Plant-Based Diets and the Incidence of Asthma Symptoms among Elderly Women, and the Mediating Role of Body Mass Index
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diet Assessment
2.3. Assessment of Asthma Symptoms Incidence
2.4. Body Mass Index
2.5. Other Variables
2.6. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Association between PDI Scores and the Incidence of Asthma Symptoms
3.2.1. Healthful Plant-Based Diet Index
3.2.2. Unhealthful Plant-based Diet Index
3.2.3. Plant-Based Diet Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Ray, A.; Wenzel, S.E. Evolving Concepts of Asthma. Am. J. Respir. Crit. Care Med. 2015, 192, 660–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, R.M.; Busse, P.J.; Wechsler, M.E. Asthma in the Elderly and Late-Onset Adult Asthma. Allergy 2018, 73, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Benfante, A.; Spatafora, M.; Scichilone, N. Asthma in the Elderly: A Different Disease? Breathe 2016, 12, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curto, E.; Crespo-Lessmann, A.; González-Gutiérrez, M.V.; Bardagí, S.; Cañete, C.; Pellicer, C.; Bazús, T.; del Carmen Vennera, M.; Martínez, C.; Plaza, V. Is Asthma in the Elderly Different? Functional and Clinical Characteristics of Asthma in Individuals Aged 65 Years and Older. Asthma Res. Pract. 2019, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, A.; Cho, S.-H.; Soriano, J.B.; Rosenwasser, L.J.; Rodrigo, G.J.; Rabe, K.F.; Peters, S.; Niimi, A.; Ledford, D.K.; Katial, R.; et al. Asthma in the Elderly: What We Know and What We Have yet to Know. World Allergy Organ. J. 2014, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Baptist, A.P.; Hamad, A.; Patel, M.R. Older Women with Asthma: Special Challenges in Treatment and Self-Management. Ann. Allergy Asthma Immunol. 2014, 113, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Alwarith, J.; Kahleova, H.; Crosby, L.; Brooks, A.; Brandon, L.; Levin, S.M.; Barnard, N.D. The Role of Nutrition in Asthma Prevention and Treatment. Nutr. Rev. 2020, 78, 928–938. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, B.; Berthon, B.S.; Wark, P.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 341. [Google Scholar] [CrossRef]
- Andrianasolo, R.M.; Hercberg, S.; Kesse-Guyot, E.; Druesne-Pecollo, N.; Touvier, M.; Galan, P.; Varraso, R. Association between Dietary Fibre Intake and Asthma (Symptoms and Control): Results from the French National e-Cohort NutriNet-Santé. Br. J. Nutr. 2019, 122, 1040–1051. [Google Scholar] [CrossRef]
- Li, Z.; Rava, M.; Bédard, A.; Dumas, O.; Garcia-Aymerich, J.; Leynaert, B.; Pison, C.; Le Moual, N.; Romieu, I.; Siroux, V.; et al. Cured Meat Intake Is Associated with Worsening Asthma Symptoms. Thorax 2017, 72, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Cespedes, E.M.; Hu, F.B. Dietary Patterns: From Nutritional Epidemiologic Analysis to National Guidelines. Am. J. Clin. Nutr. 2015, 101, 899–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane-Diallo, A.; Srour, B.; Sellem, L.; Deschasaux, M.; Latino-Martel, P.; Hercberg, S.; Galan, P.; Fassier, P.; Guéraud, F.; Pierre, F.H.; et al. Association between a pro Plant-Based Dietary Score and Cancer Risk in the Prospective NutriNet-Santé Cohort. Int. J. Cancer 2018, 143, 2168–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.A.; Sánchez-Tainta, A.