Monitoring Corn Nitrogen Concentration from Radar (C-SAR), Optical, and Sensor Satellite Data Fusion
Abstract
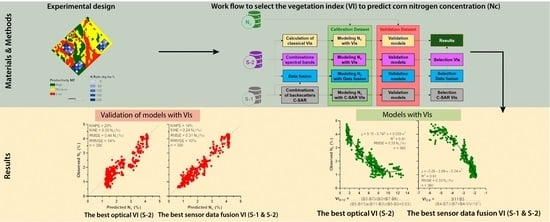
:1. Introduction
2. Materials and Methods
2.1. Description of Field Studies
2.2. Remote Sensing Data
2.2.1. Traditional Vegetation Indices
2.2.2. Combinations of Spectral Bands and C-SAR Backscatters and Data Fusion
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carciochi, W.D.; Massigoge, I.; Lapaz Olveira, A.; Reussi Calvo, N.I.; Cafaro La Menza, F.; Saínz Rozas, H.R.; Barbieri, P.A.; Di Napoli, M.; Gonzalez Montaner, J.; Ciampitti, I.A. Cover Crop Species Can Increase or Decrease the Fertilizer-nitrogen Requirement in Maize. Agron. J. 2021, 113, 5412–5423. [Google Scholar] [CrossRef]
- Orcellet, J.M.; Reussi Calvo, N.I.; Saínz Rozas, H.R.; Wyngaard, N.; Echeverría, H.E. Anaerobically Incubated Nitrogen Improved Nitrogen Diagnosis in Corn. Agron. J. 2017, 109, 291–298. [Google Scholar] [CrossRef]
- Reussi Calvo, N.I.; Wyngaard, N.; Orcellet, J.M.; Saínz Rozas, H.R.; Echeverría, H.E. Predicting Field-Apparent Nitrogen Mineralization from Anaerobically Incubated Nitrogen. Soil Sci. Soc. Am. J. 2018, 82, 502–508. [Google Scholar] [CrossRef]
- Stanford, G. Rationale for Optimum Nitrogen Fertilization in Corn Production. Environ. Qual. 1973, 2, 159–166. [Google Scholar] [CrossRef]
- Correndo, A.A.; Lanza Lopez, O.; Almeida, L.F.A.; Ciampitti, I.A. Yield Response to Nitrogen Management in a Corn-Soybean Sequence in North Central Kansas—2021 Season. Kansas Agric. Exp. Stn. Res. Rep. 2022, 8, 128. [Google Scholar] [CrossRef]
- Barbieri, P.A.; Echeverría, H.E.; Saínz Rozas, H.R. Alternatives for Nitrogen Diagnosis for Wheat with Different Yield Potentials in the Humid Pampas of Argentina. Commun. Soil Sci. Plant Anal. 2012, 43, 1512–1522. [Google Scholar] [CrossRef]
- Pagani, A.; Echeverría, H.E.; Andrade, F.H.; Saínz Rozas, H.R. Characterization of Corn Nitrogen Status with a Greenness Index under Different Availability of Sulfur. Agron. J. 2009, 101, 315–322. [Google Scholar] [CrossRef]
- Pagani, A.; Echeverría, H.E.; Saínz Rozas, H.R.; Barbieri, P.A. Dosis Óptima Económica de Nitrógeno En Maíz Bajo Siembra Directa En El Sudeste Bonaerense. Cienc. Suelo 2008, 26, 183–193. [Google Scholar]
- Saínz Rozas, H.R.; Calviño, P.A.; Echeverría, H.E.; Barbieri, P.A.; Redolatti, M. Contribution of Anaerobically Mineralized Nitrogen to the Reliability of Planting or Presidedress Soil Nitrogen Test in Maize. Agron. J. 2008, 100, 1020–1025. [Google Scholar] [CrossRef]
- Saínz Rozas, H.R.; Echeverría, H.E. Relación Entre Las Lecturas Del Medidor de Clorofila (Minolta SPAD 502) En Distintos Estadios Del Ciclo Del Cultivo de Maíz y El Rendimiento En Grano. Rev. Fac. Agron. Plata 1998, 103, 37–44. [Google Scholar]
