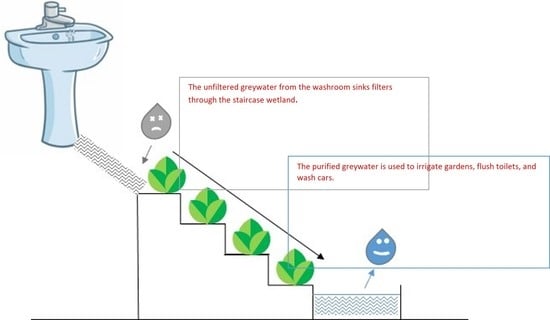
Staircase Wetlands for the Treatment of Greywater and the Effect of Greywater on Soil Microbes
Abstract
:1. Introduction
- Environmental benefits include recovering water resources and minimizing sewage production [5].
- Economic benefits are reductions in water supply costs (through water recycling), which results in reduced household water bills [14].
- Energy benefits are in the form of limited energy generation per family per year from reused GW with installed turbines, pipe systems, storage and disinfection in high-rise buildings [15].
1.1. GW Classification, Parameters and Guidelines
1.2. Soil Properties and Biodiversity
- The tea bag plantation method is used to find the decomposition rate of the soil that absorbs the GW. The tea bag index (TBI) method is a standardized and economical method used to quantify microbial-driven decomposition by measuring the tea mass after being buried in soil over a certain period [60]. This decomposition rate (k) results from increased microbial biomass (cell formations) and higher metabolic activity. Two different tea types are widely accepted for this test: rooibos and green. Each data point corresponds to a replica, i.e., a pair of tea bags includes one rooibos and one green tea bag. Rooibos tea is easy to decompose, whereas green tea is characterized by a slower rate of decomposition. The fraction of green tea that remains after the rooibos tea is fully decomposed is used to estimate the amount of biomass that is fixed in the soil, which is called stabilization (S). The TBI is calculated from both types of tea and is based on these two factors (S and k). Hence, the ‘S’ indicates the amount of material that remains in the soil, and ‘k’ is the amount lost as a byproduct of the decomposition. Both ‘S’ and ‘k’ are functions of the initial and final weights of their respective tea bags [60].
- DNA sequencing is a method used to gather information about organisms and their environment [61]. The sequencing is performed through a two-stage process. First, with commercial DNA kits, the cells are broken down, involving mechanical and chemical processes [62]. Second, short single-stranded DNA fragments, known as primers, are amplified by artificial replication [63]. The amplified DNA fragments are then sequenced, and a taxonomy of all the different kinds of bacteria is generated. Based on that taxonomy, diversity indexes are calculated, namely the alpha (α) and beta (β) [64]. α-diversity is local diversity, which counts the types of microbes in a sample [65]. As the species richness increases, the α-diversity of a particular sample also increases. β-diversity compares all the different kinds of microbes between two or more samples [66]. It gives an estimation of how similar or dissimilar the microbes of different communities are in different samples. Both α and β-diversity are determined from the phylogenetic tree, which is a representation of the evolutionary relationships among various taxa [67]. The α-diversity refers to the diversity within a particular habitat patch or ecosystem [68]. It corresponds to the number of species within a patch. Among patch diversity is the β-diversity, referring to the diversity between habitat patches or ecosystems. It corresponds to the total number of species that are unique to each of the ecosystems being compared.
2. Materials and Methods
| Testing Parameters | Measuring Method/Standards |
|---|---|
| 1. Water tests | |
| 1.1 pH | Measured by Gro Line Waterproof Portable pH/EC (Hanna Instruments, Woonsocket, RI, USA) |
| 1.2 Electrical conductivity | Measured by Gro Line Waterproof Portable pH/EC |
| 1.3 Turbidity | Measured nephelometrically using Inorg-022 a turbidimeter, in accordance with APHA latest edition, 2130-B |
| 1.4 TSS | Determined gravimetrically via filtration of the sample; samples were dried at 104 +/− 5 °C |
| 1.5 BOD | Analyzed in accordance with Inorg-091 APHA latest edition 5210 D |
| 1.6 TC | Australian standard 4276.5-2007 |
| 1.7 FC | Australian standard 4276.5-2007 |
| 2. Soil tests | |
| 2.1 Physiochemical tests | |
| 2.1.1 pH | Measured using a pH meter and electrode in accordance with APHA latest edition, 4500-H+. |
| 2.1.2 Electrical conductivity (EC) | Measured using a conductivity cell at 25 °C in accordance with APHA latest edition 2510 and Rayment and Lyons. |
| 2.1.3 Moisture content | Determined by heating at 105 °C (±5) for a minimum of 12 h |
| 2.1.4 Total organic carbon (TOC) | A titrimetric method that measures the oxidizable organic content of soils |
| 2.1.5 Total Nitrogen (TN) | Calculated as the sum of TKN (Total Kjeldahl Nitrogen) and oxidized nitrogen. Alternatively analyzed via combustion and chemiluminescence |