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; Lamuela-Raventós, R.M.; Schröder, H.; et al. A Provegetarian Food Pattern and Reduction in Total Mortality in the Prevención Con Dieta Mediterránea (PREDIMED) Study. Am. J. Clin. Nutr. 2014, 100 (Suppl. S1), 320S–328S. [Google Scholar] [CrossRef] [Green Version]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [Green Version]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and Asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo, C.A.; Weiss, S.T.; Zhang, S.; Willett, W.C.; Speizer, F.E. Prospective Study of Body Mass Index, Weight Change, and Risk of Adult-Onset Asthma in Women. Arch. Intern. Med. 1999, 159, 2582–2588. [Google Scholar] [CrossRef] [Green Version]
- Van der Plaat, D.A. Mendelian Randomisation Supports Causal Link between Obesity and Asthma. Thorax 2020, 75, 194–195. [Google Scholar] [CrossRef] [Green Version]
- Song, W.-J.; Cho, S.-H. Challenges in the Management of Asthma in the Elderly. Allergy Asthma Immunol. Res. 2015, 7, 431–439. [Google Scholar] [CrossRef]
- Boggs, D.A.; Rosenberg, L.; Rodríguez-Bernal, C.L.; Palmer, J.R. Long-Term Diet Quality Is Associated with Lower Obesity Risk in Young African American Women with Normal BMI at Baseline. J. Nutr. 2013, 143, 1636–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bédard, A.; Li, Z.; Ait-hadad, W.; Camargo, C.A.; Leynaert, B.; Pison, C.; Dumas, O.; Varraso, R. The Role of Nutritional Factors in Asthma: Challenges and Opportunities for Epidemiological Research. Int. J. Environ. Res. Public Health 2021, 18, 3013. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kesse-Guyot, E.; Dumas, O.; Garcia-Aymerich, J.; Leynaert, B.; Pison, C.; Le Moual, N.; Romieu, I.; Siroux, V.; Camargo, C.A.; et al. Longitudinal Study of Diet Quality and Change in Asthma Symptoms in Adults, According to Smoking Status. Br. J. Nutr. 2017, 117, 562–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, I.; Biswas, S.K.; Kode, A. Oxidant and Antioxidant Balance in the Airways and Airway Diseases. Eur. J. Pharmacol. 2006, 533, 222–239. [Google Scholar] [CrossRef]
- Clavel-Chapelon, F.; van Liere, M.J.; Giubout, C.; Niravong, M.Y.; Goulard, H.; Le Corre, C.; Hoang, L.A.; Amoyel, J.; Auquier, A.; Duquesnel, E. E3N, a French Cohort Study on Cancer Risk Factors. E3N Group. Etude Epidémiologique Auprès de Femmes de l’Education Nationale. Eur. J. Cancer Prev. 1997, 6, 473–478. [Google Scholar] [PubMed]
- Burney, P.G.; Luczynska, C.; Chinn, S.; Jarvis, D. The European Community Respiratory Health Survey. Eur. Respir. J. 1994, 7, 954–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauffmann, F.; Dizier, M.H.; Pin, I.; Paty, E.; Gormand, F.; Vervloet, D.; Bousquet, J.; Neukirch, F.; Annesi, I.; Oryszczyn, M.P.; et al. Epidemiological Study of the Genetics and Environment of Asthma, Bronchial Hyperresponsiveness, and Atopy: Phenotype Issues. Am. J. Respir. Crit. Care Med. 1997, 156, S123–S129. [Google Scholar] [CrossRef] [PubMed]
