The Mediterranean Diet: An Update of the Clinical Trials
Abstract
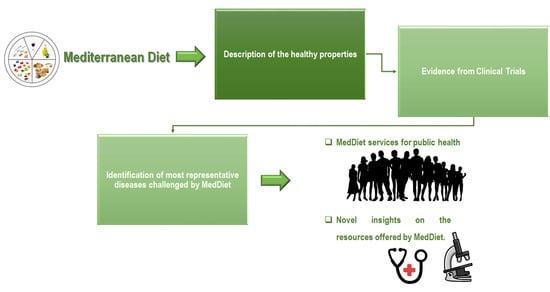
:1. Introduction
2. The MedDiet: Definition and Composition
3. MedDiet and Immunity
4. The MedDiet in Clinical Trials: An Update
5. Evidence from the Clinical Trials
5.1. The MedDiet and Metabolic Diseases
5.2. The MedDiet and Cardiovascular Diseases
5.3. The MedDiet and Cancer
6. Conclusion and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Lacatusu, C.M.; Grigorescu, E.D.; Floria, M.; Onofriescu, A.; Mihai, B.M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra-Majem, L.; Ortiz-Andrellucchi, A.; Sánchez-Villegas, A. Mediterranean Diet. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Oxford, UK, 2019; pp. 292–301. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.M. Biographical notes on Ancel Keys and Salim Yusuf: Origins and significance of the seven countries study and the INTERHEART study. J. Clin. Lipidol. 2011, 5, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Taylor, H.L.; Blackburn, H.; Brozek, J.; Anderson, J.T.; Simonson, E. Coronary Heart Disease among Minnesota Business and Professional Men Followed Fifteen Years. Circulation 1963, 28, 381–395. [Google Scholar] [CrossRef] [Green Version]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef]
- de Lorgeril, M. Mediterranean diet and cardiovascular disease: Historical perspective and latest evidence. Curr. Atheroscler. Rep. 2013, 15, 370. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [Green Version]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Macian, F.; Ordovas, J.M. Advances in Understanding the Molecular Basis of the Mediterranean Diet Effect. Annu. Rev. Food Sci. Technol. 2018, 9, 227–249. [Google Scholar] [CrossRef]
- Kargin, D.; Tomaino, L.; Serra-Majem, L. Experimental Outcomes of the Mediterranean Diet: Lessons Learned from the Predimed Randomized Controlled Trial. Nutrients 2019, 11, 2991. [Google Scholar] [CrossRef] [Green Version]
- Finicelli, M.; Squillaro, T.; Di Cristo, F.; Di Salle, A.; Melone, M.A.B.; Galderisi, U.; Peluso, G. Metabolic syndrome, Mediterranean diet, and polyphenols: Evidence and perspectives. J. Cell. Physiol. 2019, 234, 5807–5826. [Google Scholar] [CrossRef]
- Gouveri, E.; Diamantopoulos, E.J. The Mediterranean diet andmetabolic syndrome. In The Mediterranean Diet; Preedy, V., Watson, R., Eds.; Elsevier Inc.: Cambridge, UK, 2015; pp. 313–323. [Google Scholar] [CrossRef]
- Wang, C.; Enssle, J.; Pietzner, A.; Schmocker, C.; Weiland, L.; Ritter, O.; Jaensch, M.; Elbelt, U.; Pagonas, N.; Weylandt, K.H. Essential Polyunsaturated Fatty Acids in Blood from Patients with and without Catheter-Proven Coronary Artery Disease. Int. J. Mol. Sci. 2022, 23, 766. [Google Scholar] [CrossRef]
- Alexander, D.D.; Miller, P.E.; Van Elswyk, M.E.; Kuratko, C.N.; Bylsma, L.C. A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin. Proc. 2017, 92, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Schlesinger, S.; Devleesschauwer, B.; Hoffmann, G.; Bechthold, A.; Schwedhelm, C.; Iqbal, K.; Knuppel, S.; Boeing, H. Generating the evidence for risk reduction: A contribution to the future of food-based dietary guidelines. Proc. Nutr. Soc. 2018, 77, 432–444. [Google Scholar] [CrossRef] [Green Version]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef]
- Di Cristo, F.; Finicelli, M.; Digilio, F.A.; Paladino, S.; Valentino, A.; Scialo, F.; D’Apolito, M.; Saturnino, C.; Galderisi, U.; Giordano, A.; et al. Meldonium improves Huntington’s disease mitochondrial dysfunction by restoring peroxisome proliferator-activated receptor gamma coactivator 1alpha expression. J. Cell. Physiol. 2019, 234, 9233–9246. [Google Scholar] [CrossRef]