| 2.1.6 Cation Exchange Capacity (CEC)—NH4Cl | Using 1 M ammonium chloride exchange and ICP-AES (inductively coupled plasma atomic emission spectroscopy) analytical finish |
| 2.2 Biomass tests | Tea bag index (TBI) tests |
| 2.3 DNA Extraction | Soil DNA sequencing |

2.1. GW Soap Recipe
2.2. Construction and Arrangement of Staircase Wetland

2.3. Tea Bag Plantation in Staircase Wetland

2.4. Soil DNA Tests
2.5. Statistical Analysis
3. Results and Analysis
3.1. Water Tests
3.2. Soil Tests
3.3. Soil Biomass
3.3.1. Tea Bag Results
3.3.2. Soil DNA Results
4. Discussion and Conclusions
| Reference | GW Source | Filtering Media | CW Technology | Plants | Flow (m3/day) | HRT (days) | Parameters Studied | Soil BIOMASS Study | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Physical | Chemical | Microbiological | ||||||||
| [102] | Bathroom sink, shower | Sand/soil/compost | VF, HSSF | Phragmites australis | 0.48 | 2.1 | ✓ | ✓ | ✓ | - |
| [103] | Bathroom sink, shower | Gravel (HSSF) | FWS, HSSF | Typha latifolia (FWS) Scirpus acutus (HSSF) | 0.29 | 9.3–12 | ✓ | ✓ | ✓ | - |
| [132] | Secondary GW from aerobic biofilter | Light-weight aggregates | HSSF | Phragmites australis | - | 6–7 | ✓ | ✓ | ✓ | - |
| [133] | Washing machine, clean half of kitchen sink, bathroom sink, tub, shower | Sand (SF) | FWS + SF | Water hyacinth (FWS), tomatoes, peppers (SF) | 0.41 | 6 | ✓ | ✓ | ✓ | - |
| [107] | GW, nonspecific | Plastic bottles or crushed rock | HSSF | Coix lacryma-jobi | 0.005–0.01 | 2.5–7.2 | - | ✓ | ✓ | - |
| [134] | Secondary treated GW (UASB) | Sand | HSSF | Phragmites australis | - | 5 | ✓ | ✓ | ✓ | - |
| This study | Bathroom sink | Soil Washed sand Gravel Hydroponic clay pebbles | VF | Phalaris arundinacea Rhynchospora colorata H. Pfeiff Zantedeschia aethiopica Spreng | 0.01 | 7 | ✓ | ✓ | ✓ | ✓ |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Water, U. Target 6.3—Water Quality and Wastewater. Sustainable Development Goal 6. 2022. Available online: https://www.sdg6monitoring.org/indicators/target-63/ (accessed on 19 August 2022).
- IUCN. Nature-Based Solutions. 2022. Available online: https://www.iucn.org/commissions/commission-ecosystem-management/our-work/nature-based-solutions (accessed on 19 August 2022).
- Eriksson, E.; Auffarth, K.; Henze, M.; Ledin, A. Characteristics of Grey Wastewater. Urban Water 2002, 4, 85–104. [Google Scholar] [CrossRef]
- UNICEF. Water Scarcity. 2022. Available online: https://www.unicef.org/wash/water-scarcity (accessed on 20 August 2022).
- Boano, F.; Caruso, A.; Costamagna, E.; Ridolfi, L.; Fiore, S.; Demichelis, F.; Galvão, A.; Pisoeiro, J.; Rizzo, A.; Masi, F. A review of nature-based solutions for greywater treatment: Applications, hydraulic design, and environmental benefits. Sci. Total Environ. 2020, 711, 134731. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Economic Beneifts of Wetlands. 2006. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/2000D2PF.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2006+Thru+2010&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C06thru10%5CTxt%5C00000000%5C2000D2PF.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 20 August 2022).
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan Jand Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Halverson, N. Review of Constructed Subsurface Flow vs. Surface Flow Wetlands; Savannah River Site (SRS): Aiken, SC, USA, 2004. [Google Scholar]
- Byrne, J.; Dallas, S.; Anda, M.; Ho, G. Quantifying the Benefits of Residential Greywater Reuse. Water 2020, 12, 2310. [Google Scholar] [CrossRef]
- Hadad, E.; Fershtman, E.; Gal, Z.; Silberman, I.; Oron, G. Simulation of dual systems of greywater reuse in high-rise buildings for energy recovery and potential use in irrigation. Resour. Conserv. Recycl. 2022, 180, 106134. [Google Scholar] [CrossRef]
- Neal, J. Waste water reuse studies and trial in Canberra. Desalination 1996, 106, 399–405. [Google Scholar] [CrossRef]
- Hiltner, L. Uber nevere Erfahrungen und Probleme auf dem Gebiet der Boden Bakteriologie und unter besonderer Beurchsichtigung der Grundungung und Broche. Arbeit. Dtsch. Landw. Ges. Berl. 1904, 98, 59–78. [Google Scholar]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Garland, J.L.; Levine, L.H.; Yorio, N.C.; Adams, J.L.; Cook, K.L. Graywater processing in recirculating hydroponic systems: Phytotoxicity, surfactant degradation, and bacterial dynamics. Water Res. 2000, 34, 3075–3086. [Google Scholar] [CrossRef]
- Christova-Boal, D.; Eden, R.E.; McFarlane, S. An investigation into greywater reuse for urban residential properties. Desalination 1996, 106, 391–397. [Google Scholar] [CrossRef]
- Landscape South Australia. Testing Soils and Plants. 2015. Available online: https://www.landscape.sa.gov.au/mr/publications/testing-soils-and-plants (accessed on 12 February 2022).
