 2013, 24, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, D.; Car, H.; Borawska, M.H.; Nikliński, J. The anticancer activity of propolis. Folia Histochem. Cytobiol. 2012, 50, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zhangm, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [Green Version]
- Curti, V.; Zaccaria, V.; Tsetegho Sokeng, A.J.; Dacrema, M.; Masiello, I.; Mascaro, A.; D’Antona, G.; Daglia, M. Bioavailability and in vivo antioxidant activity of a standardized polyphenol mixture extracted from brown propolis. Int. J. Mol. Sci. 2019, 20, 1250. [Google Scholar] [CrossRef] [Green Version]
- Sayre, C.L.; Alrushaid, S.; Martinez, S.E.; Anderson, H.D.; Davies, N.M. Pre-clinical pharmacokinetic and pharmacodynamic characterization of selected chiral flavonoids: Pinocembrin and pinostrobin. J. Pharm. Pharm. Sci. 2015, 18, 368–395. [Google Scholar] [CrossRef] [Green Version]
- Moskwa, J.; Naliwajko, S.K.; Markiewicz-Żukowska, R.; Gromkowska-Kępka, K.J.; Nowakowski, P.; Strawa, J.W.; Borawska, M.H.; Tomczyk, M.; Socha, K. Chemical composition of Polish propolis and its antiproliferative effect in combination with Bacopa monnieri on glioblastoma cell lines. Sci. Rep. 2020, 10, 21127. [Google Scholar] [CrossRef]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its role and efficacy in human health and diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef]
- Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Bielec, B.; Wojtyczka, R.D.; Bułdak, R.J.; Wyszyńska, M.; Stawiarska-Pięta, B.; Szaflarska-Stojko, E. The Ethanol Extract of Polish Propolis Exhibits Anti-Proliferative and/or Pro-Apoptotic Effect on HCT 116 Colon Cancer and Me45 Malignant Melanoma Cells In Vitro Conditions. Adv. Clin. Exp. Med. 2015, 24, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mendonça, I.C.; Porto, I.C.; do Nascimento, T.G.; de Souza, N.S.; Oliveira, J.M.; Arruda, R.E.; Mousinho, K.C.; dos Santos, A.F.; Basílio-Júnior, I.D.; Parolia, A.; et al. Brazilian red propolis: Phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement. Altern. Med. 2015, 15, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žižić, J.B.; Vuković, N.L.; Jadranin, M.B.; Anđelković, B.D.; Tešević, V.V.; Kacaniova, M.M.; Sukdolak, S.B.; Marković, S.D. Chemical composition, cytotoxic and antioxidative activities of ethanolic extracts of propolis on HCT-116 cell line. J. Sci. Food Agric. 2013, 93, 3001–3009. [Google Scholar] [CrossRef]
- Catchpole, O.; Mitchell, K.; Bloor, S.; Davis, P.; Suddes, A. Antiproliferative activity of New Zealand propolis and phenolic compounds vs human colorectal adenocarcinoma cells. Fitoterapia 2015, 106, 167–174. [Google Scholar] [CrossRef]
- Choudhari, M.K.; Haghniaz, R.; Rajwade, J.M.; Paknikar, K.M. Anticancer activity of Indian stingless bee propolis: An in vitro study. Evid. Based Complement. Altern. Med. 2013, 2013, 928280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calhelha, R.C.; Falcão, S.; Queiroz, M.J.; Vilas-Boas, M.; Ferreira, I.C. Cytotoxicity of Portuguese propolis: The proximity of the in vitro doses for tumor and normal cell lines. Biomed. Res. Int. 2014, 2014, 897361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, M.; Naoi, K.; Hashita, M.; Itoh, Y.; Suzui, M. Growth inhibitory activity of ethanol extracts of Chinese and Brazilian propolis in four human colon carcinoma cell lines. Oncol. Rep. 2009, 22, 349–354. [Google Scholar]
- Russo, A.; Cardile, V.; Sanchez, F.; Troncoso, N.; Vanella, A.; Garbarino, J.A. Chilean propolis: Antioxidant activity and antiproliferative action in human tumor cell lines. Life Sci. 2004, 76, 545–558. [Google Scholar] [CrossRef]
- Valença, I.; Morais-Santos, F.; Miranda-Gonçalves, V.; Ferreira, A.M.; Almeida-Aguiar, C.; Baltazar, F. Portuguese propolis disturbs glycolytic metabolism of human colorectal cancer in vitro. BMC Complement. Altern. Med. 2013, 13, 184. [Google Scholar] [CrossRef] [Green Version]
