A Fish-Derived Protein Hydrolysate Induces Postprandial Aminoacidaemia and Skeletal Muscle Anabolism in an In Vitro Cell Model Using Ex Vivo Human Serum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Ethical Approval
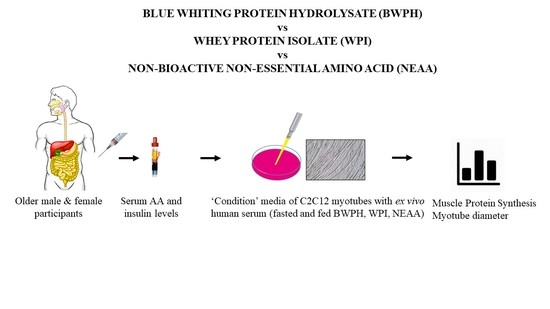
2.2. Study Design
2.3. Generation of Blue Whiting Protein Hydrolysate
2.4. Measurement of Amino Acids and Insulin Concentrations
2.5. Mammalian Cell Culture
2.6. Muscle Protein Synthesis
2.7. Immunoblotting
2.8. Myotube Thickness
2.9. Statistical Analysis
3. Results
3.1. Serum Amino Acids and Insulin
3.2. Intracellular Signaling
3.3. Muscle Protein Synthesis
3.4. Myotube Thickness
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef] [Green Version]
- Abellan Van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.M.; Gillette-Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) task force. J. Nutr. Health Aging 2009, 13, 881–889. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C.; et al. Added value of physical performance measures in predicting adverse health-related events: Results from the health, aging and body composition study. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Cruz-Jentoft, A.J.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia and mortality risk in frail olderpersons aged 80 years and older: Results from iLSIRENTE study. Age Ageing 2013, 42, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batsis, J.A.; Mackenzie, T.A.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National health and nutrition examination surveys 1999–2004. Nutr. Res. 2015, 35, 1031–1039. [Google Scholar] [CrossRef] [Green Version]
- Martone, A.; Lattanzio, F.; Abbatecola, A.; Carpia, D.; Tosato, M.; Marzetti, E.; Calvani, R.; Onder, G.; Landi, F. Treating sarcopenia in older and oldest old. Curr. Pharm. Des. 2015, 21, 1715–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shad, B.J.; Thompson, J.L.; Breen, L. Does the muscle protein synthetic response to exercise and amino acid-based nutrition diminish with advancing age? A systematic review. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E803–E817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naseeb, M.A.; Volpe, S.L. Protein and exercise in the prevention of sarcopenia and aging. Nutr. Res. 2017, 40, 1–20. [Google Scholar] [CrossRef]
- Walker, D.K.; Dickinson, J.M.; Timmerman, K.L.; Drummond, M.J.; Reidy, P.T.; Fry, C.S.; Gundermann, D.M.; Rasmussen, B.B. Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med. Sci. Sports Exerc. 2011, 43, 2249–2258. [Google Scholar] [CrossRef] [Green Version]
- Krok-Schoen, J.L.; Archdeacon Price, A.; Luo, M.; Kelly, O.J.; Taylor, C.A. Low dietary protein intakes and associated dietary patterns and functional limitations in an aging population: A NHANES analysis. J. Nutr. Health Aging 2019, 23, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Baum, J.I.; Kim, I.Y.; Wolfe, R.R. Protein consumption and the elderly: What is the optimal level of intake? Nutrients 2016, 8, 359. [Google Scholar] [CrossRef] [Green Version]
- Traylor, D.A.; Gorissen, S.H.M.; Phillips, S.M. Perspective: Protein requirements and optimal intakes in aging: Arewe ready to recommend more than the recommended daily allowance? Adv. Nutr. 2018, 9, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Richter, M.; Baerlocher, K.; Bauer, J.M.; Elmadfa, I.; Heseker, H.; Leschik-Bonnet, E.; Stangl, G.; Volkert, D.; Stehle, P. Revised reference values for the intake of protein. Ann. Nutr. Metab. 2019, 74, 242–250. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Rasmussen, B.B. Dietary protein recommendations and the prevention of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Cramer, J.T.; Cruz-Jentoft, A.J.; Landi, F.; Hickson, M.; Zamboni, M.; Pereira, S.L.; Hustead, D.S.; Mustad, V.A. Impacts of high-protein oral nutritional supplements among malnourished men and women with sarcopenia: A multicenter, randomized, double-blinded, controlled trial. J. Am. Med. Dir. Assoc. 2016, 17, 1044–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.E.; Phillips, S.M. Maximizing muscle protein anabolism: The role of protein quality. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 66–71. [Google Scholar] [CrossRef]