- Plénet, D.; Lemaire, G. Relationships between Dynamics of Nitrogen Uptake and Dry Matter Accumulation in Maize Crops. Determination of Critical N Concentration. Plant Soil 2000, 216, 65–82. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A Review of Methods for Sensing the Nitrogen Status in Plants: Advantages, Disadvantages and Recent Advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Barzin, R.; Lotfi, H.; Varco, J.J.; Bora, G.C. Machine Learning in Evaluating Multispectral Active Canopy Sensor for Prediction of Corn Leaf Nitrogen Concentration and Yield. Remote Sens. 2022, 14, 120. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Feng, G.; Yuan, F.; Yue, S.; Gao, X.; Liu, Y.; Liu, B.; Ustin, S.L.; Chen, X. Improving Estimation of Summer Maize Nitrogen Status with Red Edge-Based Spectral Vegetation Indices. Field Crops Res. 2014, 157, 111–123. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Scharf, P.C.; Vories, E.D.; Drummond, S.T.; Dunn, D.; Stevens, W.G.; Bronson, K.F.; Benson, N.R.; Hubbard, V.C.; Jones, A.S. Calibrating Canopy Reflectance Sensors to Predict Optimal Mid-Season Nitrogen Rate for Cotton. Soil Sci. Soc. Am. J. 2012, 77, 173–183. [Google Scholar] [CrossRef]
- Zhao, B.; Duan, A.; Ata-ul-karim, S.T.; Liu, Z.; Chen, Z.; Gong, Z.; Zhang, J.; Xiao, J.; Liu, Z.; Qin, A.; et al. Exploring New Spectral Bands and Vegetation Indices for Estimating Nitrogen Nutrition Index of Summer Maize. Eur. J. Agron. 2018, 93, 113–125. [Google Scholar] [CrossRef]
- Chen, P.; Nicolas, T.; Wang, J.; Philippe, V.; Huang, W.; Li, B. New Index for Crop Canopy Fresh Biomass Estimation. Spectrosc. Spectr. Anal. 2010, 30, 512–517. [Google Scholar] [CrossRef]
- Fitzgerald, G.; Rodriguez, D.; O’Leary, G. Measuring and Predicting Canopy Nitrogen Nutrition in Wheat Using a Spectral Index-The Canopy Chlorophyll Content Index (CCCI). Field Crops Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Pagani, A. Manejo Sitio-Específico de Nutriente. In Fertilidad de Suelos y Fertilización de Cultivos; Echeverría, H.E., García, F.O., Eds.; Editorial INTA: Buenos Aires, Argentina, 2014; pp. 839–870. [Google Scholar]
- Scharf, P.C.; Kitchen, N.R.; Sudduth, K.A.; Davis, J.G.; Hubbard, V.C.; Lory, J.A. Field-Scale Variability in Optimal Nitrogen Fertilizer Rate for Corn. Agron. J. 2005, 97, 452–461. [Google Scholar] [CrossRef]
- Campbell, J.B.; Wynne, R.H.; Thomas, V.A. Introduction to Remote Sensing, 6th ed.; The Guilford Press: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Misra, G.; Cawkwell, F.; Wingler, A. Status of Phenological Research Using Sentinel-2 Data: A Review. Remote Sens. 2020, 12, 2760. [Google Scholar] [CrossRef]
- Morris, T.F.; Murrell, T.S.; Beegle, D.B.; Camberato, J.J.; Ferguson, R.B.; Grove, J.; Ketterings, Q.; Kyveryga, P.M.; Laboski, C.A.M.; McGrath, J.M.; et al. Strengths and Limitations of Nitrogen Rate Recommendations for Corn and Opportunities for Improvement. Agron. J. 2018, 110, 1–37. [Google Scholar] [CrossRef] [Green Version]
- ESA. Sentinel Online-ESA. Available online: https://sentinel.esa.int/web/sentinel/home (accessed on 8 June 2019).