- Joseph, L.C.; Barca, E.; Subramanyam, P.; Komrowski, M.; Pajvani, U.; Colecraft, H.M.; Hirano, M.; Morrow, J.P. Inhibition of NAPDH Oxidase 2 (NOX2) Prevents Oxidative Stress and Mitochondrial Abnormalities Caused by Saturated Fat in Cardiomyocytes. PLoS ONE 2016, 11, e0145750. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, P.; Lozano-Sanchez, J.; Borras-Linares, I.; Emanuelli, T.; Menendez, J.A.; Segura-Carretero, A. Structure-Biological Activity Relationships of Extra-Virgin Olive Oil Phenolic Compounds: Health Properties and Bioavailability. Antioxidants 2020, 9, 685. [Google Scholar] [CrossRef]
- Karkovic Markovic, A.; Toric, J.; Barbaric, M.; Jakobusic Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function—A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Rodriguez, E.; Biel-Glesson, S.; Fernandez-Navarro, J.R.; Calleja, M.A.; Espejo-Calvo, J.A.; Gil-Extremera, B.; de la Torre, R.; Fito, M.; Covas, M.I.; Vilchez, P.; et al. Effects of Virgin Olive Oils Differing in Their Bioactive Compound Contents on Biomarkers of Oxidative Stress and Inflammation in Healthy Adults: A Randomized Double-Blind Controlled Trial. Nutrients 2019, 11, 626. [Google Scholar] [CrossRef] [Green Version]
- Colica, C.; Di Renzo, L.; Trombetta, D.; Smeriglio, A.; Bernardini, S.; Cioccoloni, G.; Costa de Miranda, R.; Gualtieri, P.; Sinibaldi Salimei, P.; De Lorenzo, A. Antioxidant Effects of a Hydroxytyrosol-Based Pharmaceutical Formulation on Body Composition, Metabolic State, and Gene Expression: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Oxid. Med. Cell. Longev. 2017, 2017, 2473495. [Google Scholar] [CrossRef]
- Delgado, A.M.; Vaz Almeida, M.D.; Parisi, S. Chemistry of the Mediterranean Diet; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Hoffman, R.; Gerber, M. Evaluating and adapting the Mediterranean diet for non-Mediterranean populations: A critical appraisal. Nutr. Rev. 2013, 71, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Bechthold, A.; Boeing, H.; Schwedhelm, C.; Hoffmann, G.; Knuppel, S.; Iqbal, K.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Schlesinger, S.; et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food. Sci. Nutr. 2019, 59, 1071–1090. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food Groups and Risk of Overweight, Obesity, and Weight Gain: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2019, 10, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knuppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.M.; Knuppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 1462–1473. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knuppel, S.; Iqbal, K.; Andriolo, V.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food Groups and Risk of Hypertension: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2017, 8, 793–803. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zec, M.; Glibetic, M. Health Benefits of Nut Consumption. In Reference Module in Food Science; Elsevier: Oxford, UK, 2018. [Google Scholar] [CrossRef]
- Kelly, J.H., Jr.; Sabate, J. Nuts and coronary heart disease: An epidemiological perspective. Br. J. Nutr. 2006, 96 (Suppl. 2), 61–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Jacobs, D.R., Jr.; Mayer-Davis, E.; Szklo, M.; Herrington, D.; Jenny, N.S.; Kronmal, R.; Barr, R.G. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2006, 163, 222–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opie, L.H.; Lecour, S. The red wine hypothesis: From concepts to protective signalling molecules. Eur. Heart J. 2007, 28, 1683–1693. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Serra-Majem, L.; Roman-Vinas, B.; Sanchez-Villegas, A.; Guasch-Ferre, M.; Corella, D.; La Vecchia, C. Benefits of the Mediterranean diet: Epidemiological and molecular aspects. Mol. Asp. Med. 2019, 67, 1–55. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [Green Version]