- Azarshinfam, N.; Tanomand, A.; Soltanzadeh, H.; Rad, F.A. Evaluation of anticancer effects of propolis extract with or without combination with layered double hydroxide nanoparticles on Bcl-2 and Bax genes expression in HT-29 cell lines. Gene Rep. 2021, 23, 101031. [Google Scholar] [CrossRef]
- Frión-Herrera, Y.; Gabbia, D.; Díaz-García, A.; Cuesta-Rubio, O.; Carrara, M. Chemosensitizing activity of Cuban propolis and nemorosone in doxorubicin resistant human colon carcinoma cells. Fitoterapia 2019, 136, 104173. [Google Scholar] [CrossRef] [PubMed]
- Memmedov, H.; Oktay, L.M.; Durmaz, B.; Günel, N.S.; Yı Ldırım, H.K.; Sözmen, E.Y. Propolis prevents inhibition of apoptosis by potassium bromate in CCD 841 human colon cell. Cell Biochem. Funct. 2020, 38, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Gutierrez, L.; Bordonaro, M.; Russo, D.; Anzelmi, F.; Hooven, J.T.; Cerra, C.; Lazarova, D.L. Effects of propolis and gamma-cyclodextrin on intestinal neoplasia in normal weight and obese mice. Cancer Med. 2016, 5, 2448–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbaz, N.M.; Khalil, I.A.; Abd-Rabou, A.A.; El-Sherbiny, I.M. Chitosan-based nano-in-microparticle carriers for enhanced oral delivery and anticancer activity of propolis. Int. J. Biol. Macromol. 2016, 92, 254–269. [Google Scholar] [CrossRef]
- Sameni, H.R.; Yosefi, S.; Alipour, M.; Pakdel, A.; Torabizadeh, N.; Semnani, V.; Bandegi, A.R. Co-administration of 5FU and propolis on AOM/DSS induced colorectal cancer in BALB-c mice. Life Sci. 2021, 276, 119390. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Fujioka, M.; Sokuza, Y.; Ohnishi, M.; Gi, M.; Takeshita, M.; Kumada, K.; Kakehashi, A.; Wanibuchi, H. Chemopreventive Action by Ethanol-extracted Brazilian Green Propolis on Post-initiation Phase of Inflammation-associated Rat Colon Tumorigenesis. In Vivo 2017, 31, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Bazo, A.P.; Rodrigues, M.A.; Sforcin, J.M.; de Camargo, J.L.; Ribeiro, L.R.; Salvadori, D.M. Protective action of propolis on the rat colon carcinogenesis. Teratog. Carcinog. Mutagen. 2002, 22, 183–194. [Google Scholar] [CrossRef]
- Yasui, Y.; Miyamoto, S.; Kim, M.; Kohno, H.; Sugie, S.; Tanaka, T. Aqueous and ethanolic extract fractions from the Brazilian propolis suppress azoxymethane-induced aberrant crypt foci in rats. Oncol. Rep. 2008, 20, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Salehi, A.; Hosseini, S.M.; Kazemi, S. Antioxidant and Anticarcinogenic Potentials of Propolis for Dimethylhydrazine-Induced Colorectal Cancer in Wistar Rats. Biomed. Res. Int. 2022, 2022, 8497562. [Google Scholar] [CrossRef]
- Braga, V.N.L.; Juanes, C.C.; Peres Júnior, H.S.; Sousa, J.R.; Cavalcanti, B.C.; Jamacaru, F.V.F.; Lemos, T.L.G.; Dornelas, C.A. Gum arabic and red propolis protecteting colorectal preneoplastic lesions in a rat model of azoxymethane1. Acta Cir. Bras. 2019, 34, e201900207. [Google Scholar] [CrossRef] [Green Version]
- Miryan, M.; Alavinejad, P.; Abbaspour, M.; Soleimani, D.; Ostadrahimi, A. Does propolis affect the quality of life and complications in subjects with irritable bowel syndrome (diagnosed with Rome IV criteria)? A study protocol of the randomized, double-blinded, placebo-controlled clinical trial. Trials 2020, 21, 698. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Goto, M.; Matsuura, N.; Murakami, Y.; Goto, C.; Sakai, T.; Kanazawa, K. A pilot, randomized, placebo-controlled, double-blind phase 0/biomarker study on effect of artepillin C-rich extract of Brazilian propolis in frequent colorectal adenoma polyp patients. J. Am. Coll. Nutr. 2012, 31, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, N.L.; Obradovic, A.D.; Vukic, M.D.; Jovanovic, D.; Djurdjevic, P.M. Cytotoxic, proapoptotic and antioxidative potential of flavonoids isolated from propolis against colon (HCT-116) and breast (MDA-MB-231) cancer cell lines. Food Res. Int. 2018, 106, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gajek, G.; Marciniak, B.; Lewkowski, J.; Kontek, R. Antagonistic Effects of CAPE (a Component of Propolis) on the Cytotoxicity and Genotoxicity of Irinotecan and SN38 in Human Gastrointestinal Cancer Cells In Vitro. Molecules 2020, 25, 658. [Google Scholar] [CrossRef] [Green Version]
