Dietary Protein Restriction Improves Metabolic Dysfunction in Patients with Metabolic Syndrome in a Randomized, Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
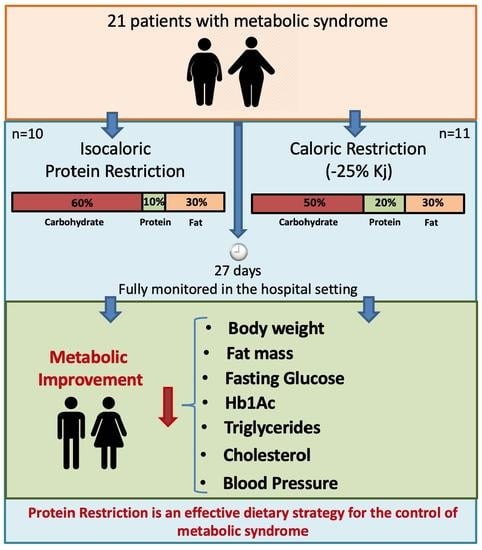
2.2. Study Participants
2.3. Intervention Groups
2.4. Endpoints
2.5. Biopsy of Subcutaneous Adipose Tissue
2.6. RNA-Seq
2.7. Single-Cell Processing and Integration
2.8. Cell Type Deconvolution
2.9. Fecal Sampling and 16S rRNA Sequencing
2.10. Metagenomics Analysis
2.11. Estimation of Participant Group Sizes
2.12. Statistical Analysis
3. Results
3.1. Study Overview and Characteristics of Participants
3.2. PR and CR Promote Weight Loss and Anthropometric Changes and Increase Energy Expenditure in Patients with Metabolic Syndrome
3.3. PR and CR Decrease Systemic Blood Pressure in Patients with Metabolic Syndrome
3.4. PR and CR Decrease Glycemia, Circulating Lipid Levels, and Inflammation in Patients with the Metabolic Syndrome
3.5. PR and CR Improves Insulin Sensitivity in Patients with Metabolic Syndrome
3.6. Metabolic Improvement Is Maintained during 1-Month Follow-Up
3.7. PR Has No Short-Term Effects on Gut Microbiota Composition
3.8. PR and CR Short-Term Dietary Restriction Effects on SAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mooradian, A.D. Dyslipidemia in type 2 diabetes mellitus. Nat. Clin. Pract. Endocrinol. Metab. 2009, 5, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speakman, J.R.; Mitchell, S.E. Caloric restriction. Mol. Asp. Med. 2011, 32, 159–221. [Google Scholar] [CrossRef] [PubMed]
- Bodkin, N.L.; Alexander, T.M.; Ortmeyer, H.K.; Johnson, E.; Hansen, B.C. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric Restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.S.; Ingram, D.K.; Lane, M.A. Caloric restriction in primates and relevance to humans. Ann. N. Y. Acad. Sci. 2006, 928, 305–315. [Google Scholar] [CrossRef]
- Anderson, R.M.; Weindruch, R. Metabolic reprogramming in dietary restriction. Interdiscip. Top. Gerontol. 2007, 35, 18–38. [Google Scholar]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- Redman, L.M.; Smith, S.R.; Burton, J.H.; Martin, C.K.; Il’Yasova, D.; Ravussin, E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab. 2018, 27, 805–815.e4. [Google Scholar] [CrossRef] [Green Version]
- Tay, J.; Luscombe-Marsh, N.; Thompson, C.H.; Noakes, M.; Buckley, J.; Wittert, G.; Yancy, W.S., Jr.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef]
- Rock, C.L.; Flatt, S.W.; Pakiz, B.; Taylor, K.S.; Leone, A.F.; Brelje, K.; Heath, D.D.; Quintana, E.L.; Sherwood, N.E. Weight Loss, Glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: A randomized controlled trial. Diabetes Care 2014, 37, 1573–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, S.J.; Le Couteur, D.; Raubenheimer, D. Putting the balance back in diet. Cell 2015, 161, 18–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagiou, P.; Sandin, S.; Weiderpass, E.; Lagiou, A.; Mucci, L.; Trichopoulos, D.; Adami, H.