Refeeding Syndrome: A Critical Reality in Patients with Chronic Disease
Abstract
:1. Introduction
2. Methodology
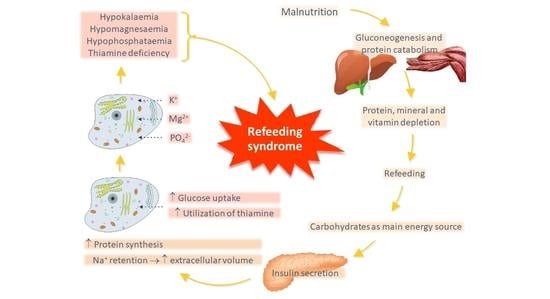
3. Pathophysiology
4. Clinical Aspects
5. State of Evidence
6. Risk Stratification
7. Management
8. Monitoring
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katona, P.; Katona-Apte, J. The Interaction between Nutrition and Infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Felder, S.; Lechtenboehmer, C.; Bally, M.; Fehr, R.; Deiss, M.; Faessler, L.; Kutz, A.; Steiner, D.; Rast, A.C.; Laukemann, S.; et al. Association of nutritional risk and adverse medical outcomes across different medical inpatient populations. Nutrition. 2015, 31, 1385–1393. [Google Scholar] [CrossRef]
- Schütz, P.; Bally, M.; Stanga, Z.; Keller, U. Loss of appetite in acutely ill medical inpatients: Physiological response or therapeutic target? Swiss Med. Wkly. 2014, 144, w13957. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Seres, D.; Lobo, D.N.; Gomes, F.; Kaegi-Braun, N.; Stanga, Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet 2021, 398, 1927–1938. [Google Scholar] [CrossRef]
- Felder, S.; Braun, N.; Stanga, Z.; Kulkarni, P.; Faessler, L.; Kutz, A.; Steiner, D.; Laukemann, S.; Haubitz, S.; Huber, A.; et al. Unraveling the Link between Malnutrition and Adverse Clinical Outcomes: Association of Acute and Chronic Malnutrition Measures with Blood Biomarkers from Different Patho-physiological States. Ann. Nutr. Metab. 2016, 68, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Imoberdorf, R.; Meier, R.; Krebs, P.; Hangartner, P.J.; Hess, B.; Stäubli, M.; Wegmann, D.; Rühlin, M.; Ballmer, P.E. Prevalence of undernutrition on admission to Swiss hospitals. Clin. Nutr. 2010, 29, 38–41. [Google Scholar] [CrossRef]
- Esposito, K.; Ciotola, M.; Sasso, F.C.; Cozzolino, D.; Saccomanno, F.; Assaloni, R.; Ceriello, A.; Giugliano, D. Effect of a single high-fat meal on endothelial function in patients with the metabolic syndrome: Role of tumor necrosis factor-α. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 274–279. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef]
- Friedli, N.; Baumann, J.; Hummel, R.; Kloter, M.; Odermatt, J.; Fehr, R.; Felder, S.; Baechli, V.; Geiser, M.; Deiss, M.; et al. Refeeding syndrome is associated with increased mortality in malnourished medical inpatients: Secondary analysis of a randomized trial. Medicine 2020, 99, e18506. [Google Scholar] [CrossRef]
- Stanga, Z.; Brunner, A.; Leuenberger, M.; Grimble, R.F.; Shenkin, A.; Allison, S.P.; Lobo, D. Nutrition in clinical practice—The refeeding syndrome: Illustrative cases and guidelines for prevention and treatment. Eur. J. Clin. Nutr. 2007, 62, 687–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crook, M.; Hally, V.; Panteli, J. The importance of the refeeding syndrome. Nutrition 2001, 17, 632–637. [Google Scholar] [CrossRef]
- Kagansky, N.; Levy, S.; Berger, D.; Knobler, H.; Koren-Morag, N. Hypophosphataemia in old patients is associated with the refeeding syndrome and reduced survival. J. Intern. Med. 2005, 257, 461–468. [Google Scholar] [CrossRef] [PubMed]
- González Avila, G.; Fajardo Rodríguez, A.; González Figueroa, E. The incidence of the refeeding syndrome in cancer patients who receive artificial nutritional treatment. Nutr. Hosp. 1996, 11, 98–101. [Google Scholar]
- Ornstein, R.M.; Golden, N.H.; Jacobson, M.S.; Shenker, I. Hypophosphatemia during nutritional rehabilitation in anorexia nervosa: Implications for refeeding and monitoring. J. Adolesc. Health 2003, 32, 83–88. [Google Scholar] [CrossRef]
- Janssen, G.; on behalf of the working group on nutrition and metabolism of the German Geriatric Society (DGG); Pourhassan, M.; Lenzen-Großimlinghaus, R.; Jäger, M.; Schäfer, R.; Spamer, C.; Cuvelier, I.; Volkert, D.; Wirth, R. The Refeeding Syndrome revisited: You can only diagnose what you know. Eur. J. Clin. Nutr. 2019, 73, 1458–1463. [Google Scholar] [CrossRef] [Green Version]
- Sevick, M.A.; Trauth, J.M.; Ling, B.S.; Anderson, R.T.; Piatt, G.A.; Kilbourne, A.M. Patients with Complex Chronic Diseases: Per-spectives on supporting self-management. J. Gen. Intern. Med. 2007, 22, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Friedli, N.; Stanga, Z.; Sobotka, L.; Culkin, A.; Kondrup, J.; Laviano, A.; Mueller, B.; Schuetz, P. Revisiting the refeeding syndrome: Results of a sys-tematic review. Nutrition 2017, 35, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Friedli, N.; Stanga, Z.; Culkin, A.; Crook, M.; Laviano, A.; Sobotka, L.; Kressig, R.W.; Kondrup, J.; Mueller, B.; Schuetz, P. Management and prevention of refeeding syndrome in medical inpatients: An evidence-based and consensus-supported algorithm. Nutrition 2018, 47, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Cahill, G.F., Jr. Fuel Metabolism in Starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Hearing, S.D. Refeeding syndrome. BMJ 2004, 328, 908–909. [Google Scholar] [CrossRef] [PubMed]
- Sasso, F.C.; Rinaldi, L.; Lascar, N.; Marrone, A.; Pafundi, P.C.; Adinolfi, L.E.; Marfella, R. Role of Tight Glycemic Control during Acute Coronary Syndrome on CV Outcome in Type 2 Diabetes. J. Diabetes Res. 2018, 2018, 3106056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boateng, A.A.; Sriram, K.; Meguid, M.M.; Crook, M. Refeeding syndrome: Treatment considerations based on collective analysis of literature case reports. Nutrition 2010, 26, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.udem.insel.ch/de/lehre-und-forschung/forschungsschwerpunkte/ernaehrungsmedizin/wichtige-abbildungen (accessed on 8 June 2022).