- Finicelli, M.; Digilio, F.A.; Galderisi, U.; Peluso, G. The Emerging Role of Macrophages in Chronic Obstructive Pulmonary Disease: The Potential Impact of Oxidative Stress and Extracellular Vesicle on Macrophage Polarization and Function. Antioxidants 2022, 11, 464. [Google Scholar] [CrossRef]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef]
- Parkinson, L.; Keast, R. Oleocanthal, a phenolic derived from virgin olive oil: A review of the beneficial effects on inflammatory disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar] [CrossRef] [Green Version]
- Scotece, M.; Gomez, R.; Conde, J.; Lopez, V.; Gomez-Reino, J.J.; Lago, F.; Smith, A.B., 3rd; Gualillo, O. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of MIP-1alpha and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [Green Version]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef] [Green Version]
- Pauls, S.D.; Rodway, L.A.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-inflammatory effects of alpha-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from alpha-linolenic and linoleic acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef]
- Ni, Y.F.; Wang, J.; Yan, X.L.; Tian, F.; Zhao, J.B.; Wang, Y.J.; Jiang, T. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respir. Res. 2010, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Montero, C.; Fraile-Martinez, O.; Gomez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; Garcia-Honduvilla, N.; Asunsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; Naya, I.; Garcia-Honduvilla, N.; Alvarez-Mon, M.; Bujan, J.; Asunsolo, A.; de la Torre, B. Type 2 Diabetes Mellitus Associated with Obesity (Diabesity). The Central Role of Gut Microbiota and Its Translational Applications. Nutrients 2020, 12, 2749. [Google Scholar] [CrossRef]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Micro-RNAs: Crossroads between the Exposure to Environmental Particulate Pollution and the Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 7221. [Google Scholar] [CrossRef]
- Lim, C.C.; Hayes, R.B.; Ahn, J.; Shao, Y.; Silverman, D.T.; Jones, R.R.; Thurston, G.D. Mediterranean Diet and the Association Between Air Pollution and Cardiovascular Disease Mortality Risk. Circulation 2019, 139, 1766–1775. [Google Scholar] [CrossRef]
- Whyand, T.; Hurst, J.R.; Beckles, M.; Caplin, M.E. Pollution and respiratory disease: Can diet or supplements help? A review. Respir. Res. 2018, 19, 79. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z. Common Limitations and Challenges of Dietary Clinical Trials for Translation into Clinical Practices. Int. J. Endocrinol. Metab. 2021, 19, 108170. [Google Scholar] [CrossRef]
- Weaver, C.M.; Miller, J.W. Challenges in conducting clinical nutrition research. Nutr. Rev. 2017, 75, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, H.N.; MacCallum, P.R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J. Cardiometab. Syndr. 2009, 4, 113–119. [Google Scholar] [CrossRef]
- Alegria Ezquerra, E.; Castellano Vazquez, J.M.; Alegria Barrero, A. Obesity, metabolic syndrome and diabetes: Cardiovascular implications and therapy. Rev. Esp. Cardiol. 2008, 61, 752–764. [Google Scholar] [CrossRef] [Green Version]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [Green Version]