- Xiang, D.; Wang, D.; He, Y.; Xie, J.; Zhong, Z.; Li, Z.; Xie, J. Caffeic acid phenethyl ester induces growth arrest and apoptosis of colon cancer cells via the beta-catenin/T-cell factor signaling. Anticancer Drugs 2006, 17, 753–762. [Google Scholar] [CrossRef]
- Fraser, S.P.; Hemsley, F.; Djamgoz, M.B.A. Caffeic acid phenethyl ester: Inhibition of metastatic cell behaviours via voltage-gated sodium channel in human breast cancer in vitro. Int. J. Biochem. Cell Biol. 2016, 71, 111–118. [Google Scholar] [CrossRef]
- Liao, H.F.; Chen, Y.Y.; Liu, J.J.; Hsu, M.L.; Shieh, H.J.; Liao, H.J.; Shieh, C.J.; Shiao, M.S.; Chen, Y.J. Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J. Agric. Food Chem. 2003, 51, 7907–7912. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A.A.; Di Carlo, G.; Maffia, P.; Russo, A.; Maiello, F.M.; Capasso, F.; Mascolo, N. Effect of a propolis extract and caffeic acid phenethyl ester on formation of aberrant crypt foci and tumors in the rat colon. Fitoterapia 2002, 73, S38–S43. [Google Scholar] [CrossRef]
- Tang, H.; Yao, X.; Yao, C.; Zhao, X.; Zuo, H.; Li, Z. Anti-colon cancer effect of caffeic acid p-nitro-phenethyl ester in vitro and in vivo and detection of its metabolites. Sci. Rep. 2017, 7, 7599. [Google Scholar] [CrossRef]
- Ha, T.K.; Kim, M.E.; Yoon, J.H.; Bae, S.J.; Yeom, J.; Lee, J.S. Galangin induces human colon cancer cell death via the mitochondrial dysfunction and caspase-dependent pathway. Exp. Biol. Med. (Maywood) 2013, 238, 1047–1054. [Google Scholar] [CrossRef]
- Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Yamada, N.; Ohta, S.; Ichihara, K.; Akao, Y. Propolis cinnamic acid derivatives induce apoptosis through both extrinsic and intrinsic apoptosis signaling pathways and modulate of miRNA expression. Phytomedicine 2014, 21, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Das, S.K.; Hashimoto, T.; Sowa, Y.; Yoshida, T.; Sakai, T.; Matsuura, Y.; Kanazawa, K. Artepillin C in Brazilian propolis induces G(0)/G(1) arrest via stimulation of Cip1/p21 expression in human colon cancer cells. Mol. Carcinog. 2005, 44, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Carballo, D.; Freistã Hler, M.; Malak, S.; Bardenheuer, W.; Reusch, H.P. Mucronulatol from Caribbean propolis exerts cytotoxic effects on human tumor cell lines. Int. J. Clin. Pharmacol. Ther. 2008, 46, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Carballo, D.; Malak, S.; Bardenheuer, W.; Freistuehler, M.; Peter Reusch, H. The contribution of plukenetione A to the anti-tumoral activity of Cuban propolis. Bioorg Med. Chem. 2008, 16, 9635–9643. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Chen, C.I.; Hsiang, Y.P.; Hsu, Y.C.; Cheng, K.C.; Chien, P.H.; Pan, H.L.; Lu, C.C.; Chen, Y.J. Chrysin Attenuates Cell Viability of Human Colorectal Cancer Cells through Autophagy Induction Unlike 5-Fluorouracil/Oxaliplatin. Int. J. Mol. Sci. 2018, 19, 1763. [Google Scholar] [CrossRef] [Green Version]
- Karim, M.; Schrader, H.; Holt, R.; Cardetti, M.; Keen, C. Honey with high levels of antioxidants can provide protection in healthy human subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Quiles, J.L.; Gil, E.; Bompadre, S.; Simal-Gandara, J.; Battino, M.; Giampieri, F. The influence of in vitro gastrointestinal digestion on the anticancer activity of manuka honey. Antioxidants 2020, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Puścion-Jakubik, A.; Borawska, M.H.; Socha, K. Modern methods for assessing the quality of bee honey and botanical origin identification. Foods 2020, 9, 1028. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Med. J. Nutr. Metab. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a potential natural antioxidant medicine: An insight into its molecular mechanisms of action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.M.; Molan, P.C.; Cursons, R.T. The controlled in vitro susceptibility of gastrointestinal pathogens to the antibacterial effect of manuka honey. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Badawy, O.F.; Shafii, S.S.A.; Tharwat, E.E.; Kamal, A.M. Antibacterial activity of bee honey and its therapeutic usefulness against Escherichia coli O157: H7 and Salmonella typhimurium infection. Rev. Sci. Tech. 2004, 23, 1011–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Long, S.; Liu, Q.; Ma, H.; Li, J.; Xiaoqing, W.; Yuan, J.; Li, M.; Hou, B. Gut microbiota is involved in the alleviation of loperamide-induced constipation by honey supplementation in mice. Food Sci. Nutr. 2020, 8, 4388–4398. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, S.; Gandhi, H.; Ustunol, Z. Effect of honey on the growth of and acid production by human intestinal Bifidobacterium spp.: An in vitro comparison with commercial oligosaccharides and inulin. J. Food Prot. 2002, 65, 214–218. [Google Scholar] [CrossRef]
- Rosendale, D.I.; Maddox, I.S.; Miles, M.C.; Rodier, M.; Skinner, M.; Sutherland, J. High-throughput microbial bioassays to screen potential New Zealand functional food ingredients intended to manage the growth of probiotic and pathogenic gut bacteria. Int. J. Food Sci. Technol. 2008, 43, 2257–2267. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: The suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct. 2018, 9, 2145–2157. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Quiles, J.L.; Battino, M. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 2: Induction of oxidative stress, alteration of mitochondrial respiration and glycolysis, and suppression of metastatic ability. Food Funct. 2018, 9, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Forbes-Hernández, T.Y.; Gasparrini, M.; Amici, A.; Cianciosi, D.; Quiles, J.L.; Battino, M. Manuka honey synergistically enhances the chemopreventive effect of 5-fluorouracil on human colon cancer cells by inducing oxidative stress and apoptosis, altering metabolic phenotypes and suppressing metastasis ability. Free Radic. Biol. Med. 2018, 126, 41–54. [Google Scholar] [CrossRef]
- T-Johari, S.A.T.; Hashim, F.; Ismail, W.I.; Ali, A.M. Combinatorial Cytotoxic Effects of Gelam Honey and 5-Fluorouracil against Human Adenocarcinoma Colon Cancer HT-29 Cells In Vitro. Int. J. Cell Biol. 2019, 2019, 3059687. [Google Scholar] [PubMed]
- Hakim, L.; Alias, E.; Makpol, S.; Ngah, W.Z.; Morad, N.A.; Yusof, Y.A. Gelam honey and ginger potentiate the anticancer effect of 5-FU against HCT 116 colorectal cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 4651–4657. [Google Scholar] [CrossRef] [Green Version]
- Wee, L.H.; Morad, N.A.; Aan, G.J.; Makpol, S.; Wan Ngah, W.Z.; Mohd Yusof, Y.A. Mechanism of Chemoprevention against Colon Cancer Cells Using Combined Gelam Honey and Ginger Extract via mTOR and Wnt/beta-catenin Pathways. Asian Pac. J. Cancer Prev. 2015, 16, 6549–6556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, N.; Ray, N.; Patil, A.R.; Saini, S.S.; Waghmode, B.; Ghosh, C.; Patil, S.B.; Patil, S.B.; Mote, C.S.; Saini, S.; et al. Inhibitory effect of selected Indian honey on colon cancer cell growth by inducing apoptosis and targeting the β-catenin/Wnt pathway. Food Funct. 2022, 13, 8283–8303. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mondhe, D.; Wani, Z.A.; Pal, H.C.; Mandal, M. Effect of honey and eugenol on Ehrlich ascites and solid carcinoma. J. Biomed. Biotechnol. 2010, 2010, 989163. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.H.; Andrae, L.; Engeseth, N.J. Antimutagenic effect of various honeys and sugars against Trp-p-1. J. Agric. Food Chem. 2002, 50, 6923–6928. [Google Scholar] [CrossRef]