- Segarra, J.; Buchaillot, M.L.; Araus, J.L.; Kefauver, S.C. Remote Sensing for Precision Agriculture: Sentinel-2 Improved Features and Applications. Agronomy 2020, 10, 641. [Google Scholar] [CrossRef]
- Madonsela, S.; Cho, M.A.; Naidoo, L.; Main, R.; Majozi, N. Exploring the Utility of Sentinel-2 for Estimating Maize Chlorophyll Content and Leaf Area Index across Different Growth Stages. J. Spat. Sci. 2021, 1–13. [Google Scholar] [CrossRef]
- Moreira, A.; Prats-Iraola, P.; Younis, M.; Krieger, G.; Hajnsek, I.; Papathanassiou, K.P. A Tutorial on Synthetic Aperture Radar. IEEE Geosci. Remote Sens. Mag. 2013, 1, 6–43. [Google Scholar] [CrossRef]
- Ulaby, F.T.; Long, D.G. (Eds.) Microwave Radar and Radiometric Remote Sensing; University of Michigan Press: Ann Arbor, MI, USA, 2014. [Google Scholar] [CrossRef]
- Baup, F.; Villa, L.; Fieuzal, R.; Ameline, M. Sensitivity of X-Band (Σ0, γ) and Optical (NDVI) Satellite Data to Corn Biophysical Parameters. Adv. Remote Sens. 2016, 5, 103–117. [Google Scholar] [CrossRef]
- Canisius, F.; Shang, J.; Liu, J.; Huang, X.; Ma, B.L.; Jiao, X.; Geng, X.; Kovacs, J.M.; Walters, D. Tracking Crop Phenological Development Using Multi-Temporal Polarimetric Radarsat-2 Data. Remote Sens. Environ. 2017, 210, 508–518. [Google Scholar] [CrossRef]
- Mandal, D.; Kumar, V.; Lopez-Sanchez, J.M.; Bhattacharya, A.; McNairn, H.; Rao, Y.S. Crop Biophysical Parameter Retrieval from Sentinel-1 SAR Data with a Multi-Target Inversion of Water Cloud Model. Int. J. Remote Sens. 2020, 41, 5503–5524. [Google Scholar] [CrossRef]
- Setiyono, T.D.; Quicho, E.D.; Holecz, F.H.; Khan, N.I.; Romuga, G.; Maunahan, A.; Garcia, C.; Rala, A.; Raviz, J.; Collivignarelli, F.; et al. Rice Yield Estimation Using Synthetic Aperture Radar (SAR) and the ORYZA Crop Growth Model: Development and Application of the System in South and South-East Asian Countries. Int. J. Remote Sens. 2018, 40, 8093–8124. [Google Scholar] [CrossRef]
- Yang, H.; Yang, G.; Gaulton, R.; Zhao, C.; Li, Z.; Taylor, J.; Wicks, D.; Minchella, A.; Chen, E.; Yang, X. In-Season Biomass Estimation of Oilseed Rape (Brassica Napus L.) Using Fully Polarimetric SAR Imagery. Precis. Agric. 2018, 20, 630–648. [Google Scholar] [CrossRef]
- El Hajj, M.; Baghdadi, N.; Bazzi, H.; Zribi, M. Penetration Analysis of SAR Signals in the C and L Bands for Wheat, Maize, and Grasslands. Remote Sens. 2019, 11, 31. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.; McNairn, H.; Mitchell, S.; Dingle Robertson, L.; Davidson, A.; Homayouni, S. Synthetic Aperture Radar and Optical Satellite Data for Estimating the Biomass of Corn. Int. J. Appl. Earth Obs. Geoinf. 2019, 83, 101933. [Google Scholar] [CrossRef]
- Liao, C.; Wang, J.; Shang, J.; Huang, X.; Liu, J.; Huffman, T. Sensitivity Study of Radarsat-2 Polarimetric SAR to Crop Height and Fractional Vegetation Cover of Corn and Wheat. Int. J. Remote Sens. 2018, 39, 1475–1490. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, E.; Li, Z.; Zhao, L.; Ji, Y.; Zhang, Y.; Liu, Z. Rape (Brassica Napus L.) Growth Monitoring and Mapping Based on Radarsat-2 Time-Series Data. Remote Sens. 2018, 10, 206. [Google Scholar] [CrossRef]
- Kaplan, G.; Fine, L.; Lukyanov, V.; Manivasagam, V.S.; Tanny, J.; Rozenstein, O. Normalizing the Local Incidence Angle in Sentinel-1 Imagery to Improve Leaf Area Index, Vegetation Height, and Crop Coefficient Estimations. Land 2021, 10, 680. [Google Scholar] [CrossRef]
- Hosseini, M.; Mcnairn, H.; Mitchell, S.; Dingle, L.; Davidson, A.; Homayouni, S. MethodsX Integration of Synthetic Aperture Radar and Optical Satellite Data for Corn Biomass Estimation. MethodsX 2020, 7, 100857. [Google Scholar] [CrossRef]
- Hosseini, M.; McNairn, H.; Mitchell, S.; Davidson, A.; Di Robertson, L. Combination of Optical and SAR Sensors for Monitoring Biomass over Corn Fields. In Proceedings of the IGARSS 2018–2018 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Valencia, Spain, 22–27 July 2018; pp. 5952–5955. [Google Scholar] [CrossRef]
- Panigatti, L. Argentina: 200 Años, 200 Suelos; INTA: Buenos Aires, Argentina, 2010; p. 345. Available online: https://inta.gob.ar/sites/default/files/script-tmp-inta-200-suelos.pdf (accessed on 7 May 2022).