- Andreoli, B.; Mantovani, A.; Andreoli, C. Type 2 Diabetes, sarcopenic obesity and Mediterranean food pattern: Considerations about the therapeutic effect and the problem of maintaining weight loss and healthy habits. The outpatient experience of two clinical cases. J. Clin. Transl. Endocrinol. Case Rep. 2020, 16, 100061. [Google Scholar] [CrossRef]
- Bagetta, D.; Maruca, A.; Lupia, A.; Mesiti, F.; Catalano, R.; Romeo, I.; Moraca, F.; Ambrosio, F.A.; Costa, G.; Artese, A.; et al. Mediterranean products as promising source of multi-target agents in the treatment of metabolic syndrome. Eur. J. Med. Chem. 2020, 186, 111903. [Google Scholar] [CrossRef]
- Santulli, G.; Pascale, V.; Finelli, R.; Visco, V.; Giannotti, R.; Massari, A.; Morisco, C.; Ciccarelli, M.; Illario, M.; Iaccarino, G.; et al. We are What We Eat: Impact of Food from Short Supply Chain on Metabolic Syndrome. J. Clin. Med. 2019, 8, 2061. [Google Scholar] [CrossRef] [Green Version]
- Salas-Salvado, J.; Guasch-Ferre, M.; Lee, C.H.; Estruch, R.; Clish, C.B.; Ros, E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J. Nutr. 2015, 146, 920S–927S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luisi, M.L.E.; Lucarini, L.; Biffi, B.; Rafanelli, E.; Pietramellara, G.; Durante, M.; Vidali, S.; Provensi, G.; Madiai, S.; Gheri, C.F.; et al. Effect of Mediterranean Diet Enriched in High Quality Extra Virgin Olive Oil on Oxidative Stress, Inflammation and Gut Microbiota in Obese and Normal Weight Adult Subjects. Front. Pharm. 2019, 10, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimak, E.; Zieba, B.; Duma, D.; Solski, J. Myeloperoxidase level and inflammatory markers and lipid and lipoprotein parameters in stable coronary artery disease. Lipids Health Dis. 2018, 17, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, A.; Finicelli, M.; Grossi, M.; Vicchio, M.; Alessio, N.; Sante, P.; De Feo, M.; Cotrufo, M.; Berrino, L.; Rossi, F.; et al. DNA damage and repair in a model of rat vascular injury. Clin. Sci. 2010, 118, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Yen, M.; Donma, O.; Yildizfer, F.; Ekmekci, O.; Asli Karatas Kul, Z.; Esat Imal, A.; Keser, Z.; Cagil, E.; Mengi, M.; Ekmekci, H.; et al. Association of fetuin A, adiponectin, interleukin 10 and total antioxidant capacity with IVF outcomes. Iran. J. Reprod. Med. 2014, 12, 747–754. [Google Scholar]
- van Dijk, S.J.; Feskens, E.J.; Bos, M.B.; de Groot, L.C.; de Vries, J.H.; Muller, M.; Afman, L.A. Consumption of a high monounsaturated fat diet reduces oxidative phosphorylation gene expression in peripheral blood mononuclear cells of abdominally overweight men and women. J. Nutr. 2012, 142, 1219–1225. [Google Scholar] [CrossRef] [Green Version]
- Bondia-Pons, I.; Martinez, J.A.; de la Iglesia, R.; Lopez-Legarrea, P.; Poutanen, K.; Hanhineva, K.; Zulet Mde, L. Effects of short- and long-term Mediterranean-based dietary treatment on plasma LC-QTOF/MS metabolic profiling of subjects with metabolic syndrome features: The Metabolic Syndrome Reduction in Navarra (RESMENA) randomized controlled trial. Mol. Nutr. Food. Res. 2015, 59, 711–728. [Google Scholar] [CrossRef]
- Mathew, A.V.; Li, L.; Byun, J.; Guo, Y.; Michailidis, G.; Jaiswal, M.; Chen, Y.E.; Pop-Busui, R.; Pennathur, S. Therapeutic Lifestyle Changes Improve HDL Function by Inhibiting Myeloperoxidase-Mediated Oxidation in Patients With Metabolic Syndrome. Diabetes Care 2018, 41, 2431–2437. [Google Scholar] [CrossRef] [Green Version]
