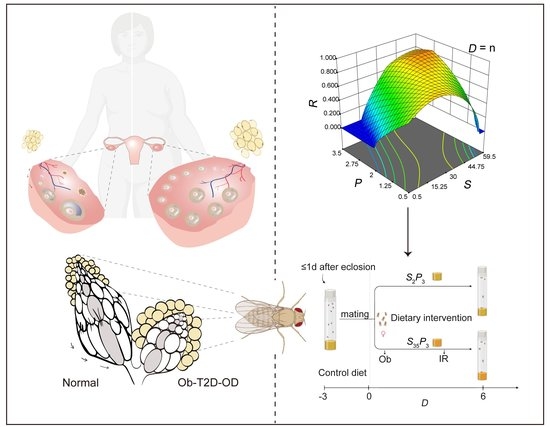
Modeling Obesity-Associated Ovarian Dysfunction in Drosophila
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Stocks and Husbandry
2.2. Central Composite Design
2.3. Measurements of Fertility and Body Fat in CCD Experiments
2.4. Statistical Modeling of CCD Data
2.5. Measurement of Continued Egg Production
2.6. Staining and Quantification of Lipid Droplets in the Fat Body
2.7. Measurement of Food and Energy Intake
2.8. Hemolymph Glucose Measurement
2.9. Ovary Morphology Imaging and Ovarian Stage Identification
2.10. Insulin Sensitivity Assay of Dissected Ovaries
2.11. mRNA-seq Library Preparation, Sequencing and Analysis
2.12. Other Statistical Information and Visualization Tools
3. Results and Discussion
3.1. CCD Analysis Evaluating the Role of Sucrose and Protein on Body Fat and Fertility
3.2. Evaluating Body Fat and Fertility as Distinct Responses or As an Integrated Response under Different Scenarios
3.3. Experimental Verification of the Ob Aspect of Pathophysiology in the Ob-T2D-OD Model
3.4. Evaluation of the OD Aspect of Pathophysiology in the Ob-T2D-OD Model
3.5. Verification of the T2D Aspect of Pathophysiology in the Ob-T2D-OD Model
3.6. Dynamic Progression of Diet-Induced Pathophysiology in Ob-T2D-OD Model
3.7. Oogenesis Defect in Ob-T2D-OD Model Resembles That of the Human Condition PCOS
3.8. Implications of the Ob-T2D-OD Disease Model in Relation to PCOS and Previous Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, J.; He, F.; Xie, G.; Deng, W. Maternal AP determinants in the Drosophila oocyte and embryo. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 562–581. [Google Scholar] [CrossRef] [PubMed]
- Sieber, M.H.; Spradling, A.C. The role of metabolic states in development and disease. Curr. Opin. Genet. Dev. 2017, 45, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Arhin, S.K.; Lu, J.; Xi, H.; Jin, X. Energy requirements in mammalian oogenesis. Cell. Mol. Biol. 2018, 64, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Olson, E.; Titi, E.S. Spectral Filtering of Interpolant Observables for a Discrete-in-Time Downscaling Data Assimilation Algorithm. SIAM J. Appl. Dyn. Syst. 2019, 18, 1118–1142. [Google Scholar] [CrossRef] [Green Version]
- Roth, S. Drosophila oogenesis: Coordinating germ line and soma. Curr. Biol. 2001, 11, R779–R781. [Google Scholar] [CrossRef] [Green Version]
- Klusza, S.; Deng, W.-M. At the crossroads of differentiation and proliferation: Precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells. BioEssays 2010, 33, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Mirth, C.K.; Alves, A.N.; Piper, M.D.W. Turning food into eggs: Insights from nutritional biology and developmental physiology of Drosophila. Curr. Opin. Insect Sci. 2019, 31, 49–57. [Google Scholar] [CrossRef]
- van Dam, E.; van Leeuwen, L.A.G.; dos Santos, E.; James, J.; Best, L.; Lennicke, C.; Vincent, A.J.; Marinos, G.; Foley, A.; Buricova, M.; et al. Sugar-Induced Obesity and Insulin Resistance Are Uncoupled from Shortened Survival in Drosophila. Cell Metab. 2020, 31, 710–725.e7. [Google Scholar] [CrossRef]
- Machtinger, R.; Combelles, C.M.H.; Missmer, S.A.; Correia, K.; Fox, J.H.; Racowsky, C. The association between severe obesity and characteristics of failed fertilized oocytes. Hum. Reprod. 2012, 27, 3198–3207. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, K.A.; Campbell, R.E.; Benrick, A.; Giacobini, P.; Dumesic, D.A.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41, bnaa010. [Google Scholar] [CrossRef]
- Musselman, L.P.; Kühnlein, R.P. Drosophilaas a model to study obesity and metabolic disease. J. Exp. Biol. 2018, 221, jeb163881. [Google Scholar] [CrossRef] [Green Version]