- Lucas, F.; Niravong, M.; Villeminot, S.; Kaaks, R.; Clavel-Chapelon, F. Estimation of Food Portion Size Using Photographs: Validity, Strengths, Weaknesses and Recommendations. J. Hum. Nutr. Diet. 1995, 8, 65–74. [Google Scholar] [CrossRef]
- Pekkanen, J.; Sunyer, J.; Anto, J.M.; Burney, P. European Community Respiratory Health Study Operational Definitions of Asthma in Studies on Its Aetiology. Eur. Respir. J. 2005, 26, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Sunyer, J.; Pekkanen, J.; Garcia-Esteban, R.; Svanes, C.; Künzli, N.; Janson, C.; de Marco, R.; Antó, J.M.; Burney, P. Asthma Score: Predictive Ability and Risk Factors. Allergy 2007, 62, 142–148. [Google Scholar] [CrossRef]
- Bédard, A.; Serra, I.; Dumas, O.; Basagaña, X.; Clavel-Chapelon, F.; Le Moual, N.; Sanchez, M.; Siroux, V.; Varraso, R.; Garcia-Aymerich, J. Time-Dependent Associations Between Body Composition, Physical Activity, and Current Asthma in Women: A Marginal Structural Modeling Analysis. Am. J. Epidemiol. 2017, 186, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varraso, R.; Oryszczyn, M.P.; Mathieu, N.; Le Moual, N.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Romieu, I.; Kauffmann, F. Farming in Childhood, Diet in Adulthood and Asthma History. Eur. Respir. J. 2012, 39, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Valeri, L.; Vanderweele, T.J. Mediation Analysis Allowing for Exposure-Mediator Interactions and Causal Interpretation: Theoretical Assumptions and Implementation with SAS and SPSS Macros. Psychol. Methods 2013, 18, 137–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, T.; Vansteelandt, S.; Bekaert, M. A Simple Unified Approach for Estimating Natural Direct and Indirect Effects. Am. J. Epidemiol. 2012, 176, 190–195. [Google Scholar] [CrossRef] [Green Version]
- VanderWeele, T. Explanation in Causal Inference: Methods for Mediation and Interaction; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Hafeman, D.M. “Proportion Explained”: A Causal Interpretation for Standard Measures of Indirect Effect? Am. J. Epidemiol. 2009, 170, 1443–1448. [Google Scholar] [CrossRef] [Green Version]
- Tomova, G.D.; Arnold, K.F.; Gilthorpe, M.S.; Tennant, P.W.G. Adjustment for Energy Intake in Nutritional Research: A Causal Inference Perspective. Am. J. Clin. Nutr. 2022, 115, 189–198. [Google Scholar] [CrossRef]
- Laouali, N.; Shah, S.; MacDonald, C.-J.; Mahamat-Saleh, Y.; El Fatouhi, D.; Mancini, F.; Fagherazzi, G.; Boutron-Ruault, M.-C. BMI in the Associations of Plant-Based Diets with Type 2 Diabetes and Hypertension Risks in Women: The E3N Prospective Cohort Study. J. Nutr. 2021, 151, 2731–2740. [Google Scholar] [CrossRef]
- Ait-hadad, W.; Bédard, A.; Chanoine, S.; Dumas, O.; Laouali, N.; Le Moual, N.; Leynaert, B.; Macdonald, C.; Siroux, V.; Boutron-Ruault, M.-C.; et al. Healthy Diet Associated with Better Asthma Outcomes in Elderly Women of the French Asthma-E3N Study. Eur. J. Nutr. 2022, 61, 2555–2569. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Jerschow, E.; Forno, E.; Hua, S.; Mossavar-Rahmani, Y.; Perreira, K.M.; Sotres-Alvarez, D.; Afshar, M.; Punjabi, N.M.; Thyagarajan, B.; et al. Dietary Patterns, Asthma, and Lung Function in the Hispanic Community Health Study/Study of Latinos. Ann. Am. Thorac. Soc. 2020, 17, 293–301. [Google Scholar] [CrossRef]
