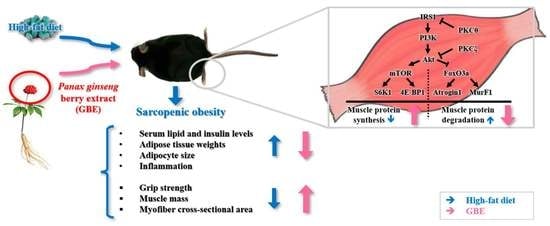
The Administration of Panax Ginseng Berry Extract Attenuates High-Fat-Diet-Induced Sarcopenic Obesity in C57BL/6 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Panax Ginseng Berry Extract (GBE) and UHPLC-ESI-MS/MS Analysis of GBE
2.2. Animals and Experimental Design
2.3. Measurement of Grip Strength
2.4. Biochemical Analysis of Serum Lipid and Insulin Levels
2.5. Histological Analysis of Muscle Cross-Sectional Area (CSA) and Adipocyte Size
2.6. Quantitative Real Time-PCR (qRT-PCR) Assay
2.7. Western Blot Assay
2.8. Statistical Analysis
3. Results
3.1. GBE Administration Significantly Decreased Food Efficiency Ratio and Increased Grip Strength
3.2. GBE Administration Significantly Increased Muscle Mass and Decreased Adipose Tissue Weights
3.3. GBE Administration Significantly Decreased Serum Lipid and Insulin Levels
3.4. GBE Administration Significantly Decreased Adipocyte Size and Significantly Increased Myofiber Cross-Sectional Area (CSA)
3.5. GBE Administration Up-Regulated the PI3K/Akt Pathway through Inactivation of PKCθ and PKCζ
3.6. GBE Administration Down-Regulated the Expression Levels of Inflammatory Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Atkins, J.L. Muscle loss and obesity: The health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015, 74, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054. [Google Scholar] [CrossRef] [Green Version]
- Zamboni, M.; Rubele, S.; Rossi, A.P. Sarcopenia and obesity. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 13–19. [Google Scholar] [CrossRef]
- Tumova, J.; Andel, M.; Trnka, J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol. Res. 2016, 65, 193. [Google Scholar] [CrossRef]
- Kitessa, S.M.; Abeywardena, M.Y. Lipid-induced insulin resistance in skeletal muscle: The chase for the culprit goes from total intramuscular fat to lipid intermediates, and finally to species of lipid intermediates. Nutrients 2016, 8, 466. [Google Scholar] [CrossRef] [Green Version]
- Schmitz-Peiffer, C.; Biden, T.J. Protein kinase C function in muscle, liver, and β-cells and its therapeutic implications for type 2 diabetes. Diabetes 2008, 57, 1774–1783. [Google Scholar] [CrossRef] [Green Version]
- Morino, K.; Petersen, K.F.; Shulman, G.I. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 2006, 55, S9–S15. [Google Scholar] [CrossRef] [Green Version]
- Lipina, C.; Hundal, H.S. Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopenia Muscle 2017, 8, 190–201. [Google Scholar] [CrossRef] [Green Version]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Galadari, A.; Thayyullathil, F. Role of ceramide in diabetes mellitus: Evidence and mechanisms. Lipids Health Dis. 2013, 12, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 2005, 37, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Sandri, M. Signaling pathways that control muscle mass. Int. J. Mol. Sci. 2020, 21, 4759. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and oleic acid: The yin and yang of fatty acids in type 2 diabetes mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Chen, P.-J.; Xiao, W.-H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Helms, S. Cancer prevention and therapeutics: Panax ginseng. Altern. Med. Rev. 2004, 9, 259–274. [Google Scholar]
- Lee, H.J.; Lee, Y.-H.; Park, S.K.; Kang, E.S.; Kim, H.-J.; Lee, Y.C.; Choi, C.S.; Park, S.E.; Ahn, C.W.; Cha, B.-S.; et al. Korean red ginseng (Panax ginseng) improves insulin sensitivity and attenuates the development of diabetes in Otsuka Long-Evans Tokushima fatty rats. Metabolism 2009, 58, 1170–1177. [Google Scholar] [CrossRef]