- FIEMG. Construction Industry Chamber. In Construction Sustainability Guide; FIEMG: Belo Horizonte, Brazil, 2008; p. 60. [Google Scholar]
- Proença, L.C.; Ghisi, E. Assessment of Potable Water Savings in Office Buildings Considering Embodied Energy. Water Resour. Manag. 2013, 27, 581–599. [Google Scholar] [CrossRef]
- do Couto, E.D.A.; Calijuri, M.L.; Assemany, P.P.; da Fonseca Santiago, A.; Lopes, L.S. Greywater treatment in airports using anaerobic filter followed by UV disinfection: An efficient and low cost alternative. J. Clean. Prod. 2015, 106, 372–379. [Google Scholar] [CrossRef]
- Leonard, M.; Gilpin, B.; Robson, B.; Wall, K. Field study of the composition of greywater and comparison of microbiological indicators of water quality in on-site systems. Environ. Monit. Assess. 2016, 188, 475. [Google Scholar] [CrossRef]
- Jefferson, B.; Palmer, A.; Jeffrey, P.; Stuetz, R.; Judd, S. Grey water characterisation and its impact on the selection and operation of technologies for urban reuse. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2004, 50, 157–164. [Google Scholar] [CrossRef]
- Hourlier, F.; Masse, A.; Jaouen, P.; Lakel, A.; Gerente, C.; Faur, C.; Le, C. Formulation of synthetic greywater as an evaluation tool for wastewater recycling technologies. Environ. Technol. 2010, 31, 215–223. [Google Scholar] [CrossRef]
- Fowdar, H.S.; Deletic, A.; Hatt, B.E.; Cook, P.L. Nitrogen removal in greywater living walls: Insights into the governing mechanisms. Water 2018, 10, 527. [Google Scholar] [CrossRef]
- Ghaitidak, D.M.; Yadav, K.D. Characteristics and treatment of greywater—A review. Environ. Sci. Pollut. Res. 2013, 20, 2795–2809. [Google Scholar] [CrossRef]
- Nolde, E. Greywater reuse systems for toilet flushing in multi-storey buildings—Over ten years experience in Berlin. Urban Water 2000, 1, 275–284. [Google Scholar] [CrossRef]
- Noah, M. Graywater use still a gray area. J. Environ. Health 2002, 64, 22. [Google Scholar]
- Morel, A. Greywater Management in Low and Middle-Income Countries; Swiss Federal Institute of Aquatic Science and Technology: Dubenforf, Switzerland, 2006. [Google Scholar]
- Li, F.; Wichmann, K.; Otterpohl, R. Review of the technological approaches for grey water treatment and reuses. Sci. Total Environ. 2009, 407, 3439–3449. [Google Scholar] [CrossRef]
- Eklund, O.C.; Tegelberg, L. Small-Scale Systems for Greywater Reuse and Disposal: A Case Study in Ouagadougou; Department of Energy and Technology, Swedish University of Agricultural Sciences: Uppsala, Sweden, 2010; 136p. [Google Scholar]
- Noutsopoulos, C.; Andreadakis, A.; Kouris, N.; Charchousi, D.; Mendrinou, P.; Galani, A.; Mantziaras, I.; Koumaki, E. Greywater characterization and loadings—Physicochemical treatment to promote onsite reuse. J. Environ. Manag. 2018, 216, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Spychała, M.; Nieć, J.; Zawadzki, P.; Matz, R.; Nguyen, T.H. Removal of volatile solids from greywater using sand filters. Appl. Sci. 2019, 9, 770. [Google Scholar] [CrossRef]
- Babaei, F.; Ehrampoush, M.H.; Eslami, H.; Ghaneian, M.T.; Fallahzadeh, H.; Talebi, P.; Fard, R.F.; Ebrahimi, A.A. Removal of linear alkylbenzene sulfonate and turbidity from greywater by a hybrid multi-layer slow sand filter microfiltration ultrafiltration system. J. Clean. Prod. 2019, 211, 922–931. [Google Scholar] [CrossRef]
- Loh, M.; Coghlan, P. Domestic Water Use Study in Perth, Western Australia 1998–2001; Water Corporation: Perth, Australia, 2003. [Google Scholar]
- Gopalsamy, P.; Edwin, G.; Muthu, N. Constructed Wetlands for the Treatment of Domestic Grey Water: An Instrument of the Green Economy to Realize the Millennium Development Goals. In The Economy of Green Cities: A World Compendium on the Green Urban Economy; Springer: Dordrecht, The Netherlands, 2013; pp. 313–321. [Google Scholar]
- Edwin, G.; Gopalsamy, P.; Muthu, N. Characterization of domestic gray water from point source to determine the potential for urban residential reuse: A short review. Appl. Water Sci. 2014, 4, 39–49. [Google Scholar] [CrossRef]
- Friedler, E. Quality of Individual Domestic Greywater Streams and its Implication for On-Site Treatment and Reuse Possibilities. Environ. Technol. 2004, 25, 997–1008. [Google Scholar] [CrossRef]
- Shaikh, I.N.; Ahammed, M.M. Quantity and quality characteristics of greywater: A review. J. Environ. Manag. 2020, 261, 110266. [Google Scholar] [CrossRef]
- Banach, J.L.; van der Fels-Klerx, H.J. Microbiological Reduction Strategies of Irrigation Water for Fresh Produce. J. Food Prot. 2020, 83, 1072–1087. [Google Scholar] [CrossRef]