- Menezes, A.M.B.; Schneider, B.C.; Oliveira, V.P.; Prieto, F.B.; Silva, D.L.R.; Lerm, B.R.; da Costa, T.B.; Bouilly, R.; Wehrmeister, F.C.; Gonçalves, H.; et al. Longitudinal Association Between Diet Quality and Asthma Symptoms in Early Adult Life in a Brazilian Birth Cohort. J. Asthma Allergy 2020, 13, 493–503. [Google Scholar] [CrossRef]
- Varraso, R.; Chiuve, S.E.; Fung, T.T.; Barr, R.G.; Hu, F.B.; Willett, W.C.; Camargo, C.A. Alternate Healthy Eating Index 2010 and Risk of Chronic Obstructive Pulmonary Disease among US Women and Men: Prospective Study. BMJ 2015, 350, h286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrianasolo, R.M.; Kesse-Guyot, E.; Adjibade, M.; Hercberg, S.; Galan, P.; Varraso, R. Associations between Dietary Scores with Asthma Symptoms and Asthma Control in Adults. Eur. Respir. J. 2018, 52, 1702572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.-Y.; Forno, E.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Celedón, J.C. The Dietary Inflammatory Index and Current Wheeze among Children and Adults in the United States. J. Allergy Clin. Immunol. Pract. 2018, 6, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Shivappa, N.; Berthon, B.S.; Gibson, P.G.; Hebert, J.R. Dietary Inflammatory Index Is Related to Asthma Risk, Lung Function and Systemic Inflammation in Asthma. Clin. Exp. Allergy 2015, 45, 177–183. [Google Scholar] [CrossRef]
- Valente, M.J.; Rijnhart, J.J.M.; Smyth, H.L.; Muniz, F.B.; MacKinnon, D.P. Causal Mediation Programs in R, Mplus, SAS, SPSS, and Stata. Struct. Equ. Model. 2020, 27, 975–984. [Google Scholar] [CrossRef]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef] [Green Version]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative Stress in Asthma. World Allergy Organ. J. 2011, 4, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian Diet Improves Insulin Resistance and Oxidative Stress Markers More than Conventional Diet in Subjects with Type 2 Diabetes. Diabet. Med. 2011, 28, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Haghighatdoost, F.; Bellissimo, N.; Totosy de Zepetnek, J.O.; Rouhani, M.H. Association of Vegetarian Diet with Inflammatory Biomarkers: A Systematic Review and Meta-Analysis of Observational Studies. Public Health Nutr. 2017, 20, 2713–2721. [Google Scholar] [CrossRef]
- Varraso, R.; Camargo, C.A. Processed Meat Consumption and Lung Health: More Evidence for Harm. Eur. Respir. J. 2014, 43, 943–946. [Google Scholar] [CrossRef]
- Wang, Y.B.; Shivappa, N.; Hébert, J.R.; Page, A.J.; Gill, T.K.; Melaku, Y.A. Association between Dietary Inflammatory Index, Dietary Patterns, Plant-Based Dietary Index and the Risk of Obesity. Nutrients 2021, 13, 1536. [Google Scholar] [CrossRef] [PubMed]