- Matteucci, S.D. Ecorregión Pampa; Morello, J., Matteucci, S., Rodríguez, A., Silva, M., Eds.; Orientación Gráfica Editora: Buenos Aires, Argentina, 2012; pp. 391–446. [Google Scholar]
- Saínz Rozas, H.R.; Echeverría, H.E.; Angelini, H.P. Niveles de Carbono Orgánico y Ph En Suelos Agrícolas de Las Regiones Pampeana y Extrapampeana Argentina. Cienc. Suelo 2011, 29, 29–37. [Google Scholar]
- Córdoba, M.A.; Bruno, C.I.; Costa, J.L.; Peralta, N.R.; Balzarini, M.G. Protocol for Multivariate Homogeneous Zone Delineation in Precision Agriculture. Biosyst. Eng. 2016, 143, 95–107. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Hanway, J.J. How a Corn Plant Develops; Special Report No. 48; Iowa State University of Science and Technology, Cooperative Extension Service: Ames, IA, USA, 1986; pp. 1–21. [Google Scholar]
- Baret, F.; Weiss, M.; Allard, D.; Garrigues, S.; Leroy, M.; Jeanjean, H.; Fernandes, R.; Myneni, R.; Privette, J.; Morisette, J. VALERI: A Network of Sites and a Methodology for the Validation of Medium Spatial Resolution Land Satellite Products. Remote Sens. Environ. 2021, hal-03221068. Available online: https://hal.inrae.fr/hal-03221068 (accessed on 10 June 2022).
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis, Part 2: Chemical Methods; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Filipponi, F. Sentinel-1 GRD Preprocessing Workflow. Proceedings 2019, 18, 11. [Google Scholar] [CrossRef]
- Cochrane, D.; Orcutt, G.H. Application of Least Squares Regression to Relationships Containing Auto-Correlated Error Terms. J. Am. Stat. Assoc. 1949, 44, 32–61. [Google Scholar] [CrossRef]
- Verbeek, M. Heteroskedasticity and Autocorrelation. In A Guide to Modern Econometerics; Verbeek, M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 97–138. [Google Scholar]
- Spada, S.; Quartagno, M.; Tamburini, M.; Robinson, D. Package ‘orcutt’: Estimate Procedure in Case of First Order Autocorrelatio. Available online: https://mirror.rcg.sfu.ca/mirror/CRAN/web/packages/orcutt/orcutt.pdf (accessed on 15 July 2022).