- Parjane, S.B.; Sane, M.G. Performance of greywater treatment plant by the economical way for Indian rural development. Int. J. ChemTech Res. 2011, 3, 1808–1815. [Google Scholar]
- Samayamanthula, D.R.; Sabarathinam, C.; Bhandary, H. Treatment and effective utilization of greywater. Appl. Water Sci. 2019, 9, 90. [Google Scholar] [CrossRef]
- Oktor, K.; Çelik, D. Treatment of wash basin and bathroom greywater with Chlorella variabilis and reusability. J. Water Process Eng. 2019, 31, 100857. [Google Scholar] [CrossRef]
- Zipf, M.S.; Pinheiro, I.G.; Conegero, M.G. Simplified greywater treatment systems: Slow filters of sand and slate waste followed by granular activated carbon. J. Environ. Manag. 2016, 176, 119–127. [Google Scholar] [CrossRef]
- Al-Jayyousi, O.R. Greywater reuse: Towards sustainable water management. Desalination 2003, 156, 181–192. [Google Scholar] [CrossRef]
- Jamrah, A.; Al-Futaisi, A.; Prathapar, S.; Harrasi, A.A. Evaluating greywater reuse potential for sustainable water resources management in Oman. Environ. Monit. Assess. 2007, 137, 315. [Google Scholar] [CrossRef]
- Ziemba, C.; Larivé, O.; Reynaert, E.; Morgenroth, E. Chemical composition, nutrient-balancing and biological treatment of hand washing greywater. Water Res. 2018, 144, 752–762. [Google Scholar] [CrossRef]
- Municipal Support Division, Office of Wastewater Management, Office of Water; Technology Transfer and Support Division, National Risk Management Research Laboratory, Office of Research and Development; U.S. Agency for International Development. Guidelines for Water Reuse; U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
- New South Wales Ministry of Health. Greywater Reuse in Sewered Single Domestic Premises; NSW Government: Sydney, Australia, 2000.
- Donovan, J.F.; Bates, J.E. Guidelines for Water Refuse; United States Environmental Protection Agency: Cincinnati, OH, USA, 1980. [Google Scholar]
- James, D.; Ganjian, E.; Surendran, S.; Ifelebuegu, A.; Kinuthia, J. Grey Water Reclamation for Urban Non-Potable Reuse—Challenges and Solutions: A Review. In Proceedings of the 7th International Conference on Sustainable Built Environment, Kandy, Sri Lanka, 16–18 December 2016. [Google Scholar]
- Sanz, L.A.; Gawlik, B.M. Water Reuse in Europe; European Commission, Joint Research Centre–Institute for Environment and Sustainability: Brussels, Belgium, 2014. [Google Scholar]
- Bastian, R.; Murray, D. Guidelines for Water Reuse; EPA Office of Research and Development: Washington, DC, USA, 2012. [Google Scholar]
- Soil Quality Pty Ltd. Fact Sheets Microbial Biomass. 2020. Available online: http://www.soilquality.org.au/factsheets/microbial-biomass-qld#:~:text=Soil%20microbial%20biomass%20(bacteria%2C%20fungi,dioxide%20and%20plant%20available%20nutrients (accessed on 15 March 2022).
- Kandeler, E. Chapter 7—Physiological and Biochemical Methods for Studying Soil Biota and Their Functions. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Paul, E.A., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 187–222. [Google Scholar]
- Phillips, K. Methods of Soil Analysis Using Spectrophotometric Technology. 2015. Available online: https://blog.hunterlab.com/blog/color-measurement/methods-soil-analysis-using-spectrophotometric-technology/#:~:text=Compared%20to%20other%20methods%20of,of%20various%20soil%20materials%20simultaneously (accessed on 10 March 2022).
- Ben-Dor, E.; Taylor, R.G.; Hill, J.; Demattê, J.A.M.; Whiting, M.L.; Chabrillat, S.; Sommer, S. Imaging Spectrometry for Soil Applications. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2008; pp. 321–392. [Google Scholar]
- Assadi, Y.; Farajzadeh, M.A.; Bidari, A. 2.10—Dispersive Liquid–Liquid Microextraction. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 181–212. [Google Scholar]
- Quideau, S.A.; McIntosh, A.C.; Norris, C.E.; Lloret, E.; Swallow, M.J.; Hannam, K. Extraction and Analysis of Microbial Phospholipid Fatty Acids in Soils. J. Vis. Exp. JoVE 2016, 114, 54360. [Google Scholar]
- Keuskamp, J.A.; Dingemans, B.J.J.; Lehtinen, T.; Sarneel, J.M.; Hefting, M.M. Tea Bag Index: A novel approach to collect uniform decomposition data across ecosystems. Methods Ecol. Evol. 2013, 4, 1070–1075. [Google Scholar] [CrossRef]
- Zwolinski, M. DNA Sequencing: Strategies for Soil Microbiology. Soil Sci. Soc. Am. J. SSSAJ 2007, 71, 592–600. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Isolating, Cloning, and Sequencing DNA. In Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Shchelochkov, O.A. Primer. 2022. Available online: https://www.genome.gov/genetics-glossary/Primer (accessed on 20 August 2022).