- Bestetti, I.; Barbieri, C.; Sironi, A.; Specchia, V.; Yatsenko, S.A.; De Donno, M.D.; Caslini, C.; Gentilini, D.; Crippa, M.; Larizza, L.; et al. Targeted whole exome sequencing and Drosophila modelling to unveil the molecular basis of primary ovarian insufficiency. Hum. Reprod. 2021, 36, 2975–2991. [Google Scholar] [CrossRef]
- Brookheart, R.T.; Swearingen, A.R.; Collins, C.A.; Cline, L.M.; Duncan, J.G. High-sucrose-induced maternal obesity disrupts ovarian function and decreases fertility in Drosophila melanogaster. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1255–1263. [Google Scholar] [CrossRef]
- Baker, K.D.; Thummel, C.S. Diabetic Larvae and Obese Flies—Emerging Studies of Metabolism in Drosophila. Cell Metab. 2007, 6, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Morris, S.N.; Coogan, C.; Chamseddin, K.; Fernandez-Kim, S.O.; Kolli, S.; Keller, J.N.; Bauer, J.H. Development of diet-induced insulin resistance in adult Drosophila melanogaster. Biochim. Biophys. Act. 2012, 1822, 1230–1237. [Google Scholar] [CrossRef] [Green Version]
- Lenaerts, C.; Monjon, E.; Van Lommel, J.; Verbakel, L.; Broeck, J.V. Peptides in insect oogenesis. Curr. Opin. Insect Sci. 2018, 31, 58–64. [Google Scholar] [CrossRef]
- Lin, K.-Y.; Hsu, H.-J. Regulation of adult female germline stem cells by nutrient-responsive signaling. Curr. Opin. Insect Sci. 2020, 37, 16–22. [Google Scholar] [CrossRef]
- Grönke, S.; Clarke, D.-F.; Broughton, S.; Andrews, T.D.; Partridge, L. Molecular Evolution and Functional Characterization of Drosophila Insulin-Like Peptides. PLoS Genet. 2010, 6, e1000857. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Chen, W.; Kang, K.; Pang, R.; Dong, Y.; Liu, K.; Zhang, W. FoxO directly regulates the expression of TOR/S6K and vitellogenin to modulate the fecundity of the brown planthopper. Sci. China Life Sci. 2020, 64, 133–143. [Google Scholar] [CrossRef]
- Silvestris, E.; De Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, K.M. Obesity and Lifestyle Management in Polycystic Ovary Syndrome. Clin. Obstet. Gynecol. 2007, 50, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Bonora, E.; Moghetti, P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: A comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum. Reprod. 2017, 32, 2515–2521. [Google Scholar] [CrossRef] [Green Version]
- Dag, Z.O.; Dilbaz, B. Impact of obesity on infertility in women. J. Turk. Gynecol. Assoc. 2015, 16, 111–117. [Google Scholar] [CrossRef]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef]
- Wellons, M.F.; Matthews, J.J.; Kim, C. Ovarian aging in women with diabetes: An overview. Maturitas 2016, 96, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Gambineri, A.; Patton, L.; Altieri, P.; Pagotto, U.; Pizzi, C.; Manzoli, L.; Pasquali, R. Polycystic Ovary Syndrome Is a Risk Factor for Type 2 Diabetes. Diabetes 2012, 61, 2369–2374. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Yang, J.; Xiao, Z.; Zhou, S.; Wang, L. A diet-induced type 2 diabetes model in Drosophila. Sci. China Life Sci. 2020, 64, 326–329. [Google Scholar] [CrossRef]
- Alfa, R.W.; Kim, S.K. Using Drosophila to discover mechanisms underlying type 2 diabetes. Dis. Model. Mech. 2016, 9, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Ait-Amir, B.; Pougnet, P.; El Hami, A. Meta-Model Development. In Embedded Mechatronic Systems 2; El Hami, A., Pougnet, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 151–179. [Google Scholar] [CrossRef]
- Lesperance, D.N.A.; Broderick, N.A. Meta-analysis of Diets Used in Drosophila Microbiome Research and Introduction of theDrosophila Dietary Composition Calculator (DDCC). G3 Genes Genomes Genetics 2020, 10, 2207–2211. [Google Scholar] [CrossRef]