-O. Low carbohydrate?high protein diet and mortality in a cohort of Swedish women. J. Intern. Med. 2007, 261, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Marliss, E.; Cahill, G.F., Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 1969, 281, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, J.; Angel, A.; Farkas, J. Metabolic fate of leucine: A significant sterol precursor in adipose tissue and muscle. Am. J. Physiol. 1974, 226, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Fabbiano, S.; Suárez-Zamorano, N.; Chevalier, C.; Lazarevic, V.; Kieser, S.; Rigo, D.; Leo, S.; Veyrat-Durebex, C.; Gaïa, N.; Maresca, M.; et al. Functional Gut Microbiota Remodeling Contributes to the Caloric Restriction-Induced Metabolic Improvements. Cell Metab. 2018, 28, 907–921.e7. [Google Scholar] [CrossRef] [Green Version]
- Thaiss, C.A.; Itav, S.; Rothschild, D.; Meijer, M.; Levy, M.; Moresi, C.; Dohnalová, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef]
- Suárez-Zamorano, N.; Fabbiano, S.; Chevalier, C.; Stojanovic, O.; Colin, D.J.; Stevanović, A.; Veyrat-Durebex, C.; Tarallo, V.; Rigo, D.; Germain, S.; et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat. Med. 2015, 21, 1497–1501. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [CrossRef]
- Ferrannini, E.; Mari, A. How to measure insulin sensitivity. J. Hypertens. 1998, 16, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Dall, M.; Hassing, A.S.; Niu, L.; Nielsen, T.S.; Ingerslev, L.R.; Sulek, K.; Trammell, S.A.; Gillum, M.P.; Barrès, R.; Larsen, S.; et al. Hepatocyte-specific perturbation of NAD+ biosynthetic pathways in mice induces reversible nonalcoholic steatohepatitis–like phenotypes. J. Biol. Chem. 2021, 297, 101388. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Dong, H.; Balaz, M.; Slyper, M.; Drokhlyansky, E.; Colleluori, G.; Giordano, A.; Kovanicova, Z.; Stefanicka, P.; Balazova, L.; et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 2020, 587, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M., 3rd; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated analysis of multimodal single-cell data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ren, J. Caloric restriction and heart function: Is there a sensible link? Acta Pharmacol. Sin. 2010, 31, 1111–1117. [Google Scholar] [CrossRef] [Green Version]
- Kirchner, H.; Hofmann, S.M.; Fischer-Rosinský, A.; Hembree, J.; Abplanalp, W.; Ottaway, N.; Donelan, E.; Krishna, R.; Woods, S.C.; Müller, T.D.; et al. Caloric restriction chronically impairs metabolic programming in mice. Diabetes 2012, 61, 2734–2742. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.K.; Bhapkar, M.; Pittas, A.G.; Pieper, C.F.; Das, S.K.; Williamson, D.A.; Scott, T.; Redman, L.M.; Stein, R.; Gilhooly, C.H.; et al. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Nonobese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 743–752. [Google Scholar] [CrossRef] [Green Version]
- McPherron, A.C.; Guo, T.; Bond, N.D.; Gavrilova, O. Increasing muscle mass to improve metabolism. Adipocyte 2013, 2, 92–98. [Google Scholar] [CrossRef]