- Lawrence, K.D.; Klimberg, R.K.; Lawrence, S.M. Fundamentals of Forecasting Using Excel; Industrial Press: New York, NY, USA, 2009. [Google Scholar]
- Lu, J.; Cheng, D.; Geng, C.; Zhang, Z.; Xiang, Y.; Hu, T. Combining Plant Height, Canopy Coverage and Vegetation Index from UAV-Based RGB Images to Estimate Leaf Nitrogen Concentration of Summer Maize. Biosyst. Eng. 2021, 202, 42–54. [Google Scholar] [CrossRef]
- Li, D.; Miao, Y.; Ransom, C.J.; Bean, G.M.; Kitchen, N.R.; Fernández, F.G.; Sawyer, J.E.; Camberato, J.J.; Carter, P.R.; Ferguson, R.B.; et al. Corn Nitrogen Nutrition Index Prediction Improved by Integrating Genetic, Environmental, and Management Factors with Active Canopy Sensing Using Machine Learning. Remote Sens. 2022, 14, 394. [Google Scholar] [CrossRef]
- Ameline, M.; Fieuzal, R.; Betbeder, J.; Berthoumieu, J.F.; Baup, F. Estimation of Corn Yield by Assimilating SAR and Optical Time Series into a Simplified Agro-Meteorological Model: From Diagnostic to Forecast. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 4747–4760. [Google Scholar] [CrossRef]
- Xu, M.; Liu, R.; Chen, J.M.; Liu, Y.; Shang, R.; Ju, W.; Wu, C.; Huang, W. Retrieving Leaf Chlorophyll Content Using a Matrix-Based Vegetation Index Combination Approach. Remote Sens. Environ. 2019, 224, 60–73. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Gao, D.; Qiao, L.; Liu, N.; Li, M.; Zhang, Y. Detection of Canopy Chlorophyll Content of Corn Based on Continuous Wavelet Transform Analysis. Remote Sens. 2020, 12, 2741. [Google Scholar] [CrossRef]
- Holzman, M.E.; Rivas, R.E.; Bayala, M.I. Relationship between Tir and Nir-Swir as Indicator of Vegetation Water Availability. Remote Sens. 2021, 13, 3371. [Google Scholar] [CrossRef]
- Guerrero, A.; De Neve, S.; Mouazen, A.M. Chapter One-Current sensor technologies for in situ and on-line measurement of soil nitrogen for variable rate fertilization: A review. Adv. Agron. 2021, 168, 1–38. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Camberato, J.J.; Murrell, S.T.; Vyn, T.J. Maize Nutrient Accumulation and Partitioning in Response to Plant Density and Nitrogen Rate: I. Macronutrients. Agron. J. 2013, 105, 783–795. [Google Scholar] [CrossRef]
- Fernandez, J.A.; DeBruin, J.; Messina, C.D.; Ciampitti, I.A. Late-Season Nitrogen Fertilization on Maize Yield: A Meta-Analysis. Field Crops Res. 2020, 247, 107586. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-Based Plant Height from Crop Surface Models, Visible, and near Infrared Vegetation Indices for Biomass Monitoring in Barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Woebbecke, D.M.; Meyer, G.E.; Von Bargen, K.; Mortensen, D.A. Color Indices for Weed Identification Under Various Soil, Residue, and Lighting Conditions. Trans. ASAE Am. Soc. Agric. Eng. 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Metternicht, G. Vegetation Indices Derived from High-Resolution Airborne Videography for Precision Crop Management. Int. J. Remote Sens. 2003, 24, 2855–2877. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel Algorithms for Remote Estimation of Vegetation Fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Michez, A.; Bauwens, S.; Brostaux, Y.; Hiel, M.-P.; Garré, S.; Lejeune, P.; Dumont, B. How Far Can Consumer-Grade UAV RGB Imagery Describe Crop Production? A 3D and Multitemporal Modeling Approach Applied to Zea Mays. Remote Sens. 2018, 10, 1798. [Google Scholar] [CrossRef]