- Wolfe, R.R. Regulation of muscle protein by amino acids. J. Nutr. 2002, 132, 3219S–3224S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, L.M.; Winter, J.A.; Cameron-Smith, D.; Enslen, M.; Farnfield, M.; Decombaz, J. Effect of intake of different dietary protein sources on plasma amino acid profiles at rest and after exercise. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 452–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [Green Version]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E381–E387. [Google Scholar] [CrossRef] [Green Version]
- West, D.W.D.; Burd, N.A.; Coffey, V.G.; Baker, S.K.; Burke, L.M.; Hawley, J.A.; Moore, D.R.; Stellingwerff, T.; Phillips, S.M. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am. J. Clin. Nutr. 2011, 94, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Fry, C.; Rasmussen, B. Skeletal muscle protein balance and metabolism in the elderly. Curr. Aging Sci. 2012, 4, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, R.W.; McGlory, C.; Phillips, S.M. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Traylor, D.A.; Gorissen, S.H.M.; Hopper, H.; Prior, T.; McGlory, C.; Phillips, S.M. Aminoacidemia following ingestion of native whey protein, micellar casein, and a whey-casein blend in young men. Appl. Physiol. Nutr. Metab. 2019, 44, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Sanbongi, C.; Ikegami, S. Effects of whey protein hydrolysate ingestion on postprandial aminoacidemia compared with a free amino acid mixture in young men. Nutrients 2018, 10, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012, 9, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees, M.J.; Carson, B.P. The potential role of fish-derived protein hydrolysates on metabolic health, skeletal muscle mass and function in ageing. Nutrients 2020, 12, 2434. [Google Scholar] [CrossRef]
- Rondanelli, M.; Rigon, C.; Perna, S.; Gasparri, C.; Iannello, G.; Akber, R.; Alalwan, T.A.; Freije, A.M. Novel insights on intake of fish and prevention of sarcopenia: All reasons for an adequate consumption. Nutrients 2020, 12, 307. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Cordeiro, E.M.; de Oliveira, G.V.; Volino-Souza, M.; do Couto Velozo, O.; Alvares, T.S. Effects of fish protein hydrolysate ingestion on postexercise aminoacidemia compared with whey protein hydrolysate in young individuals. J. Food Sci. 2020, 85, 21–27. [Google Scholar] [CrossRef]
- Carson, B.P.; Patel, B.; Amigo-Benavent, M.; Pauk, M.; Kumar Gujulla, S.; Murphy, S.M.; Kiely, P.A.; Jakeman, P.M. Regulation of muscle protein synthesis in an in vitro cell model using ex vivo human serum. Exp. Physiol. 2018, 103, 783–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.; Pauk, M.; Amigo-Benavent, M.; Nongonierma, A.B.; Fitzgerald, R.J.; Jakeman, P.M.; Carson, B.P. A cell-based evaluation of a non-essential amino acid formulation as a non-bioactive control for activation and stimulation of muscle protein synthesis using ex vivo human serum. PLoS ONE 2019, 14, e0220757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogan, K.E.; Carson, B.P.; Patel, B.; Amigo-Benavent, M.; Jakeman, P.M.; Egan, B. Regulation of GLUT4 translocation in an in vitro cell model using postprandial human serum ex vivo. Exp. Physiol. 2019, 104, 800–807. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Franchi, M.V.; Brook, M.S.; Narici, M.V.; Williams, J.P.; Mitchell, W.K.; Szewczyk, N.J.; Greenhaff, P.L.; Atherton, P.J.; Smith, K. A validation of the application of D2O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E571–E579. [Google Scholar] [CrossRef] [Green Version]
- Brook, M.S.; Wilkinson, D.J.; Mitchell, W.K.; Lund, J.N.; Phillips, B.E.; Szewczyk, N.J.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans. J. Physiol. 2016, 594, 7399–7417. [Google Scholar] [CrossRef] [Green Version]