- Kim, D.Y.; Yang, W.M. Panax ginseng ameliorates airway inflammation in an ovalbumin-sensitized mouse allergic asthma model. J. Ethnopharmacol. 2011, 136, 230–235. [Google Scholar] [CrossRef]
- Li, Z.; Ji, G.E. Ginseng and obesity. J. Ginseng Res. 2018, 42, 1–8. [Google Scholar] [CrossRef]
- Sun, S.; Qi, L.-W.; Du, G.-J.; Mehendale, S.R.; Wang, C.-Z.; Yuan, C.-S. Red notoginseng: Higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food Chem. 2011, 125, 1299–1305. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.C.; Chung, W.-S.; Lee, S.K.; Leung, A.W.; Cheng, C.H.; Yue, K.K. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2006, 550, 173–179. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.-S.; Kim, M.-Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-K.; Cho, D.H.; Lee, K.-S.; Lee, D.-K.; Park, C.-W.; Kim, W.G.; Lee, S.J.; Ha, K.-S.; Taeg, O.G.; Kwon, Y.-G.; et al. Ginseng berry extract prevents atherogenesis via anti-inflammatory action by upregulating phase II gene expression. Evid.-Based Complementary Altern. Med. 2012, 2012, 490301. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yoo, D.S.; Xu, H.; Park, N.I.; Kim, H.H.; Choi, J.E.; Park, S.U. Ginsenoside content of berries and roots of three typical Korean ginseng (Panax ginseng) cultivars. Nat. Prod. Commun. 2009, 4, 1934578X0900400704. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.S.; Park, C.W.; Kim, C.-K.; Jeon, H.Y.; Kim, W.G.; Lee, S.J.; Kim, Y.; Lee, J.; Choi, Y. Effects of Korean ginseng berry extract (GB0710) on penile erection: Evidence from in vitro and in vivo studies. Asian J. Androl. 2013, 15, 503. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zhou, Y.-P.; Dey, L.; Attele, A.; Wu, J.; Gu, M.; Polonsky, K.; Yuan, C.-S. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine 2002, 9, 254–258. [Google Scholar] [CrossRef]
- Attele, A.S.; Zhou, Y.-P.; Xie, J.-T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.-S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.; Kim, S.; Lee, S.J.; Oh, B.-C.; Jun, H.-S. Ginseng berry extract supplementation improves age-related decline of insulin signaling in mice. Nutrients 2015, 7, 3038–3053. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.-S.; You, B.H.; Choi, J.; Chin, Y.-W.; Kim, H.; Choi, H.S.; Choi, Y.H. Ginseng berry extract enhances metformin efficacy against obesity and hepatic steatosis in mice fed high-fat diet through increase of metformin uptake in liver. J. Funct. Foods 2019, 62, 103551. [Google Scholar] [CrossRef]
- Witteveen, E.; Hoogland, I.C.; Wieske, L.; Weber, N.C.; Verhamme, C.; Schultz, M.J.; Van Schaik, I.N.; Horn, J. Assessment of intensive care unit-acquired weakness in young and old mice: An E. coli septic peritonitis model. Muscle Nerve 2016, 53, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.R.; Kim, J.E.; Choi, J.Y.; Park, J.J.; Kim, H.R.; Song, B.R.; Choi, Y.W.; Kim, K.M.; Song, H.; Hwang, D.Y. Anti-obesity effect in high-fat-diet-induced obese C57BL/6 mice: Study of a novel extract from mulberry (Morus alba) leaves fermented with Cordyceps militaris. Exp. Ther. Med. 2019, 17, 2185–2193. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Sun, Q.; Xu, W.; He, Y.; Jin, W.; Yuan, L.; Gao, R. Vitexin ameliorates high fat diet-induced obesity in male C57BL/6J mice via the AMPKα-mediated pathway. Food Funct. 2019, 10, 1940–1947. [Google Scholar] [CrossRef]
- Tong, T.; Kim, M.; Park, T. α-Ionone attenuates high-fat diet-induced skeletal muscle wasting in mice via activation of cAMP signaling. Food Funct. 2019, 10, 1167–1178. [Google Scholar] [CrossRef]