- Masood, S.; Cappelli, C.; Li, Y.; Tanenbaum, H.; Chou, C.-P.; Spruijt-Metz, D.; Palmer, P.H.; Johnson, C.A.; Xie, B. Cigarette Smoking Is Associated with Unhealthy Patterns of Food Consumption, Physical Activity, Sleep Impairment, and Alcohol Drinking in Chinese Male Adults. Int. J. Public Health 2015, 60, 891–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Vaart, H.; Postma, D.; Timens, W.; Ten, H. Acute Effects of Cigarette Smoke on Inflammation and Oxidative Stress: A Review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Lee, K.; Park, S.; Shin, N.; Kim, H.; Kim, J. Association between Unhealthful Plant-Based Diets and Possible Risk of Dyslipidemia. Nutrients 2021, 13, 4334. [Google Scholar] [CrossRef] [PubMed]
- Baden, M.Y.; Shan, Z.; Wang, F.; Li, Y.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B.; Rexrode, K.M. Quality of Plant-Based Diet and Risk of Total, Ischemic, and Hemorrhagic Stroke. Neurology 2021, 96, e1940–e1953. [Google Scholar] [CrossRef] [PubMed]
- Sotos-Prieto, M.; Struijk, E.A.; Fung, T.T.; Rodríguez-Artalejo, F.; Willett, W.C.; Hu, F.B.; Lopez-Garcia, E. Association between the Quality of Plant-Based Diets and Risk of Frailty. J. Cachexia Sarcopenia Muscle 2022, 13, 2854–2862. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Drouin-Chartier, J.-P.; Li, Y.; Baden, M.Y.; Manson, J.E.; Willett, W.C.; Voortman, T.; Hu, F.B.; Bhupathiraju, S.N. Changes in Plant-Based Diet Indices and Subsequent Risk of Type 2 Diabetes in Women and Men: Three U.S. Prospective Cohorts. Diabetes Care 2021, 44, 663–671. [Google Scholar] [CrossRef]
- Van Liere, M.J.; Lucas, F.; Clavel, F.; Slimani, N.; Villeminot, S. Relative Validity and Reproducibility of a French Dietary History Questionnaire. Int. J. Epidemiol. 1997, 26 (Suppl. S1), S128–S136. [Google Scholar] [CrossRef] [Green Version]
- Tehard, B.; van Liere, M.J.; Com Nougué, C.; Clavel-Chapelon, F. Anthropometric Measurements and Body Silhouette of Women: Validity and Perception. J. Am. Diet. Assoc. 2002, 102, 1779–1784. [Google Scholar] [CrossRef]
- Pekkanen, J.; Sunyer, J. Problems in Using Incidence to Analyze Risk Factors in Follow-Up Studies. Eur. J. Epidemiol. 2008, 23, 581–584. [Google Scholar] [CrossRef]



| hPDI Diet Score | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Age-Adjusted p for Trend | |
| hPDI diet score, min-max | 31–50.5 | 51–54 | 54.5–57.5 | 58–61 | 61.5–78.5 | |
| hPDI diet score, m(sd) | 47.5 (2.7) | 52.6 (1.03) | 55.9 (1.01) | 59.4 (1.00) | 64.5 (2.7) | |
| uPDI diet score, m (sd) | 54.0 (5.8) | 52.7 (6.0) | 51.8 (5.9) | 50.7 (5.9) | 49.3 (5.6) | |
| Component score of hPDI (g/d) | ||||||
| Fiber in whole grains | 2.0 (2.5) | 2.6 (2.9) | 3.3 (3.3) | 4.0 (3.2) | 4.9 (3.7) | <0.0001 |
| Fruits | 223.2 (128.3) | 260.3 (142.2) | 283.2 (140.2) | 312.9 (153.1) | 361.1 (161.0) | <0.0001 |
| Vegetables | 16.9 (67.7) | 29.3 (102.7) | 18.3 (78.2) | 21.5 (96.1) | 28.8 (113.4) | 0.01 |
| Nuts | 7.0 (8.0) | 7.2 (8.7) | 6.9 (8.4) | 7.7 (9.7) | 8.9 (11.9) | <0.0001 |
| Legumes | 23.0 (21.4) | 23.8 (22.3) | 23.7 (22.2) | 23.6 (22.5) | 27.6 (27.6) | <0.0001 |
| Vegetable oils | 21.4 (9.5) | 23.2 (10.5) | 24.7 (10.3) | 25.4 (10.9) | 30.2 (12.1) | <0.0001 |
| Tea and coffee | 436.7 (243.1) | 487.0 (279.9) | 509.8 (270.9) | 551.2 (294.8) | 622.7 (317.3) | <0.0001 |
| Fruit juices | 97.2 (92.9) | 76.7 (88.2) | 67.4 (84.3) | 57.3 (74.7) | 46.0 (80.6) | <0.0001 |