- Hunt, E.R.; Daughtry, C.S.T.; Eitel, J.U.H.; Long, D.S. Remote Sensing Leaf Chlorophyll Content Using a Visible Band Index. Agron. J. 2011, 103, 1090–1099. [Google Scholar] [CrossRef]
- Rouse, J.W.J.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite Symposium, Greenbelt, MD, USA, 10–14 December 1973; Freden, S.C., Becker, M.A., Eds.; Scientific and Technical Information Office, National Aeronautics and Space Administration: Washington, DC, USA, 1974; Volume 1, pp. 309–317. [Google Scholar]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Huete, A.R. A Soil-Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Signature Analysis of Leaf Reflectance Spectra: Algorithm Development for Remote Sensing of Chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of Soil-Adjusted Vegetation Indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A Modified Soil Adjusted Vegetation Index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Gao, B. NDWI A Normalized Difference Water Index for Remote Sensing of Vegetation Liquid Water From Space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Raper, T.B.; Varco, J.J. Canopy-Scale Wavelength and Vegetative Index Sensitivities to Cotton Growth Parameters and Nitrogen Status. Precis. Agric. 2015, 16, 62–76. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS Terrestrial Chlorophyll Index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Narrow Band Vegetation Indices Overcome the Saturation Problem in Biomass Estimation. Int. J. Remote Sens. 2004, 25, 3999–4014. [Google Scholar] [CrossRef]
- Chang, J.; Shoshany, M. Red-Edge Ratio Normalized Vegetation Index for Remote Estimation of Green Biomass. In Proceedings of the 2016 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Beijing, China, 10–15 July 2016; pp. 1337–1339. [Google Scholar]
- Clevers, J.G.P.W.; Jong, S.M.D.E.; Epema, G.F. Derivation of the Red Edge Index Using the MERIS Standard. Int. J. Remote Sens. 2002, 23, 3169–3184. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco Tejada, P.J.; Dextraze, L. Integrated Narrow-Band Vegetation Indices for Prediction of Crop Chlorophyll Content for Application to Precision Agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Chen, P.; Haboudane, D.; Tremblay, N.; Wang, J.; Vigneault, P.; Li, B. New Spectral Indicator Assessing the Efficiency of Crop Nitrogen Treatment in Corn and Wheat. Remote Sens. Environ. 2010, 114, 1987–1997. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Brown de Colstoun, E.; McMurtrey, J.E., III. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]






| Site | Exp (MZ) | Sowing Date | Harvest Date | Plant Density Plants m−2 | Soil Texture (0–20 cm) | SOM (0–20 cm) g kg−1 | NO3−–N (0–60 cm) kg ha−1 |
|---|---|---|---|---|---|---|---|
| 1 | 1(VH) | 22 September | 23 March | 9.0 | Sandy loam | 3.8 | 72 |
| 2(L) | 22 September | 23 March | 8.0 | Loamy sand | 2.5 | 42 | |
| 3(H) | 23 September | 23 March | 9.0 | Sandy loam | 3.7 | 70 | |
| 4(M) | 23 September | 23 March | 8.5 | Sandy loam | 3.5 | 46 | |
| 2 | 5(H) | 24 September | 23 March | 8.9 | Sandy loam | 2.9 | 48 |