- Wen, C.T.P.; Hussein, S.Z.; Abdullah, S.; Karim, N.A.; Makpol, S.; Yusof, Y.A.M. Gelam and Nenas honeys inhibit proliferation of ht 29 colon cancer cells by inducing dna damage and apoptosis while suppressing inflammation. Asian Pac. J Cancer Prev. 2012, 13, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, S.K.; Mazumdar, A.; Mondhe, D.; Mandal, M. Apoptotic effect of eugenol in human colon cancer cell lines. Cell Biol. Int. 2011, 35, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Chow, P.H.; Kourghi, M.; Pei, J.V.; Nourmohammadi, S.; Yool, A.J. 5-Hydroxymethyl-Furfural and Structurally Related Compounds Block the Ion Conductance in Human Aquaporin-1 Channels and Slow Cancer Cell Migration and Invasion. Mol. Pharmacol. 2020, 98, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K. Growth inhibition by caffeic acid, one of the phenolic constituents of honey, in HCT 15 colon cancer cells. Sci. World J. 2012, 2012, 372345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. 2013, 19, 7726–7734. [Google Scholar] [CrossRef]
- Cai, G.; Wu, Y.; Wusiman, A.; Gu, P.; Mao, N.; Xu, S.; Zhu, T.; Feng, Z.; Liu, Z.; Wang, D. Alhagi honey polysaccharides attenuate intestinal injury and immune suppression in cyclophosphamide-induced mice. Food Funct. 2021, 12, 6863–6877. [Google Scholar] [CrossRef]
- Lai, C.S.; Yang, G.; Li, S.; Lee, P.S.; Wang, B.N.; Chung, M.C.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. 3′-Hydroxypterostilbene Suppresses Colitis-Associated Tumorigenesis by Inhibition of IL-6/STAT3 Signaling in Mice. J. Agric. Food Chem. 2017, 65, 9655–9664. [Google Scholar] [CrossRef]
- Prdun, S.; Svecnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods 2021, 10, 2103. [Google Scholar] [CrossRef] [PubMed]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Wang, B.; Diao, Q.; Zhang, Z.; Liu, Y.; Gao, Q.; Zhou, Y.; Li, S. Antitumor activity of bee pollen polysaccharides from Rosa rugosa. Mol. Med. Rep. 2013, 7, 1555–1558. [Google Scholar] [CrossRef] [Green Version]
- Uțoiu, E.; Matei, F.; Toma, A.; Diguță, C.F.; Ștefan, L.M.; Mănoiu, S.; Vrăjmașu, V.V.; Moraru, I.; Oancea, A.; Israel-Roming, F.; et al. Bee collected pollen with enhanced health benefits, produced by fermentation with a Kombucha consortium. Nutrients 2018, 10, 1365. [Google Scholar] [CrossRef] [Green Version]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharmacol. 2017, 8, 412. [Google Scholar] [CrossRef] [Green Version]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.C.; Chou, W.M.; Widowati, D.A.; Lin, I.-P.; Peng, C.-C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Khoob, M.S.; Hosseini, S.M.; Kazem, S. In vitro and in vivo antioxidant and anticancer potentials of royal jelly for dimethylhydrazine-induced colorectal cancer in wistar rats. Oxid. Med. Cell. Longev. 2022, 2022, 9506026. [Google Scholar]
- Zheng, J.; Lee, H.L.; Ham, Y.W.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom on colon cancer cell growth by activation of death receptors and inhibition of nuclear factor kappa B. Oncotarget 2015, 6, 44437–44451. [Google Scholar] [CrossRef] [Green Version]
- Yaacoub, C.; Rifi, M.; El-Obeid, D.; Mawlawi, H.; Sabatier, J.-M.; Coutard, B.; Fajloun, Z. The cytotoxic effect of Apis mellifera venom with a synergistic potential of its two main components—Melittin and PLA2—On colon cancer HCT116 cell lines. Molecules 2021, 26, 2264. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskwa, J.; Naliwajko, S.K.; Dobiecka, D.; Socha, K. Bee Products and Colorectal Cancer—Active Components and Mechanism of Action. Nutrients 2023, 15, 1614. https://doi.org/10.3390/nu15071614
Moskwa J, Naliwajko SK, Dobiecka D, Socha K. Bee Products and Colorectal Cancer—Active Components and Mechanism of Action. Nutrients. 2023; 15(7):1614. https://doi.org/10.3390/nu15071614
Chicago/Turabian StyleMoskwa, Justyna, Sylwia Katarzyna Naliwajko, Dominika Dobiecka, and Katarzyna Socha. 2023. "Bee Products and Colorectal Cancer—Active Components and Mechanism of Action" Nutrients 15, no. 7: 1614. https://doi.org/10.3390/nu15071614