- Yoo, A.; Jang, Y.J.; Ahn, J.; Jung, C.H.; Seo, H.D.; Ha, T.Y. Chrysanthemi Zawadskii var. Latilobum Attenuates Obesity-Induced Skeletal Muscle Atrophy via Regulation of PRMTs in Skeletal Muscle of Mice. Int. J. Mol. Sci. 2020, 21, 2811. [Google Scholar] [CrossRef]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef]
- Han, D.-H.; Kim, S.H.; Higashida, K.; Jung, S.-R.; Polonsky, K.S.; Klein, S.; Holloszy, J.O. Ginsenoside Re rapidly reverses insulin resistance in muscles of high-fat diet fed rats. Metabolism 2012, 61, 1615–1621. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, X.; Peng, X.; Sun, L.; Jia, S.; Wang, P.; Ma, S.; Zhao, H.; Yu, Q.; Huo, H. Ginsenoside Rg1 prevents starvation-induced muscle protein degradation via regulation of AKT/mTOR/FoxO signaling in C2C12 myotubes. Exp. Ther. Med. 2017, 14, 1241–1247. [Google Scholar] [CrossRef] [Green Version]
- Kandarian, S.C.; Jackman, R.W. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 2006, 33, 155–165. [Google Scholar] [CrossRef]
- Argadine, H.M.; Mantilla, C.B.; Zhan, W.-Z.; Sieck, G.C. Intracellular signaling pathways regulating net protein balance following diaphragm muscle denervation. Am. J. Physiol.-Cell Physiol. 2011, 300, C318–C327. [Google Scholar] [CrossRef] [Green Version]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. [Google Scholar] [CrossRef]
- Kang, S.-H.; Lee, H.-A.; Kim, M.; Lee, E.; Sohn, U.D.; Kim, I. Forkhead box O3 plays a role in skeletal muscle atrophy through expression of E3 ubiquitin ligases MuRF-1 and atrogin-1 in Cushing’s syndrome. Am. J. Physiol.-Endocrinol. Metab. 2017, 312, E495–E507. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Tian, Z.; Torigoe, D.; Zhao, J.; Xie, P.; Sugizaki, T.; Sato, M.; Horiguchi, H.; Terada, K.; Kadomatsu, T.; et al. Aging-and obesity-related peri-muscular adipose tissue accelerates muscle atrophy. PLoS ONE 2019, 14, 0221366. [Google Scholar] [CrossRef] [Green Version]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Pellegrinelli, V.; Rouault, C.; Rodriguez-Cuenca, S.; Albert, V.; Edom-Vovard, F.; Vidal-Puig, A.; Clément, K.; Butler-Browne, G.S.; Lacasa, D. Human adipocytes induce inflammation and atrophy in muscle cells during obesity. Diabetes 2015, 64, 3121–3134. [Google Scholar] [CrossRef] [Green Version]






| Group | Normal | HFD | GBE 50 | GBE 100 | GBE 200 | |
|---|---|---|---|---|---|---|
| Factors | ||||||
| TC (mg/dL) | 105.59 ± 6.74 | 14.04 ± 14.19 ## | 135.20 ± 13.04 | 125.33 ± 9.87 | 119.41 ± 11.79 * | |
| HDL-c (mg/dL) | 66.74 ± 3.62 | 80.18 ± 11.06 | 82.38 ± 9.45 | 81.28 ± 1.05 | 79.63 ± 4.65 | |
| HDL-c/TC ratio(mg/dL) | 0.66 ± 0.03 | 0.55 ± 0.04 ## | 0.61 ± 0.03 | 0.65 ± 0.04 * | 0.67 ± 0.04 ** | |
| LDL-c (mg/dL) | 29.10 ± 3.80 | 49.88 ± 11.20 ## | 42.37 ± 5.01 | 34.73 ± 11.01 | 33.41 ± 7.50 * | |
| TG (mg/dL) | 44.00 ± 8.64 | 71.00 ± 16.77 # | 64.00 ± 12.65 | 47.00 ± 8.87 | 44.50 ± 8.81 * | |
| Insulin (ng/mL) | 0.30 ± 0.03 | 0.99 ± 0.01 ### | 1.09 ± 0.15 | 0.96 ± 0.04 | 0.71 ± 0.16 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-E.; Jeon, S.-H.; Lee, S.-J.; Choung, S.-Y. The Administration of Panax Ginseng Berry Extract Attenuates High-Fat-Diet-Induced Sarcopenic Obesity in C57BL/6 Mice. Nutrients 2022, 14, 1747. https://doi.org/10.3390/nu14091747
Shin J-E, Jeon S-H, Lee S-J, Choung S-Y. The Administration of Panax Ginseng Berry Extract Attenuates High-Fat-Diet-Induced Sarcopenic Obesity in C57BL/6 Mice. Nutrients. 2022; 14(9):1747. https://doi.org/10.3390/nu14091747
Chicago/Turabian StyleShin, Ji-Eun, So-Hyun Jeon, Sang-Jun Lee, and Se-Young Choung. 2022. "The Administration of Panax Ginseng Berry Extract Attenuates High-Fat-Diet-Induced Sarcopenic Obesity in C57BL/6 Mice" Nutrients 14, no. 9: 1747. https://doi.org/10.3390/nu14091747