- Sardeli, A.V.; Komatsu, T.R.; Mori, M.A.; Gáspari, A.F.; Chacon-Mikahil, M.P.T. Resistance Training Prevents Muscle Loss Induced by Caloric Restriction in Obese Elderly Individuals: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 423. [Google Scholar] [CrossRef] [Green Version]
- Bárcena, C.; Quirós, P.M.; Durand, S.; Mayoral, P.; Rodríguez, F.; Caravia, X.M.; Mariño, G.; Garabaya, C.; Fernández-García, M.T.; Kroemer, G.; et al. Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism. Cell Rep. 2018, 24, 2392–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanders, D.; Stone, K.P.; Forney, L.A.; Cortez, C.C.; Dille, K.N.; Simon, J.; Xu, M.; Hotard, E.C.; Nikonorova, I.A.; Pettit, A.P.; et al. Role of GCN2-Independent Signaling through a Noncanonical PERK/NRF2 Pathway in the Physiological Responses to Dietary Methionine Restriction. Diabetes 2016, 65, 1499–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flint, A.J.; Rexrode, K.; Hu, F.B.; Glynn, R.J.; Caspard, H.; Manson, J.E.; Willett, W.C.; Rimm, E.B. Body mass index, waist circumference, and risk of coronary heart disease: A prospective study among men and women. Obes. Res. Clin. Pract. 2010, 4, e171–e181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steven, S.; Hollingsworth, K.G.; Al-Mrabeh, A.; Avery, L.; Aribisala, B.; Caslake, M.; Taylor, R. Very Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiological Changes in Responders and Nonresponders. Diabetes Care 2016, 39, 808–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandhorst, S.; Longo, V.D. Dietary Restrictions and Nutrition in the Prevention and Treatment of Cardiovascular Disease. Circ. Res. 2019, 124, 952–965. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S.; et al. Impact of Dietary Resistant Starch on the Human Gut Microbiome, Metaproteome, and Metabolome. mBio 2017, 8, e01343-17. [Google Scholar] [CrossRef] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vangay, P.; Johnson, A.; Ward, T.L.; Al-Ghalith, G.A.; Shields-Cutler, R.R.; Hillmann, B.M.; Lucas, S.K.; Beura, L.K.; Thompson, E.A.; Till, L.M.; et al. US Immigration Westernizes the Human Gut Microbiome. Cell 2018, 175, 962–972.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwick, R.K.; Guerrero-Juarez, C.F.; Horsley, V.; Plikus, M.V. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018, 27, 68–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Characteristic | CR (n = 11) | PR (n = 10) | Difference between CR and PR Effect | p-Value for Difference between CR and PR Effect |
|---|---|---|---|---|
| Age, mean (SD), y | 49 (8.5) | 51.6 (8.9) | 2.6 | 0.52 |
| Female, no. (%) | 7 (63) | 5 (50) | 2 | - |
| T2DM diagnostic, mean (SD), y | 9 (1.3) | 8 (1.4) | 1 | 0.24 |
| Height, mean (SD), m | 1.64 (0.08) | 1.65 (0.08) | −0.01 | 0.96 |
| Primary Outcomes | ||||
| Δ Fasting glucose, mean (SD), mmol/L | −8.3 (3.0) | −7.0 (3.6) | 1.3 | 0.20 |
| Δ Hemoglobin A1c, mean (SD), mmol/mol | −17.5 (7.9) | −9.2 (6.5) | 8.3 | 0.02 |