- Martins, I.S.; Ortega JC, G.; Guerra, V.; Costa MM, S.; Martello, F.; Schmidt, F.A. Ant taxonomic and functional beta-diversity respond differently to changes in forest cover and spatial distance. Basic Appl. Ecol. 2022, 60, 89–102. [Google Scholar] [CrossRef]
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef]
- Knight, C.A.L.R. Species Divergence and the Measurement of Microbial Diversity. FEMS. Microbiol. Rev. 2008, 32, 557–578. [Google Scholar]
- Choudhuri, S. Chapter 9—Phylogenetic Analysis. In Bioinformatics for Beginners; Choudhuri, S., Ed.; Academic Press: Oxford, UK, 2014; pp. 209–218. [Google Scholar]
- Sirami, C. Biodiversity in heterogeneous and dynamic landscapes. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Hajlaoui, H.; Akrimi, R.; Guesmi, A.; Hachicha, M. Assessing the Reliability of Treated Grey Water Irrigation on Soil and Tomatoes (Solanum lycopersicum L.). Horticulturae 2022, 8, 981. [Google Scholar] [CrossRef]
- Aburahma, A.; Mahmoud, N. Impact of Treated Grey Water on Physical and Chemical Soil Characteristics. Master’s Thesis, Faculty of Graduate Studies, Birzeit University, Birzeit, West Bank, Palestine, 2013. [Google Scholar]
- Prabhakar, D.; Singh, A.; Sarkar, S.; Kumar, A.; Kumar, R. Effect of grey water application on physicochemical properties of tomato growing soil. J. Pharmacogn. Phytochem. 2019, 8, 3278–3280. [Google Scholar]
- Arden, S.; Ma, X. Constructed wetlands for greywater recycle and reuse: A review. Sci. Total Environ. 2018, 630, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Sijimol, M.; Joseph, S. Constructed wetland systems for greywater treatment and reuse: A review. Int. J. Energy Water Resour. 2021, 5, 357–369. [Google Scholar] [CrossRef]
- Zhao, Y.; Ji, B.; Liu, R.; Ren, B.; Wei, T. Constructed treatment wetland: Glance of development and future perspectives. Water Cycle 2020, 1, 104–112. [Google Scholar] [CrossRef]
- Oteng-Peprah, M.; Acheampong, M.A.; DeVries, N.K. Greywater characteristics, treatment systems, reuse strategies and user perception—A review. Water Air Soil Pollut. 2018, 229, 255. [Google Scholar] [CrossRef]
- Ghunmi, L.; Zeeman, A.G.; Fayyad, M.; van Lier, J.B. Grey water treatment systems: A review. Crit. Rev. Environ. Sci. Technol. 2011, 41, 657–698. [Google Scholar] [CrossRef]
- Elhegazy, H.; Eid, M.M. A state-of-the-art-review on grey water management: A survey from 2000 to 2020s. Water Sci. Technol. 2020, 82, 2786–2797. [Google Scholar] [CrossRef]
- Maimon, A.; Gross, A. Greywater: Limitations and perspective. Curr. Opin. Environ. Sci. Health 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Al-Husseini, T.H.; Al-Anbari, R.H.; AL-Obaidy, A.H.M. Greywater environmental management: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012100. [Google Scholar] [CrossRef]
- Redwood, M. Greywater irrigation: Challenges and opportunities. CABI Rev. 2008, 2008, 1–7. [Google Scholar] [CrossRef]
- Misra, R.K.; Sivongxay, A. Reuse of laundry greywater as affected by its interaction with saturated soil. J. Hydrol. 2009, 366, 55–61. [Google Scholar] [CrossRef]
- Misra, R.; Patel, J.H.; Baxi, V. Reuse potential of laundry greywater for irrigation based on growth, water and nutrient use of tomato. J. Hydrol. 2010, 386, 95–102. [Google Scholar] [CrossRef]
- Shi, K.-W.; Wang, C.-W.; Jiang, S.C. Quantitative microbial risk assessment of Greywater on-site reuse. Sci. Total Environ. 2018, 635, 1507–1519. [Google Scholar] [CrossRef]
- Clark, J.M.; Kenna, M.P. CHAPTER 5—Lawn and Turf: Management and Environmental Issues of Turfgrass Pesticides. In Handbook of Pesticide Toxicology, 2nd ed.; Krieger, R.I., Krieger, W.C., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 203–241. [Google Scholar]
- Janssen, D. Choosing Clay or Plastic Pots for Plants. 2022. Available online: https://lancaster.unl.edu/hort/articles/2002/clay-or-plastic-pots (accessed on 26 June 2022).
- Bondall. Silasec—Waterproofing Cement Paint. 2021. Available online: https://www.bondall.com/concrete-additives/silasec/ (accessed on 26 June 2022).