- Jia, D.; Xu, Q.; Xie, Q.; Mio, W.; Deng, W.-M. Automatic stage identification of Drosophila egg chamber based on DAPI images. Sci. Rep. 2016, 6, srep18850. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Luiz, M.T.; Viegas, J.S.R.; Abriata, J.P.; Viegas, F.; Vicentini, F.T.M.D.C.; Bentley, M.V.L.B.; Chorilli, M.; Marchetti, J.M.; Tapia-Blácido, D.R. Design of experiments (DoE) to develop and to optimize nanoparticles as drug delivery systems. Eur. J. Pharm. Biopharm. 2021, 165, 127–148. [Google Scholar] [CrossRef]
- Rani, L.; Saini, S.; Shukla, N.; Chowdhuri, D.K.; Gautam, N.K. High sucrose diet induces morphological, structural and functional impairments in the renal tubules of Drosophila melanogaster: A model for studying type-2 diabetes mediated renal tubular dysfunction. Insect Biochem. Mol. Biol. 2020, 125, 103441. [Google Scholar] [CrossRef]
- Colinet, H.; Larvor, V.; Bical, R.; Renault, D. Dietary sugars affect cold tolerance of Drosophila melanogaster. Metabolomics 2012, 9, 608–622. [Google Scholar] [CrossRef]
- Villanueva, J.E.; Livelo, C.; Trujillo, A.S.; Chandran, S.; Woodworth, B.; Andrade, L.; Le, H.D.; Manor, U.; Panda, S.; Melkani, G.C. Time-restricted feeding restores muscle function in Drosophila models of obesity and circadian-rhythm disruption. Nat. Commun. 2019, 10, 2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skorupa, D.A.; Dervisefendic, A.; Zwiener, J.; Pletcher, S.D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell 2008, 7, 478–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra-Peralbo, E.; Talamillo, A.; Barrio, R. Origin and Development of the Adipose Tissue, a Key Organ in Physiology and Disease. Front. Cell Dev. Biol. 2021, 9, 786129. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.M.; Bratu, D.P. Drosophila melanogaster Oogenesis: An Overview. In Drosophila Oogenesis. Methods in Molecular Biology; Bratu, D., McNeil, G., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1328, pp. 1–20. [Google Scholar] [CrossRef]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. Ovarian antral folliculogenesis during the human menstrual cycle: A review. Hum. Reprod. Updat. 2011, 18, 73–91. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Wei, C.; Wu, H.; Cheung, D.; Jiao, R.; Ma, J. Fundamental origins and limits for scaling a maternal morphogen gradient. Nat. Commun. 2015, 6, 6679. [Google Scholar] [CrossRef] [Green Version]
- Terashima, J.; Bownes, M. A microarray analysis of genes involved in relating egg production to nutritional intake in Drosophila melanogaster. Cell Death Differ. 2005, 12, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Kilpatrick, K.A. Starvation Induces Polycystic Ovarian Syndrome (PCOS) Like Symptoms in Drosophila Melanogaster; Mississippi College: Clinton, MI, USA, 2016. [Google Scholar]
- Barth, J.M.I.; Szabad, J.; Hafen, E.; Köhler, K. Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ. 2010, 18, 915–924. [Google Scholar] [CrossRef]
- Jevitt, A.; Chatterjee, D.; Xie, G.; Wang, X.-F.; Otwell, T.; Huang, Y.-C.; Deng, W.-M. A single-cell atlas of adult Drosophila ovary identifies transcriptional programs and somatic cell lineage regulating oogenesis. PLoS Biol. 2020, 18, e3000538. [Google Scholar] [CrossRef]
- Dompe, C.; Kulus, M.; Stefańska, K.; Kranc, W.; Chermuła, B.; Bryl, R.; Pieńkowski, W.; Nawrocki, M.; Petitte, J.; Stelmach, B.; et al. Human Granulosa Cells—Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells 2021, 10, 1396. [Google Scholar] [CrossRef]
- Burks, D.J.; De Mora, J.F.; Schubert, M.; Withers, D.J.; Myers, M.G.; Towery, H.H.; Altamuro, S.L.; Flint, C.L.; White, M.F. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 2000, 407, 377–382. [Google Scholar] [CrossRef]