- Rennie, M.J.; Edwards, R.H.T.; Halliday, D.; Matthews, D.E.; Wolman, S.L.; Millward, D.J. Muscle protein synthesis measured by stable isotope techniques in man: The effects of feeding and fasting. Clin. Sci. 1982, 63, 519–523. [Google Scholar] [CrossRef] [Green Version]
- Greig, C.A.; Young, A.; Skelton, D.A.; Pippet, E.; Butler, F.M.M.; Mahmud, S.M. Exercise studies with elderly volunteers. Age Ageing 1994, 23, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J. Funct. Foods 2018, 40, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Le Maux, S.; Nongonierma, A.B.; Barre, C.; Fitzgerald, R.J. Enzymatic generation of whey protein hydrolysates under pH-controlled and non pH-controlled conditions: Impact on physicochemical and bioactive properties. Food Chem. 2016, 199, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Spellman, D.; Kenny, P.; O’Cuinn, G.; Fitzgerald, R.J. Aggregation properties of whey protein hydrolysates generated with Bacillus licheniformis proteinase activities. J. Agric. Food Chem. 2005, 53, 1258–1265. [Google Scholar] [CrossRef]
- Power-Grant, O.; McCormack, W.G.; Ramia De Cap, M.; Amigo-Benavent, M.; Fitzgerald, R.J.; Jakeman, P. Evaluation of the antioxidant capacity of a milk protein matrix in vitro and in vivo in women aged 50-70 years. Int. J. Food Sci. Nutr. 2016, 67, 325–334. [Google Scholar] [CrossRef]
- Murphy, S.M.; Kiely, M.; Jakeman, P.M.; Kiely, P.A.; Carson, B.P. Optimization of an in vitro bioassay to monitor growth and formation of myotubes in real time. Biosci. Rep. 2016, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, C.A.; Mabrey, D.M.; Frey, J.W.; Miu, M.H.; Schmidt, E.K.; Pierre, P.; Hornberger, T.A. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011, 25, 1028–1039. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.A.; Hornberger, T.A. Measuring protein synthesis with SUnSET: A valid alternative to traditional techniques? Exerc. Sport Sci. Rev. 2013, 41, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Gürtler, A.; Kunz, N.; Gomolka, M.; Hornhardt, S.; Friedl, A.A.; McDonald, K.; Kohn, J.E.; Posch, A. Stain-free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013, 433, 105–111. [Google Scholar] [CrossRef]
- Pauk, M.; Amigo-Benavent, M.; Patel, B.; Jakeman, P.M.; Carson, B.P. Casein protein hydrolysate fed human serum from older adults regulates in vitro muscle protein synthesis, muscle protein breakdown and myotube growth. Preprints 2020. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum: New York, NY, USA, 1988. [Google Scholar]
- Wilkinson, D.J.; Bukhari, S.S.I.; Phillips, B.E.; Limb, M.C.; Cegielski, J.; Brook, M.S.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Williams, J.P.; et al. Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clin. Nutr. 2018, 37, 2011–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohé, J.; Aili Low, J.F.; Wolfe, R.R.; Rennie, M.J. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J. Physiol. 2001, 532, 575–579. [Google Scholar] [CrossRef]
- Rennie, M.J. Exercise- and nutrient-controlled mechanisms involved in maintenance of the musculoskeletal mass. Biochem. Soc. Trans. 2007, 35, 1302–1305. [Google Scholar] [CrossRef]
- Grimble, G.K. The significance of peptides in clinical nutrition. Annu. Rev. Nutr. 1994, 14, 419–447. [Google Scholar] [CrossRef] [PubMed]
- Greenhaff, P.L.; Karagounis, L.G.; Peirce, N.; Simpson, E.J.; Hazell, M.; Layfield, R.; Wackerhage, H.; Smith, K.; Atherton, P.; Selby, A.; et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E595–E604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groen, B.B.L.; Horstman, A.M.H.; Hamer, H.M.; de Haan, M.; van Kranenburg, J.; Bierau, J.; Poeze, M.; Wodzig, W.K.W.H.; Rasmussen, B.B.; van Loon, L.J.C. Increasing insulin availability does not augment postprandial muscle protein synthesis rates in healthy young and older men. J. Clin. Endocrinol. Metab. 2016, 101, 3978–3988. [Google Scholar] [CrossRef]