- Wolverton, B.C.; Wolverton, J.D. Growing Clean Water: Nature’s Solution to Water Pollution; WES, Incorporated: Derwood, MD, USA, 2001. [Google Scholar]
- Chowdhury, R.; Sulaiman Abaya, J. An Experimental Study of Greywater Irrigated Green Roof Systems in an Arid Climate. J. Water Manag. Model. 2018, 26, 1–10. [Google Scholar] [CrossRef]
- Pradhan, S.; Al-Ghamdi, S.G.; Mackey, H.R. Greywater treatment by ornamental plants and media for an integrated green wall system. Int. Biodeterior. Biodegrad. 2019, 145, 104792. [Google Scholar] [CrossRef]
- Oh, K.S.; Poh, P.E.; Chong, M.N.; Gouwanda, D.; Lam, W.H.; Chee, C.Y. Optimizing the in-line ozone injection and delivery strategy in a multistage pilot-scale greywater treatment system: System validation and cost-benefit analysis. J. Environ. Chem. Eng. 2015, 3, 1146–1151. [Google Scholar] [CrossRef]
- Dossou-Yovo, W.; Parent, S.E.; Ziadi, N.; Parent, E.; Parent, L.E. Tea Bag Index to Assess Carbon Decomposition Rate in Cranberry Agroecosystems. Soil Syst. 2021, 5, 44. [Google Scholar] [CrossRef]
- Prescott, C.E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 2010, 101, 133–149. [Google Scholar] [CrossRef]
- Berg, B.; Meentemeyer, V. Litter quality in a north European transect versus carbon storage potential. Plant Soil 2002, 242, 83–92. [Google Scholar] [CrossRef]
- Soest, P.V. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar] [CrossRef]
- Metagen Australia. 2021. Available online: https://metagen.com.au/ (accessed on 24 March 2022).
- MicrobiomeAnalyst. 2021. Available online: https://www.microbiomeanalyst.ca/ (accessed on 20 May 2022).
- R-Project. 2021. Available online: https://www.r-project.org/ (accessed on 2 January 2022).
- McInnes, L.; Healy, J.; Melville, J. Umap: Uniform manifold approximation and projection for dimension reduction. arXiv 2018, arXiv:1802.03426. [Google Scholar]
- Van der Maaten, L.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 11. [Google Scholar]
- Byleveld, P.; Leask, S.; Jarvis, L.; Wall, K.; Henderson, W.; Tickell, J. Safe drinking water in regional NSW, Australia. Public Health Res. Pract. 2016, 26, e2621615. [Google Scholar] [CrossRef]
- Virginia Brunton, O. Irrigation Water Quality. 2011. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0005/433643/Irrigation-water-quality.pdf (accessed on 7 April 2022).
- IWA Publishing Association. Simple Options to Remove Turbidity. 2022. Available online: https://www.iwapublishing.com/news/simple-options-remove-turbidity (accessed on 1 April 2022).
- Winward, G.P.; Avery, L.M.; Frazer-Williams, R.; Pidou, M.; Jeffrey, P.; Stephenson, T.; Jefferson, B. A study of the microbial quality of grey water and an evaluation of treatment technologies for reuse. Ecol. Eng. 2008, 32, 187–197. [Google Scholar] [CrossRef]
- Jokerst, A.; Sharvelle, S.E.; Hollowed, M.E.; Roesner, L.A. Seasonal performance of an outdoor constructed wetland for graywater treatment in a temperate climate. Water Environ. Res. 2011, 83, 2187–2198. [Google Scholar] [CrossRef]
- Pinto, U.; Maheshwari, B.L.; Grewal, H.S. Effects of greywater irrigation on plant growth, water use and soil properties. Resour. Conserv. Recycl. 2010, 54, 429–435. [Google Scholar] [CrossRef]
- Merhaut, D.J. Get Cultured: Monitoring Electrical Conductivity of Irrigation Water and Rooting Media. 2022. Available online: https://ucnfanews.ucanr.edu/Articles/Get_Cultured/Monitoring_electrical_conductivity_of_irrigation_water_and_rooting_media_593/ (accessed on 26 February 2022).
- Delzer, G.; McKenzie, S. Chapter A7. Section 7.0. Five-Day Biochemical Oxygen Demand; US Geological Survey Techniques of Water-Resources Investigations: Reston, VA, USA, 2003; pp. 1–21. [Google Scholar]
- Dallas, S.; Ho, G. Subsurface flow reedbeds using alternative media for the treatment of domestic greywater in Monteverde, Costa Rica, Central America. Water Sci. Technol. 2005, 51, 119–128. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.; Knight, R.L. Treatment Wetlands; Taylor & Francis: Abingdon, UK, 1995. [Google Scholar]
- Kritzberg, E.S.; Hasselquist, E.M.; Škerlep, M.; Löfgren, S.; Olsson, O.; Stadmark, J.; Valinia, S.; Hansson, L.-A.; Laudon, H. Browning of freshwaters: Consequences to ecosystem services, underlying drivers, and potential mitigation measures. Ambio 2020, 49, 375–390. [Google Scholar] [CrossRef]
- Bolan, N.S.; Kandaswamy, K. pH. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 196–202. [Google Scholar]
- Fearnside, P.M. The effects of cattle pasture on soil fertility in the Brazilian Amazon: Consequences for beef production sustainability. Trop. Ecol. 1980, 21, 125–137. [Google Scholar]
- Bai, W.; Kong, L.; Guo, A. Effects of physical properties on electrical conductivity of compacted lateritic soil. J. Rock Mech. Geotech. Eng. 2013, 5, 406–411. [Google Scholar] [CrossRef]
- Burdekin Productivity Services. Electrical Conductivity Conversion Chart. 2022. Available online: http://bps.net.au/cms/wp-content/uploads/2015/04/EC-Conversion-Chart.pdf (accessed on 10 March 2022).