- Fernemark, H.; Jaredsson, C.; Bunjaku, B.; Rosenqvist, U.; Nystrom, F.H.; Guldbrand, H. A randomized cross-over trial of the postprandial effects of three different diets in patients with type 2 diabetes. PLoS ONE 2013, 8, e79324. [Google Scholar] [CrossRef]
- Fortin, A.; Rabasa-Lhoret, R.; Lemieux, S.; Labonte, M.E.; Gingras, V. Comparison of a Mediterranean to a low-fat diet intervention in adults with type 1 diabetes and metabolic syndrome: A 6-month randomized trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1275–1284. [Google Scholar] [CrossRef]
- Maiorino, M.I.; Bellastella, G.; Petrizzo, M.; Gicchino, M.; Caputo, M.; Giugliano, D.; Esposito, K. Effect of a Mediterranean diet on endothelial progenitor cells and carotid intima-media thickness in type 2 diabetes: Follow-up of a randomized trial. Eur. J. Prev. Cardiol. 2017, 24, 399–408. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Keys, A. Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease; Harvard University Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Becerra-Tomas, N.; Blanco Mejia, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelic, D.; Sievenpiper, J.L.; Salas-Salvado, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Gonzalez-Manzano, S.; Gonzalez-Paramas, A.M. Wine, Polyphenols, and Mediterranean Diets. What Else Is There to Say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, 34. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Fernandez-Gandara, C.; Garcia-Rios, A.; Rangel-Zuniga, O.A.; Gutierrez-Mariscal, F.M.; Torres-Pena, J.D.; Marin, C.; Lopez-Moreno, J.; Castano, J.P.; Delgado-Lista, J.; et al. Mediterranean diet and endothelial function in patients with coronary heart disease: An analysis of the CORDIOPREV randomized controlled trial. PLoS Med. 2020, 17, e1003282. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Pena, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Berezin, A.; Zulli, A.; Kerrigan, S.; Petrovic, D.; Kruzliak, P. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin. Biochem. 2015, 48, 562–568. [Google Scholar] [CrossRef]
- Przybylska, S.; Tokarczyk, G. Lycopene in the Prevention of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef]
- Gajendragadkar, P.R.; Hubsch, A.; Maki-Petaja, K.M.; Serg, M.; Wilkinson, I.B.; Cheriyan, J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: A randomised controlled trial. PLoS ONE 2014, 9, e99070. [Google Scholar] [CrossRef]
- Bedard, A.; Dodin, S.; Corneau, L.; Lemieux, S. Impact of the traditional Mediterranean diet on the Framingham risk score and the metabolic syndrome according to sex. Metab. Syndr. Relat. Disord. 2014, 12, 95–101. [Google Scholar] [CrossRef]
- Carruba, G.; Granata, O.M.; Pala, V.; Campisi, I.; Agostara, B.; Cusimano, R.; Ravazzolo, B.; Traina, A. A traditional Mediterranean diet decreases endogenous estrogens in healthy postmenopausal women. Nutr. Cancer 2006, 56, 253–259. [Google Scholar] [CrossRef]
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef]
- Bedard, A.; Riverin, M.; Dodin, S.; Corneau, L.; Lemieux, S. Sex differences in the impact of the Mediterranean diet on cardiovascular risk profile. Br. J. Nutr. 2012, 108, 1428–1434. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile: CARDIVEG Study (Cardiovascular Prevention With Vegetarian Diet). Circulation 2018, 137, 1103–1113. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Veronesi, M.; Rizzo, M.; Giovannini, M.; Borghi, C. Short-Term Effects of a Combined Nutraceutical on Lipid Level, Fatty Liver Biomarkers, Hemodynamic Parameters, and Estimated Cardiovascular Disease Risk: A Double-Blind, Placebo-Controlled Randomized Clinical Trial. Adv. Ther. 2017, 34, 1966–1975. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A Review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef] [Green Version]