- Da Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr. Clin. Pract. 2020, 35, 178–195. [Google Scholar] [CrossRef] [Green Version]
- Adika, E.; Jia, R.; Li, J.; Seres, D.; Freedberg, D.E. Evaluation of The ASPEN Guidelines for Refeeding Syndrome among Hospitalized Patients Receiving Enteral Nutrition: A Retrospective Cohort Study. J. Parenter. Enter. Nutr. 2022. [Google Scholar] [CrossRef]
- Doig, G.S.; Simpson, F.; Heighes, P.T.; Bellomo, R.; Chesher, D.; Caterson, I.D.; Reade, M.C.; Harrigan, P.W.J. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: A randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir. Med. 2015, 3, 943–952. [Google Scholar]
- Olthof, L.E.; Koekkoek, W.; van Setten, C.; Kars, J.C.N.; van Blokland, D.; van Zanten, A.R.H. Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: A retrospective study. Clin. Nutr. 2018, 37, 1609–1617. [Google Scholar] [CrossRef] [Green Version]
- Rio, A.; Whelan, K.; Goff, L.; Reidlinger, D.P.; Smeeton, N. Occurrence of refeeding syndrome in adults started on artificial nutrition support: Prospective cohort study. BMJ Open 2013, 3, e002173. [Google Scholar] [CrossRef] [Green Version]
- Olsen, S.U.; Hesseberg, K.; Aas, A.-M.; Ranhoff, A.H.; Bye, A. Refeeding syndrome occurs among older adults regardless of refeeding rates: A systematic review. Nutr. Res. 2021, 91, 1–12. [Google Scholar] [CrossRef]
- Cioffi, I.; Ponzo, V.; Pellegrini, M.; Evangelista, A.; Bioletto, F.; Ciccone, G.; Pasanisi, F.; Ghigo, E.; Bo, S. The incidence of the refeeding syndrome. A sys-tematic review and meta-analyses of literature. Clin. Nutr. 2021, 40, 3688–3701. [Google Scholar] [CrossRef]
- Friedli, N.; Odermatt, J.; Reber, E.; Schuetz, P.; Stanga, Z. Refeeding syndrome: Update and clinical advice for prevention, diagnosis and treatment. Curr. Opin. Gastroenterol. 2020, 36, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Fierz, Y.C.; Kenmeni, R.; Gonthier, A.; Lier, F.; Pralong, F.; Coti Bertrand, P. Severe and prolonged hypophosphatemia after intra-venous iron administration in a malnourished patient. Eur J. Clin. Nutr. 2014, 68, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Reber, E.; Friedli, N.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Management of Refeeding Syndrome in Medical Inpatients. J. Clin. Med. 2019, 8, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubry, E.; Friedli, N.; Schuetz, P.; Stanga, Z. Refeeding syndrome in the frail elderly population: Prevention, diagnosis and management. Clin. Exp. Gastroenterol. 2018, 11, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofer, M.; Pozzi, A.; Joray, M.L.; Ott, R.; Hähni, F.; Leuenberger, M.S.; Von Känel, R.; Stanga, Z. Safe refeeding management of anorexia nervosa inpatients: An evidence-based protocol. Nutrition 2014, 30, 524–530. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krutkyte, G.; Wenk, L.; Odermatt, J.; Schuetz, P.; Stanga, Z.; Friedli, N. Refeeding Syndrome: A Critical Reality in Patients with Chronic Disease. Nutrients 2022, 14, 2859. https://doi.org/10.3390/nu14142859
Krutkyte G, Wenk L, Odermatt J, Schuetz P, Stanga Z, Friedli N. Refeeding Syndrome: A Critical Reality in Patients with Chronic Disease. Nutrients. 2022; 14(14):2859. https://doi.org/10.3390/nu14142859
Chicago/Turabian StyleKrutkyte, Gabija, Leyla Wenk, Jonas Odermatt, Philipp Schuetz, Zeno Stanga, and Natalie Friedli. 2022. "Refeeding Syndrome: A Critical Reality in Patients with Chronic Disease" Nutrients 14, no. 14: 2859. https://doi.org/10.3390/nu14142859