- South Dakota Soil Health Coolition. Soil Electrical Conductivity. 2022. Available online: https://www.sdsoilhealthcoalition.org/technical-resources/chemical-properties/soil-electrical-conductivity/ (accessed on 10 March 2022).
- Doran, J.W.; Parkin, T.B. Quantitative Indicators of Soil Quality: A Minimum Data Set. In Methods for Assessing Soil Quality; Soil Science Society of America: Madison, WI, USA, 1997; pp. 25–37. [Google Scholar]
- Lal, R. Soil health and carbon management. Food Energy Secur. 2016, 5, 212–222. [Google Scholar] [CrossRef]
- Lal, R. Societal value of soil carbon. J. Soil Water Conserv. 2014, 69, 186A–192A. [Google Scholar] [CrossRef]
- Binkley, D. How Nitrogen-Fixing Trees Change Soil Carbon. In Tree Species Effects on Soils: Implications for Global Change; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Soil Quality Organization. Cations and Cation Exchange Capacity. 2022. Available online: https://www.soilquality.org.au/factsheets/cations-and-cec-tas (accessed on 10 March 2022).
- Chao, A.; Chiu, C.-H. Species Richness: Estimation and Comparison; Wiley Online Library: Hoboken, NJ, USA, 2006; pp. 1–26. [Google Scholar]
- Xia, Y.; Sun, J.; Chen, D.-G. Statistical Analysis of Microbiome Data with R; Springer: Berlin/Heidelberg, Germany, 2018; Volume 847. [Google Scholar]
- Whitehead, H.; Dufault, S. Techniques for analyzing vertebrate social structure using identified individuals. Adv. Stud. Behav. 1999, 28, 33–74. [Google Scholar]
- Zheng, T.; Li, W.; Ma, Y.; Liu, J.; Ren, J. Greywater: Understanding biofilm bacteria succession, pollutant removal and low sulfide generation in small diameter gravity sewers. J. Clean. Prod. 2020, 268, 122426. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, K.; Struewing, I.; Buse, H.; Domingo, J.S.; Lytle, D.; Lu, J. The Bacterial Community Diversity of Bathroom Hot Tap Water Was Significantly Lower Than That of Cold Tap and Shower Water. Front. Microbiol. 2021, 12, 625324. [Google Scholar] [CrossRef]
- Janssen, P.H. Identifying the Dominant Soil Bacterial Taxa in Libraries of 16S rRNA and 16S rRNA Genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J. 2009, 3, 992–1000. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Wolińska, A.; Kuźniar, A.; Zielenkiewicz, U.; Izak, D.; Szafranek-Nakonieczna, A.; Banach, A.; Błaszczyk, M. Bacteroidetes as a sensitive biological indicator of agricultural soil usage revealed by a culture-independent approach. Appl. Soil Ecol. 2017, 119, 128–137. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, N.; Faisal, S.; Ali, A.; Khan, F.U.; You, S.; Bokhari, A.; et al. Role of silica-based porous cellulose nanocrystals in improving water absorption and mechanical properties. Environ. Res. 2023, 222, 115253. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, P.D.; Vråle, L. Greywater treatment in combined biofilter/constructed wetlands in cold climate. In Ecosan—Closing the Loop: Proceedings of the 2nd International Symposium, Ecological Sanitation, Lübeck, Germany, 7–11 April 2003; Werner, C., Avedaño, V., Demsat, S., Eicher, I., Hernández, L., Jung, C., Kraus, S., Lacayo, I., Neupane, K., Rabiega, A., et al., Eds.; GTZ: Lübeck, Germany, 2003. [Google Scholar]
- Gerba, C.P.; Straub, T.M.; Rose, J.B.; Karpiscak, M.M.; Foster, K.E.; Brittain, R.G. Quality study of graywater treatment systems. JAWRA J. Am. Water Resour. Assoc. 1995, 31, 109–116. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; El-Khateeb, M.A.; Regelsberger, M.; El-Sheikh, R.; Shehata, M. Integrated system for the treatment of blackwater and greywater via UASB and constructed wetland in Egypt. Desalin. Water Treat. 2009, 8, 272–278. [Google Scholar] [CrossRef]
- Zegarra, E.K.S. Water Efficiency Evaluation Analysis among Environmental Certification Methods: LEED, BREEAM, DGNB, HQE, EDGE, and BONO VERDE. In Advancements in Sustainable Architecture and Energy Efficiency; IGI Global: Hershey, PA, USA, 2021; pp. 275–291. [Google Scholar]