| Refined grains | 223.7 (89.3) | 198.0 (94.8) | 173.7 (88.4) | 151.0 (86.5) | 123.4 (73.1) | <0.0001 |
| Potatoes | 87.7 (46.7) | 72.6 (41.5) | 66.3 (42.5) | 55.1 (36.8) | 46.6 (36.1) | <0.0001 |
| Sugar-sweetened beverages | 12.6 (33.3) | 7.7 (27.7) | 3.7 (15.2) | 2.1 (9.3) | 1.5 (8.4) | <0.0001 |
| Sweets and desserts | 78.7 (40.6) | 68.0 (39.1) | 62.9 (38.2) | 56.1 (37.7) | 48.3 (36.8) | <0.0001 |
| Animal fat | 22.4 (16.9) | 18.9 (16.8) | 15.3 (15.2) | 13.2 (13.9) | 10.2 (12.4) | <0.0001 |
| Dairy | 341.2 (164.9) | 318.9 (178.6) | 305.4 (160.9) | 307.0 (176.0) | 268.4 (160.8) | <0.0001 |
| Egg | 30.0 (18.9) | 25.4 (17.5) | 22.3 (15.5) | 19.5 (15.0) | 17.6 (14.3) | <0.0001 |
| Fish or Seafood | 44.6 (24.4) | 41.9 (25.9) | 39.8 (23.9) | 37.9 (24.6) | 35.7 (25.0) | <0.0001 |
| Meat | 127.8 (43.8) | 113.1 (42.2) | 104.4 (40.1) | 94.1 (40.9) | 81.6 (42.2) | <0.0001 |
| Miscellaneous animal-based food | 104.7 (52.9) | 87.1 (48.8) | 70.5 (40.8) | 62.6 (39.3) | 48.5 (34.5) | <0.0001 |
| Age (years) | 61.3 (5.3) | 61.8 (5.5) | 62.3 (5.7) | 62.6 (5.5) | 62.7 (5.5) | |
| Energy intake (kcal/d) | 2581 (518) | 2376 (497) | 2233 (463) | 2124 (443) | 2020 (436) | <0.0001 |
| Leisure-time physical activity (METs/week) | 63.9 (48.9) | 59.5 (49.7) | 61.4 (49.3) | 64.6 (53.2) | 63.8 (48.7) | 0.30 |
| Smoking status | <0.0001 | |||||
| Never smoker | 606 (56.2) | 628 (56.0) | 674 (53.8) | 628 (53.8) | 532 (49.3) | |
| Ex-smoker | 396 (36.7) | 411 (36.6) | 499 (39.8) | 464 (39.7) | 477 (44.2) | |
| Current smoker | 76 (7.1) | 83 (7.4) | 80 (6.4) | 76 (6.5) | 70 (6.5) | |
| Educational level | 0.0003 | |||||
| <high school diploma | 109 (10.1) | 115 (10.3) | 116 (9.3) | 92 (7.9) | 79 (7.3) | |
| High school to 2-level university | 537 (49.8) | 549 (48.9) | 630 (50.3) | 616 (52.7) | 507 (47.0) | |
| 3–4-level university | 226 (20.1) | 234 (20.8) | 234 (18.7) | 213 (18.2) | 225 (20.9) | |
| ≥5-level university | 173 (16.1) | 188 (16.8) | 242 (19.3) | 218 (18.7) | 234 (21.7) | |
| Missing | 33 (3.1) | 36 (3.2) | 31 (2.4) | 29 (2.5) | 34 (3.1) | |
| Marital status | 0.36 | |||||
| No | 257 (23.8) | 245 (24.0) | 302 (24.1) | 284 (24.3) | 273 (25.3) | |
| Yes | 821 (76.2) | 877 (78.2) | 950 (75.8) | 884 (75.7) | 806 (74.7) | |
| Missing | 1 (0.1) | |||||
| Having farmer parents | 0.42 | |||||
| No | 941 (87.3) | 959 (85.4) | 1086 (86.7) | 1001 (85.7) | 921 (85.4) | |
| Yes | 109 (10.1) | 143 (12.8) | 142 (11.3) | 149 (12.8) | 125 (11.6) | |
| Missing | 28 (2.6) | 20 (1.8) | 25 (2.0) | 18 (1.5) | 33 (3.0) | |
| BMI (kg/m2) | <0.0001 | |||||
| <20 | 147 (13.6) | 185 (16.5) | 202 (16.1) | 223 (19.1) | 233 (21.6) | |
| 20–24.9 | 614 (57.0) | 656 (58.5) | 732 (58.4) | 685 (58.6) | 617 (57.2) | |
| 25–29.9 | 261 (24.2) | 240 (21.4) | 265 (21.2) | 217 (18.6) | 194 (18.0) | |
| ≥30 | 56 (5.2) | 41 (3.6) | 54 (4.3) | 43 (3.7) | 35 (3.2) | |
| BMI (kg/m2) | 23.6 (3.9) | 23.2 (3.6) | 23.3 (4.0) | 23.0 (3.5) | 22.7 (3.6) | <0.0001 |
| uPDI Diet Score | ||||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Age-Adjusted p for Trend | |
| uPDI diet score, min-max | 31–46.5 | 47–50.5 | 51–53.5 | 54–57 | 57.5–72.5 | |