| 6(L) | 25 September | 22 March | 7.8 | Loamy sand | 2.2 | 34 | |
| 7(M) | 29 September | 22 March | 8.3 | Sandy loam | 2.7 | 36 | |
| 3 | 8(H) | 7 November | 19 May | 6.7 | Loam | 5.9 | 46 |
| 9(L) | 7 November | 19 May | 4.7 | Clay loam | 5.6 | 40 | |
| 4 | 10(H) | 21 November | 26 May | 4.4 | Loam | 5.5 | 78 |
| 11(L) | 21 November | 26 May | 3.4 | Clay loam | 5.5 | 90 |
| Exp | Sampling Stage | Sampling Date | Satellite Observation Acquisition | Cumulative Precipitation (mm) | |
|---|---|---|---|---|---|
| Sentinel-1 | Sentinel-2 | ||||
| 1, 2, 3, and 4 (Site 1) | V6 | 10 and 11 November | 6 and 12 November | 7 and 12 November | 118 |
| V10 | 3 and 4 December | 30 November and 6 December | 27 November and 7 December | 153 | |
| V14 | 21 and 22 December | 18 and 24 December | 17 and 22 December | 194 | |
| R1 | 5 and 6 January | 5 January | 1 January | 198 | |
| 5, 6, and 7 (Site 2) | V6 | 11 November | 6 and 12 November | 7 and 17 November | 94 |
| V10 | 4 December | 30 November and 6 December | 7 and 27 November | 114 | |
| V14 | 20 December | 18 and 24 December | 17 and 22 December | 114 | |
| R1 | 6 January | 5 January | 1 January | 163 | |
| 8 and 9 (Site 3) | V6 | 14 December | 14 December | 14 December | 25 |
| V10 | 2 January | 1 January | 31 December and 3 January | 33 | |
| V14 | 23 January | 19 and 25 January | 23 January | 181 | |
| R1 | 4 February | 31 January and 6 February | 4 February | 181 | |
| 10 and 11 (Site 4) | V6 | 29 and 30 December | 1 January | 26 and 30 December | 96 |
| V10 | 22 January | 19 and 25 January | 20 and 23 January | 182 | |
| V14 | 5 February | 6 February | 4 February | 205 | |
| R1 | 20 February | 18 and 24 February | 22 February | 306 | |
| Vegetation Index (VI) | Equation |
|---|---|
| VI1 | a − b |
| VI2 | a/b |
| VI3 | (a − b)/(a + b) |
| VI4 | (a − b)/(a + b + 0.25) × 1.25 |
| VI5 | (a − b)/(a + b + 0.5) × 1.5 |
| VI6 | (a − b)/(a + b + 0.75) × 1.75 |
| VI7 | (2a + 1 − (√((2a + 1)2 − 8 × (a − b)))/2 |
| VI8 | (a − b)/(a + b − c) |
| VI9 | (a2 − b × c)/(a2 + b × c) |
| VI10 | 100 × (a − b) − 10 × (a − c) |
| VI11 | (a − b) − 0.2 × (a − c) × (a/b) |
| VI12 | (a − b) × (b − c)/(a − c + 0.03) |
| VI13 | (a − b)/(a + b)/(a − c)/(a + c) |
| VI14 | 2.5 × (a − b)/(a + 6 × b − 7.5 × c) + 1 |
| Combined | VI1-14/VI2-14 (with a, b, and c randomly assigned) |
| N Rate (kg ha−1) | Exp 1 | Exp 2 | Exp 3 | Exp 4 | Exp 5 | Exp 6 | Exp 7 | Exp 8 | Exp 9 | Exp 10 | Exp 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nc (%) | Nc (%) | Nc (%) | Nc (%) | Nc (%) | Nc (%) | Nc (%) | Nc (%) | Nc (%) | Nc (%) | Nc (%) | |
| V6 | |||||||||||
| 0 | 3.76 d 1 | 3.42 d | 3.71 c | 3.70 c | 3.57 b | 3.64 c | 3.48 b | 2.95 b | 3.07 b | 3.26 c | 3.38 b |
| 60 | 4.09 c | 4.17 c | 4.04 b | 4.19 b | 3.93 a | 3.98 b | 3.99 b | 2.98 b | 3.49 a | 3.11 c | 3.64 a |
| 120 | 4.18 bc | 4.41 ab | 4.23 ab | 4.21 b | 3.98 a | 4.14 a | 4.13 ab | 3.26 a | 3.18 b | 3.54 b | 3.69 a |
| 180 | 4.30 ab | 4.22 bc | 4.32 a | 4.19 b | 4.08 a | 3.92 b | 4.28 a | 2.86 ab | 3.22 b | 3.80 a | 3.58 a |
| 240 | 4.44 a | 4.48 a | 4.31 a | 4.60 a | 4.03 a | 4.04 ab | 4.14 ab | 3.41 a | 3.09 b | 3.69 ab | 3.66 a |
| V10 | |||||||||||
| 0 | 2.04 c | 2.14 d | 2.21 d | 2.50 d | 1.54 c | 1.54 c | 1.59 c | 2.04 | 2.45 a | 2.01 d | 1.85 b |