- Sánchez, F.; Smitz, J. Molecular control of oogenesis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 1896–1912. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Liu, H.; Gu, X.; Boots, C.; Moley, K.H.; Wang, Q. Metabolic control of oocyte development: Linking maternal nutrition and reproductive outcomes. Cell. Mol. Life Sci. 2015, 72, 251–271. [Google Scholar] [CrossRef]
- Armstrong, A.R. Drosophila melanogaster as a model for nutrient regulation of ovarian function. Reproduction 2020, 159, R69–R82. [Google Scholar] [CrossRef]
- de Medeiros, S.F.; Rodgers, R.J.; Norman, R.J. Adipocyte and steroidogenic cell cross-talk in polycystic ovary syndrome. Hum. Reprod. Updat. 2021, 27, 771–796. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.L.; Chen, W.; Dissen, G.A.; Ojeda, S.R.; Cowley, M.; Garcia-Rudaz, C.; Enriori, P.J. Excess of Nerve Growth Factor in the Ovary Causes a Polycystic Ovary-Like Syndrome in Mice, which Closely Resembles Both Reproductive and Metabolic Aspects of the Human Syndrome. Endocrinology 2014, 155, 4494–4506. [Google Scholar] [CrossRef]
- Dissen, G.A.; Garcia-Rudaz, C.; Paredes, A.; Mayer, C.; Mayerhofer, A.; Ojeda, S.R. Excessive Ovarian Production of Nerve Growth Factor Facilitates Development of Cystic Ovarian Morphology in Mice and Is a Feature of Polycystic Ovarian Syndrome in Humans. Endocrinology 2009, 150, 2906–2914. [Google Scholar] [CrossRef]
- Garcia-Rudaz, C.; Dorfman, M.; Nagalla, S.; Svechnikov, K.; Söder, O.; Ojeda, S.R.; Dissen, G.A. Excessive ovarian production of nerve growth factor elicits granulosa cell apoptosis by setting in motion a tumor necrosis factor α/stathmin-mediated death signaling pathway. Reproduction 2011, 142, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, Y.F.; Alorabi, M.; Abdelzaher, W.Y.; Toni, N.D.; Thabet, K.; Hegazy, A.; Bahaa, H.A.; Batiha, G.E.-S.; Welson, N.N.; Morsy, M.A.; et al. Diacerein ameliorates letrozole-induced polycystic ovarian syndrome in rats. Biomed. Pharmacother. 2022, 149, 112870. [Google Scholar] [CrossRef]
- Wang, Z.; Nie, K.; Su, H.; Tang, Y.; Wang, H.; Xu, X.; Dong, H. Berberine improves ovulation and endometrial receptivity in polycystic ovary syndrome. Phytomedicine 2021, 91, 153654. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Lee, S.-H. Effectiveness of Lifestyle Modification in Polycystic Ovary Syndrome Patients with Obesity: A Systematic Review and Meta-Analysis. Life 2022, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.B.; Nichols, C.D. Human Disease Models in Drosophila melanogaster and the Role of the Fly in Therapeutic Drug Discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, V.R.; Solon-Biet, S.M.; Senior, A.M.; Edwards, M.C.; Desai, R.; Tedla, N.; Cox, M.J.; Ledger, W.L.; Gilchrist, R.B.; Simpson, S.J.; et al. Defining the impact of dietary macronutrient balance on PCOS traits. Nat. Commun. 2020, 11, 5262. [Google Scholar] [CrossRef]
- Roberts, J.S.; Perets, R.A.; Sarfert, K.S.; Bowman, J.J.; Ozark, P.A.; Whitworth, G.B.; Blythe, S.N.; Toporikova, N. High-fat high-sugar diet induces polycystic ovary syndrome in a rodent model. Biol. Reprod. 2017, 96, 551–562. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, J.; Chang, X.; He, F.; Ma, J. Modeling Obesity-Associated Ovarian Dysfunction in Drosophila. Nutrients 2022, 14, 5365. https://doi.org/10.3390/nu14245365
Liu H, Li J, Chang X, He F, Ma J. Modeling Obesity-Associated Ovarian Dysfunction in Drosophila. Nutrients. 2022; 14(24):5365. https://doi.org/10.3390/nu14245365
Chicago/Turabian StyleLiu, Huanju, Jiajun Li, Xinyue Chang, Feng He, and Jun Ma. 2022. "Modeling Obesity-Associated Ovarian Dysfunction in Drosophila" Nutrients 14, no. 24: 5365. https://doi.org/10.3390/nu14245365