| uPDI diet score, m(sd) | 43.4 (2.7) | 48.8 (1.16) | 52.2 (0.86) | 55.5 (1.02) | 60.5 (2.7) | |
| hPDI diet score, m(sd) | 58.0 (2.7) | 56.6 (6.0) | 55.9 (6.0) | 54.7 (5.7) | 53.4 (5.5) | |
| Component score of uPDI (g/d) | ||||||
| Fiber in whole grains | 4.7 (3.6) | 4.0 (3.4) | 3.1 (3.1) | 2.8 (3.1) | 1.9 (2.4) | <0.0001 |
| Fruits | 349.0 (153.9) | 317.7 (150.5) | 291.4 (146.7) | 258.3 (143.8) | 219.0 (130.9) | <0.0001 |
| Vegetables | 36.9 (127.8) | 19.4 (85.3) | 18.4 (83.4) | 17.0 (72.2) | 23.2 (86.1) | 0.0001 |
| Nuts | 10.5 (10.7) | 8.3 (9.6) | 7.4 (9.7) | 6.1 (7.7) | 4.3 (6.4) | <0.0001 |
| Legumes | 34.1 (27.0) | 27.6 (24.3) | 24.0 (23.2) | 19.0 (18.2) | 16.1 (17.9) | <0.0001 |
| Vegetable oils | 33.0 (10.9) | 27.6 (10.7) | 24.7 (9.8) | 21.5 (8.8) | 17.4 (8.0) | <0.0001 |
| Tea and coffee | 656.7 (317.2) | 538.9 (279.5) | 516.4 (287.0) | 466.6 (255.8) | 424.7 (245.3) | <0.0001 |
| Fruit juices | 60.1 (85.8) | 68.4 (82.4) | 66.1 (79.1) | 70.8 (88.8) | 78.4 (92.4) | <0.0001 |
| Refined grains | 157.1 (90.6) | 164.8 (92.8) | 176.2 (96.9) | 179.3 (93.0) | 193.4 (89.0) | <0.0001 |
| Potatoes | 65.0 (44.1) | 65.3 (45.3) | 63.8 (40.1) | 64.9 (41.4) | 68.8 (44.1) | 0.07 |
| Sugar-sweetened beverages | 2.7 (13.7) | 3.9 (17.5) | 4.7 (16.9) | 5.1 (16.4) | 11.1 (35.3) | <0.0001 |
| Sweets and desserts | 57.1 (39.4) | 60.6 (39.6) | 62.3 (40.0) | 63.7 (37.2) | 70.4 (41.5) | <0.0001 |
| Animal fat | 23.1 (19.9) | 17.4 (15.3) | 15.1 (14.5) | 13.1 (13.1) | 10.7 (11.2) | <0.0001 |
| Dairy | 379.1 (185.8) | 331.2 (172.0) | 305.4 (165.3) | 279.2 (146.4) | 241.3 (141.7) | <0.0001 |
| Egg | 32.5 (18.9) | 25.2 (17.5) | 22.1 (15.4) | 19.2 (13.9) | 15.2 (12.2) | <0.0001 |
| Fish or Seafood | 55.8 (28.3) | 45.1 (25.9) | 37.2 (21.0) | 33.7 (19.7) | 26.7 (16.9) | <0.0001 |
| Meat | 122.3 (47.4) | 109.2 (45.5) | 103.4 (42.1) | 95.2 (40.6) | 89.4 (38.9) | <0.0001 |
| Miscellaneous animal-based food | 89.0 (54.1) | 76.6 (47.0) | 73.0 (45.6) | 69.3 (45.4) | 63.8 (41.7) | <0.0001 |
| Age (years) | 61.5 (5.1) | 62.2 (5.5) | 62.2 (5.5) | 62.3 (5.6) | 62.7 (6.0) | |
| Energy intake (kcal/d) | 2553 (528) | 2355 (495) | 2238 (471) | 2129 (443) | 2027 (436) | <0.0001 |
| Leisure-time physical activity (METs/week) | 67.7 (54.1) | 64.3 (50.4) | 61.0 (46.2) | 61.7 (48.1) | 58.2 (50.6) | <0.0001 |
| Smoking status | <0.0001 | |||||
| Never smoker | 527 (47.7) | 710 (54.3) | 591 (55.0) | 613 (54.7) | 627 (57.4) | |
| Ex-smoker | 502 (45.5) | 513 (39.3) | 421 (39.2) | 439 (39.2) | 372 (34.0) | |
| Current smoker | 75 (6.8) | 84 (6.4) | 63 (6.9) | 69 (6.2) | 94 (8.6) | |
| Educational level | 0.33 | |||||
| <high school diploma | 100 (9.1) | 111 (8.6) | 89 (8.3) | 95 (8.5) | 115 (10.5) | |
| High school to 2-level university | 546 (49.4) | 657 (50.3) | 547 (50.9) | 571 (51.0) | 518 (47.4) | |
| 3–4-level university | 216 (19.6) | 259 (19.8) | 217 (20.2) | 212 (18.9) | 228 (20.9) | |
| ≥5-level university | 206 (18.6) | 232 (17.7) | 197 (18.3) | 219 (19.5) | 201 (18.4) | |
| Missing | 36 (3.3) | 47 (3.6) | 25 (2.3) | 24 (2.1) | 31 (2.8) | |
| Marital status | 0.84 | |||||
| No | 263 (23.8) | 298 (22.8) | 272 (25.3) | 259 (23.1) | 269 (24.6) | |