| 60 | 2.57 b | 2.36 c | 2.45 c | 2.99 c | 2.05 b | 2.09 b | 2.00 b | 2.08 | 2.08 b | 2.29 c | 2.00 b |
| 120 | 2.55 b | 2.58 b | 2.62 bc | 3.06 c | 2.08 b | 2.33 ab | 2.34 a | 2.05 | 2.44 a | 2.25 c | 2.29 a |
| 180 | 2.89 a | 2.78 b | 2.69 b | 3.26 b | 2.24 ab | 2.26 ab | 2.52 a | 2.12 | 2.43 a | 2.49 b | 2.24 a |
| 240 | 2.82 a | 2.86 a | 2.90 a | 3.46 a | 2.33 a | 2.42 a | 2.37 a | 2.20 | 2.58 a | 2.72 a | 2.32 a |
| V14 | |||||||||||
| 0 | 1.41 c | 1.34 d | 1.42 c | 0.82 d | 0.96 c | 1.20 d | 1.07 d | 1.21 c | 1.29 d | 1.05 c | 0.95 c |
| 60 | 1.48 c | 1.65 c | 1.60 c | 1.62 c | 1.54 a | 1.53 c | 1.44 c | 1.57 b | 1.32 cd | 1.26 b | 1.21 b |
| 120 | 1.67 bc | 1.94 b | 1.94 b | 1.83 b | 1.34 b | 1.81 ab | 1.78 b | 1.87 a | 1.49 c | 1.40 b | 1.29 ab |
| 180 | 1.81 ab | 1.96 b | 1.90 b | 1.84 ab | 1.73 a | 1.68 bc | 2.01 a | 1.76 ab | 1.85 b | 1.41 b | 1.46 a |
| 240 | 1.85 a | 2.25 a | 2.14 a | 2.03 a | 1.58 a | 1.93 a | 1.88 ab | 1.82 ab | 2.08 a | 1.62 a | 1.33 ab |
| R1 | |||||||||||
| 0 | 0.81 b | 0.95 d | 0.99 b | 0.97 c | 0.66 d | 0.66 c | 0.68 c | 0.89 c | 0.92 d | 0.98 b | 1.08 c |
| 60 | 0.94 b | 1.15 c | 1.08 b | 1.15 c | 0.78 cd | 0.87 b | 0.83 c | 1.12 a | 1.04 cd | 1.37 a | 1.43 b |
| 120 | 1.18 a | 1.21 bc | 1.38 a | 1.35 b | 0.86 bc | 1.20 a | 1.17 b | 1.19 a | 1.17 bc | 1.43 a | 1.43 b |
| 180 | 1.30 a | 1.38 b | 1.52 a | 1.51 b | 1.02 b | 1.37 a | 1.38 b | 1.23 a | 1.30 ab | 1.49 a | 1.56 b |
| 240 | 1.36 a | 1.66 a | 1.54 a | 1.71 a | 1.27 a | 1.38 a | 1.49 a | 1.26 a | 1.44 a | 1.50 a | 1.78 a |
| Ranking Position | Vegetation Indices | Calibration (n = 360) | Validation (n = 300) | ||||
|---|---|---|---|---|---|---|---|
| R2 | RMSE (%Nc) | RMSE (%Nc) | RRMSE (%) | MAE (%Nc) | MAPE (%) | ||
| 1st | RVI2 | 0.76 | 0.54 | 0.51 | 15 | 0.41 | 30 |
| 2nd | MNDVI | 0.80 | 0.48 | 0.56 | 17 | 0.46 | 31 |
| 3rd | SRRE | 0.75 | 0.54 | 0.53 | 16 | 0.43 | 31 |
| 4th | DCNI | 0.73 | 0.57 | 0.53 | 16 | 0.41 | 32 |
| 5th | RVI1 | 0.69 | 0.60 | 0.55 | 17 | 0.45 | 32 |
| 6th | RERNDVI | 0.65 | 0.64 | 0.52 | 16 | 0.42 | 32 |
| 7th | REP | 0.75 | 0.55 | 0.57 | 17 | 0.45 | 33 |
| 8th | NDWI | 0.53 | 0.75 | 0.61 | 19 | 0.51 | 35 |
| 9th | SCCCI | 0.70 | 0.60 | 0.62 | 17 | 0.49 | 37 |
| 10th | NDRE | 0.63 | 0.66 | 0.65 | 20 | 0.51 | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapaz Olveira, A.; Saínz Rozas, H.; Castro-Franco, M.; Carciochi, W.; Nieto, L.; Balzarini, M.; Ciampitti, I.; Reussi Calvo, N. Monitoring Corn Nitrogen Concentration from Radar (C-SAR), Optical, and Sensor Satellite Data Fusion. Remote Sens. 2023, 15, 824. https://doi.org/10.3390/rs15030824
Lapaz Olveira A, Saínz Rozas H, Castro-Franco M, Carciochi W, Nieto L, Balzarini M, Ciampitti I, Reussi Calvo N. Monitoring Corn Nitrogen Concentration from Radar (C-SAR), Optical, and Sensor Satellite Data Fusion. Remote Sensing. 2023; 15(3):824. https://doi.org/10.3390/rs15030824
Chicago/Turabian StyleLapaz Olveira, Adrián, Hernán Saínz Rozas, Mauricio Castro-Franco, Walter Carciochi, Luciana Nieto, Mónica Balzarini, Ignacio Ciampitti, and Nahuel Reussi Calvo. 2023. "Monitoring Corn Nitrogen Concentration from Radar (C-SAR), Optical, and Sensor Satellite Data Fusion" Remote Sensing 15, no. 3: 824. https://doi.org/10.3390/rs15030824