- Everman, S.; Meyer, C.; Tran, L.; Hoffman, N.; Carroll, C.C.; Dedmon, W.L.; Katsanos, C.S. Insulin does not stimulate muscle protein synthesis during increased plasma branched-chain amino acids alone but still decreases whole body proteolysis in humans. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E671–E677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulla, H.; Smith, K.; Atherton, P.J.; Idris, I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: A systematic review and meta-analysis. Diabetologia 2016, 59, 44–55. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Fry, C.S.; Drummond, M.J.; Gundermann, D.M.; Walker, D.K.; Glynn, E.L.; Timmerman, K.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J. Nutr. 2011, 141, 856–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Amino Acid (g·100 g Powder) | NEAA | WPI | BWPH |
|---|---|---|---|
| Essential | |||
| Leucine | 0.00 | 9.87 | 5.78 |
| Isoleucine | 0.00 | 7.26 | 3.35 |
| Valine | 0.00 | 6.62 | 3.89 |
| Lysine | 0.00 | 9.75 | 7.44 |
| Histidine | 0.00 | 1.54 | 1.53 |
| Methionine | 0.00 | 2.15 | 1.94 |
| Phenylalanine | 0.00 | 2.80 | 2.87 |
| Threonine | 0.00 | 7.07 | 3.70 |
| Tryptophan | 0.00 | 1.60 | 0.65 |
| Non-essential | |||
| Alanine | 13.90 | 5.25 | 5.41 |
| Arginine | 0.00 | 1.89 | 5.94 |
| Aspartic Acid | 20.70 | 10.60 | 8.56 |
| Cysteine | 0.00 | 2.37 | 0.61 |
| Glutamic Acid | 28.70 | 18.10 | 12.50 |
| Glycine | 14.20 | 1.51 | 5.52 |
| Proline | 8.02 | 6.04 | 3.41 |
| Serine | 10.60 | 4.96 | 4.10 |
| Tyrosine | 7.40 | 2.67 | 3.23 |
| ΣBCAAs | 0.00 | 23.75 | 13.02 |
| ΣEAAs | 0.00 | 48.66 | 31.15 |
| ΣNEAAs | 103.52 | 53.39 | 49.28 |
| ΣAAs | 103.52 | 102.05 | 80.43 |
| Variable | NEAA | WPI | BWPH |
|---|---|---|---|
| Leucine | |||
| Cmax (µM) | 141 ± 15 | 580 ± 71 a | 399 ± 47 a, b |
| Tmax (min) | 15 ± 12 | 73 ± 16 a | 41 ± 11 a, b |
| AUC (µM·3 h) | 19,977 ± 3376 | 67,614 ± 5105 a | 48,848 ± 1546 a, b |
| ΣBCAAs | |||
| Cmax (µM) | 432 ± 51 | 1488 ± 192 a | 1021 ± 117 a, b |
| Tmax (min) | 19 ± 11 | 73 ± 16 a | 45 ± 13 a, b |
| AUC (µM·3 h) | 63,674 ± 11,134 | 182,551 ± 14,036 a | 134,220 ± 3777 a, b |
| ΣEAAs | |||
| Cmax (µM) | 1092 ± 113 | 2855 ± 350 a | 2168 ± 180 a, b |
| Tmax (min) | 28 ± 10 | 69 ± 15 a | 45 ± 12 a, b |
| AUC (µM·3 h) | 168,288 ± 19,649 | 358,693 ± 28,225 a | 290,640 ± 13,419 a, b |
| ΣNEAAs | |||
| Cmax (µM) | 3154 ± 344 | 2519 ± 369 a | 2651 ± 210a |
| Tmax (min) | 84 ± 49 | 60 ± 15 | 58 ± 31 |
| AUC (µM·3 h) | 446,214 ± 38,004 | 361,362 ± 37,120 a | 382,311 ± 18,305 a |
| ΣAAs | |||
| Cmax (µM) | 4154 ± 347 | 5354 ± 693 a | 4795 ± 362 a |
| Tmax (min) | 81 ± 50 | 66 ± 17 | 58 ± 31 |
| AUC (µM·3 h) | 615,410 ± 42,914 | 720,015 ± 59,992 a | 672,951 ± 31,073 a |
| Insulin | |||
| Cmax (µM) | 30 ± 20 | 64 ± 43 a | 62 ± 43a |
| Tmax (min) | 34 ± 14 | 41 ± 14 | 36 ± 12 |
| AUC (µM·3 h) | 2697 ± 2229 | 4100 ± 2939 a | 4305 ± 3538 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lees, M.J.; Nolan, D.; Amigo-Benavent, M.; Raleigh, C.J.; Khatib, N.; Harnedy-Rothwell, P.; FitzGerald, R.J.; Egan, B.; Carson, B.P. A Fish-Derived Protein Hydrolysate Induces Postprandial Aminoacidaemia and Skeletal Muscle Anabolism in an In Vitro Cell Model Using Ex Vivo Human Serum. Nutrients 2021, 13, 647. https://doi.org/10.3390/nu13020647
Lees MJ, Nolan D, Amigo-Benavent M, Raleigh CJ, Khatib N, Harnedy-Rothwell P, FitzGerald RJ, Egan B, Carson BP. A Fish-Derived Protein Hydrolysate Induces Postprandial Aminoacidaemia and Skeletal Muscle Anabolism in an In Vitro Cell Model Using Ex Vivo Human Serum. Nutrients. 2021; 13(2):647. https://doi.org/10.3390/nu13020647
Chicago/Turabian StyleLees, Matthew J., David Nolan, Miryam Amigo-Benavent, Conor J. Raleigh, Neda Khatib, Pádraigín Harnedy-Rothwell, Richard J. FitzGerald, Brendan Egan, and Brian P. Carson. 2021. "A Fish-Derived Protein Hydrolysate Induces Postprandial Aminoacidaemia and Skeletal Muscle Anabolism in an In Vitro Cell Model Using Ex Vivo Human Serum" Nutrients 13, no. 2: 647. https://doi.org/10.3390/nu13020647