| Yes | 841 (76.2) | 1009 (77.2) | 803 (74.7) | 861 (76.8) | 824 (75.4) | |
| Missing | 1 (0.09) | |||||
| Having farmer parents | 0.73 | |||||
| No | 931 (84.3) | 1120 (85.7) | 929 (86.4) | 967 (86.3) | 961 (87.9) | |
| Yes | 150 (13.6) | 153 (11.7) | 126 (11.7) | 133 (11.8) | 106 (9.7) | |
| Missing | 23 (2.1) | 34 (2.6) | 20 (1.9) | 21 (1.9) | 26 (2.4) | |
| BMI (kg/m2) | <0.0001 | |||||
| <20 | 148 (13.4) | 195 (15.0) | 201 (18.7) | 218 (19.5) | 228 (20.8) | |
| 20–24.9 | 623 (56.4) | 751 (57.5) | 611 (56.8) | 669 (59.7) | 650 (59.5) | |
| 25–29.9 | 265 (24.0) | 304 (22.3) | 218 (20.3) | 200 (17.8) | 190 (17.4) | |
| ≥30 | 68 (6.2) | 57 (4.4) | 45 (4.2) | 34 (3.0) | 25 (2.3) | |
| BMI (kg/m2) | 23.8 (3.9) | 23.4 (3.8) | 23.2 (3.9) | 22.7 (3.5) | 22.6 (3.5) | <0.0001 |
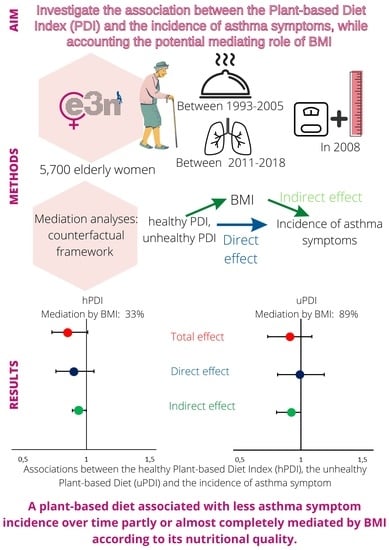
| hPDI | No. | Total Effect | Direct Effect | Indirect Effect | Proportion Mediated |
|---|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |||
| Continuous | 551/5149 | 0.85 (0.73–1.01) | 0.90 (0.76–1.06) | 0.94 (0.89–1.00) | 33% |
| Quintile 1 | 107/969 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 40% |
| Quintile 2 | 108/1008 | 0.85 (0.63–1.11) | 0.87 (0.65–1.14) | 0.98 (0.95–0.99) | |
| Quintile 3 | 127/1119 | 0.87 (0.66–1.14) | 0.91 (0.69–1.20) | 0.96 (0.91–0.98) | |
| Quintile 4 | 110/1055 | 0.84 (0.62–1.09) | 0.89 (0.66–1.16) | 0.94 (0.89–0.98) | |
| Quintile 5 | 99/998 | 0.85 (0.62–1.11) | 0.91 (0.66–1.22) | 0.93 (0.87–0.97) |
| uPDI | No. | Total Effect | Direct Effect | Indirect Effect | Proportion Mediated |
|---|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | |||
| Continuous | 551/5149 | 0.91 (0.73–1.09) | 0.99 (0.81–1.19) | 0.92 (0.70–1.00) | 89% |
| Quintile 1 | 106/1017 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 83% |
| Quintile 2 | 134/1138 | 1.07 (0.82–1.39) | 1.11 (0.86–1.46) | 0.96 (0.90–0.99) | |
| Quintile 3 | 98/973 | 0.91 (0.65–1.23) | 0.97 (0.72–1.31) | 0.93 (0.85–0.98) | |
| Quintile 4 | 109/1016 | 0.99 (0.73–1.30) | 1.09 (0.77–1.45) | 0.91 (0.83–0.97) | |
| Quintile 5 | 104/1005 | 0.88 (0.65–1.19) | 0.98 (0.73–1.32) | 0.90 (0.81–0.96) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait-hadad, W.; Bédard, A.; Delvert, R.; Orsi, L.; Chanoine, S.; Dumas, O.; Laouali, N.; Le Moual, N.; Leynaert, B.; Siroux, V.; et al. Plant-Based Diets and the Incidence of Asthma Symptoms among Elderly Women, and the Mediating Role of Body Mass Index. Nutrients 2023, 15, 52. https://doi.org/10.3390/nu15010052
Ait-hadad W, Bédard A, Delvert R, Orsi L, Chanoine S, Dumas O, Laouali N, Le Moual N, Leynaert B, Siroux V, et al. Plant-Based Diets and the Incidence of Asthma Symptoms among Elderly Women, and the Mediating Role of Body Mass Index. Nutrients. 2023; 15(1):52. https://doi.org/10.3390/nu15010052
Chicago/Turabian StyleAit-hadad, Wassila, Annabelle Bédard, Rosalie Delvert, Laurent Orsi, Sébastien Chanoine, Orianne Dumas, Nasser Laouali, Nicole Le Moual, Bénédicte Leynaert, Valérie Siroux, and et al. 2023. "Plant-Based Diets and the Incidence of Asthma Symptoms among Elderly Women, and the Mediating Role of Body Mass Index" Nutrients 15, no. 1: 52. https://doi.org/10.3390/nu15010052