| Δ Hemoglobin A1c, mean (SD), % | −1.6 (0.7) | −0.85 (0.6) | 0.75 | 0.02 |
| Δ Weight loss, mean (SD), Kg | −6.9 (1.9) | −5.4 (3.6) | 1.5 | 0.12 |
| Weight loss, mean (SD), % | 8 (2.1) | 6.6 (4.5) | 1.4 | 0.29 |
| Secondary Outcomes | ||||
| Δ Cholesterol, mmol/L | −2.2 (1.0) | −1.9 (1.3) | 0.3 | 0.33 |
| Δ LDL cholesterol, mmol/L | −1.4 (0.6) | −1.2 (0.7) | 0.2 | 0.29 |
| Δ HDL cholesterol, mmol/L | −0.2 (0.2) | −0.2 (0.2) | 0 | 0.48 |
| Δ Triglycerides, mmol/L | −1.5 (0.8) | −0.9 (0.7) | 0.6 | 0.03 |
| Δ BP Systolic, mmHg | −46.4 (25) | −44.6 (24) | 1.8 | 0.38 |
| Δ BP Diastolic, mmHg | −57.3 (31) | −58.5 (31) | −1.2 | 0.42 |
| Variable | Caloric Restriction (n = 11) | Protein Restriction (n = 10) | CR vs. PR | p, CR vs. PR | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | Effect | Effect | |
| BMI, mean (SD), kg/m2 | 32.0 (5.4) | 29.9 (5.1) | <0.0001 | 29.1 (8.2) | 27.3 (7.0) | 0.010 | 0.3 | 0.05 |
| Insulin, mean (SD), pmol/L | 77.6 (53.3) | 73.3 (37.8) | 0.96 | 71.4 (57.8) | 71.9 (37.3) | 0.99 | 3.8 | 0.37 |
| CPK, mean (SD), µkat/L | 1.9 (1.2) | 1.0 (0.4) | 0.04 | 2.1 (1.2) | 1.0 (0.3) | 0.03 | −0.2 | 0.0001 |
| CRP, mg/L | 9.2 (5.5) | 3.9 (3.8) | 0.0002 | 8.5 (5.3) | 2.6 (2.3) | 0.0041 | −0.6 | 0.48 |
| Free fatty acids, mean (SD) * | 0.7 (0.2) | 0.5 (0.1) | 0.02 | 0.9 (0.2) | 0.7 (0.2) | 0.04 | 0 | 0.48 |
| Glycerol, mean (SD) * | 3.0 (1.3) | 1.9 (0.5) | 0.004 | 1.7 (0.9) | 1.1 (0.6) | 0.03 | 0.5 | 0.0001 |
| Urinary creatinine, mean (SD) * | 6.0 (2.3) | 6.4 (2.3) | 0.37 | 4.9 (2.0) | 5.8 (5.2) | 0.91 | −0.5 | 0.01 |
| Urinary urea, mean (SD) * | 345.7 (150.6) | 347 (144.5) | 0.99 | 247 (85.1) | 207.9 (99.6) | 0.62 | −42.0 | <0.0001 |
| Hb, mean (SD), g/L | 133 (10) | 132 (9) | 0.26 | 135 (11) | 134 (8) | 0.18 | 0 | 0.50 |
| Ht, mean (SD), % | 41.0 (3.5) | 40.4 (3.1) | 0.18 | 41.4 (4.7) | 41.1 (4.0) | 0.09 | 0.3 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz-Bannitz, R.; Beraldo, R.A.; Peluso, A.A.; Dall, M.; Babaei, P.; Foglietti, R.C.; Martins, L.M.; Gomes, P.M.; Marchini, J.S.; Suen, V.M.M.; et al. Dietary Protein Restriction Improves Metabolic Dysfunction in Patients with Metabolic Syndrome in a Randomized, Controlled Trial. Nutrients 2022, 14, 2670. https://doi.org/10.3390/nu14132670
Ferraz-Bannitz R, Beraldo RA, Peluso AA, Dall M, Babaei P, Foglietti RC, Martins LM, Gomes PM, Marchini JS, Suen VMM, et al. Dietary Protein Restriction Improves Metabolic Dysfunction in Patients with Metabolic Syndrome in a Randomized, Controlled Trial. Nutrients. 2022; 14(13):2670. https://doi.org/10.3390/nu14132670
Chicago/Turabian StyleFerraz-Bannitz, Rafael, Rebeca A. Beraldo, A. Augusto Peluso, Morten Dall, Parizad Babaei, Rayana Cardoso Foglietti, Larissa Marfori Martins, Patricia Moreira Gomes, Julio Sergio Marchini, Vivian Marques Miguel Suen, and et al. 2022. "Dietary Protein Restriction Improves Metabolic Dysfunction in Patients with Metabolic Syndrome in a Randomized, Controlled Trial" Nutrients 14, no. 13: 2670. https://doi.org/10.3390/nu14132670