- Farras, M.; Almanza-Aguilera, E.; Hernaez, A.; Agusti, N.; Julve, J.; Fito, M.; Castaner, O. Beneficial effects of olive oil and Mediterranean diet on cancer physio-pathology and incidence. Semin. Cancer Biol. 2021, 73, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Serrano, M.; Blasco, M.A. The common biology of cancer and ageing. Nature 2007, 448, 767–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiseman, M.J. Nutrition and cancer: Prevention and survival. Br. J. Nutr. 2019, 122, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Bruno, E.; Oliverio, A.; Paradiso, A.V.; Daniele, A.; Tommasi, S.; Tufaro, A.; Terribile, D.A.; Magno, S.; Filippone, A.; Venturelli, E.; et al. A Mediterranean Dietary Intervention in Female Carriers of BRCA Mutations: Results from an Italian Prospective Randomized Controlled Trial. Cancers 2020, 12, 3732. [Google Scholar] [CrossRef]
- Dumais, V.; Lumingu, J.; Bedard, M.; Paquet, L.; Verma, S.; Fontaine-Bisson, B. Prevalence of Insulin Resistance, Metabolic Syndrome, and Type 2 Diabetes in Canadian Women at High Risk for Breast Cancer. Breast J 2017, 23, 482–483. [Google Scholar] [CrossRef]
- Pasanisi, P.; Bruno, E.; Venturelli, E.; Manoukian, S.; Barile, M.; Peissel, B.; De Giacomi, C.; Bonanni, B.; Berrino, J.; Berrino, F. Serum levels of IGF-I and BRCA penetrance: A case control study in breast cancer families. Fam. Cancer 2011, 10, 521–528. [Google Scholar] [CrossRef]
- Ruiz-Vozmediano, J.; Lohnchen, S.; Jurado, L.; Recio, R.; Rodriguez-Carrillo, A.; Lopez, M.; Mustieles, V.; Exposito, M.; Arroyo-Morales, M.; Fernandez, M.F. Influence of a Multidisciplinary Program of Diet, Exercise, and Mindfulness on the Quality of Life of Stage IIA-IIB Breast Cancer Survivors. Integr. Cancer Ther. 2020, 19, 1534735420924757. [Google Scholar] [CrossRef]
- Majed, B.; Moreau, T.; Asselain, B.; Curie Institute Breast Cancer, G. Overweight, obesity and breast cancer prognosis: Optimal body size indicator cut-points. Breast. Cancer Res. Treat. 2009, 115, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Cho, A.R.; Choi, W.J.; Kwon, Y.J.; Lee, H.S.; Ahn, S.G.; Lee, J.W. Mediterranean Diet and Naltrexone/Bupropion Treatment for Weight Loss in Overweight and Obese Breast Cancer Survivors and Non-Cancer Participants: A Pilot Randomized Controlled Trial. Diabetes Metab. Syndr. Obes. 2020, 13, 3325–3335. [Google Scholar] [CrossRef]
- Rojas Gil, A.P.; Kodonis, I.; Ioannidis, A.; Nomikos, T.; Dimopoulos, I.; Kosmidis, G.; Katsa, M.E.; Melliou, E.; Magiatis, P. The Effect of Dietary Intervention With High-Oleocanthal and Oleacein Olive Oil in Patients With Early-Stage Chronic Lymphocytic Leukemia: A Pilot Randomized Trial. Front Oncol. 2021, 11, 810249. [Google Scholar] [CrossRef]
- Dernini, S.; Berry, E.M.; Serra-Majem, L.; La Vecchia, C.; Capone, R.; Medina, F.X.; Aranceta-Bartrina, J.; Belahsen, R.; Burlingame, B.; Calabrese, G.; et al. Med Diet 4.0: The Mediterranean diet with four sustainable benefits. Public Health Nutr. 2017, 20, 1322–1330. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. https://doi.org/10.3390/nu14142956
Finicelli M, Di Salle A, Galderisi U, Peluso G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients. 2022; 14(14):2956. https://doi.org/10.3390/nu14142956
Chicago/Turabian StyleFinicelli, Mauro, Anna Di Salle, Umberto Galderisi, and Gianfranco Peluso. 2022. "The Mediterranean Diet: An Update of the Clinical Trials" Nutrients 14, no. 14: 2956. https://doi.org/10.3390/nu14142956