- Montgomery, D.R.; Biklé, A. Soil health and nutrient density: Beyond organic vs. conventional farming. Front. Sustain. Food Syst. 2021, 5, 417. [Google Scholar] [CrossRef]















| GW Class | Origin | Products | Percentage of Total GW |
|---|---|---|---|
| Class A (LGW) | Washbasin | Hand washing soap, toothpaste, body care products, shaving waste and hair | 50–60% [3] |
| Class B (LGW) | Bathroom | Body wash soap, shampoos, body care products, hair, body fats, lint and traces of urine | |
| Class C (DGW) | Kitchen Sink | Food residues, high amounts of oil and fat and dishwashing detergents. | 10% [27,28,29,30,31,32,33,34] |
| Class D (DGW) | Laundry and all other washing required spaces | Laundry soap, bleaches, oils, paints, solvents, non-biodegradable fibers from clothing and microplastics. | 25–30% [35,36] |
| Physical Parameters [Units] | Values | Range |
|---|---|---|
| Turbidity [Nephelometric Turbidity unit, NTU] | 164 [9], 84.3 [37], 35–164 [39] | Irrigation water quality standard < 10 [40] Fairly turbid (15–25) Rather turbid (25–35) Turbid (35–50) Very turbid > 50 |
| Total solids (TS) | 835 [9], 204 [41], 450.3 [37] | – |
| TSS [mg/L] | 153–259 [9], 141.2 [37], 25–181 [39] | Irrigation water quality standard (≤33) |
| Total dissolved solids (TDS) [ppm] | 473.3 [37] | Ideal drinking (0–40) Acceptable (40–100) Borderline (100–200) Average tap water (200–300) Possibly hazardous (300–400) Potentially hazardous (400–500+) |
| Chemical parameters | ||
| pH | 7–7.3 [9], 7.43 [42], 7.96 [43], 7.2 [37], 6.72–9.82 [39], 6.7–9.8 [44] | Adequate for irrigation (6–8) |
| Biochemical oxygen demand (BOD) [mg/L] | 155–205 [9], 109 [45], 155 [25], 100 [46], 568 [43], 138.5 [37], 33–305 [39], 35–92 [44] | Irrigation water quality standard (≤50) |
| Chemical oxygen demand (COD) [mg/L] | 386–587 [9], 263 [45], 587 [25], 110 [46], 58 [41], 1171 [43], 340.5 [37], 47–587 [39], 47–350 [44] | Irrigation water quality standard (≤50) |
| Chlorides [mg/L] | 237 [9] | Irrigation water quality standard (≤70) |
| Methylene blue active substances (MBAS) [mg/L] | 3.3 [9] | – |
| Oil and grease (O&G) [mg/L] | 135 [9] | – |
| Total organic carbon (TOC) [mg/L] | 99 [25], 63 [46], 155.28 [47], 60.8 [37] | |
| Total Nitrogen (TN)/NH3 [mg/L] | 10.4 [9], 9.6 [45], 10.4 [25], 10.2 [46], 2.22 [47], 0.21 [41], 14.3 [43], 0.6 [37], 2.5–10.4 [39] | Irrigation water quality standard (≤5) |
| Total Phosphorous (TP) [mg/L] | 2.58 [45], 0.13 [25], 0.15 [47], 2.25 [43], 1.1 [37], 0.3–2.6 [39] | Irrigation water quality standard (≤0.8) |
| N/TOC [mg/mg] | 0.11 [25], 0.16 [46] | – |
| P/NOC [mg/mg] | 0.001 [25] | – |
| Microbiological parameters | ||
| Total coliform (TC) (Most Probable Number, [MPN] | 9.42 × 104 [9], 0.0–1.7 × 106 [44] | – |
| Fecal coliform (FC) [MPN] | 3.50 × 104 [9] | – |
| Escherichia coli (E. coli) [MPN] | 10 [9] | <1000 per 100 mL |
| Ground elements and heavy metals | ||
| Boron (B) [mg/L] | 0.44 [9] | Irrigation water quality standard (≤0.75) |
| Calcium (Ca) [mg/L] | 51.19 [47], 0 [41], 5.1 [37] | Irrigation water quality standard (≤120) |
| Magnesium (Mg) [mg/L] | 7.25 [47], 0 [41], 1.8 [37] | Irrigation water quality standard (≤24) |
| Sodium (Na) [mg/L] | 131 [9], 17.11 [41], 19.2 [37] | Irrigation water quality standard (≤30) |
| Sulfur (S) [mg/L] | 27.70 [47], 2.12 [37] | |
| Copper (Cu) [mg/L] | 0.005 [47] | Irrigation water quality standard (≤0.02) |
| Zinc (Zn) [mg/L] | 0.020 [47], 2.03 [37] | Irrigation water quality standard (≤2) |
| Potassium (K) [mg/L] | 1.55 [47], 1.98 [41], 3.6 [37] | Irrigation water quality standard (≤20) |
| Iron (Fe) [mg/L] | 2 [47], 0.17 [37] | Irrigation water quality standard (≤5) |
| Required Parameters for Reuse of GW | USEPA Standards [50] | UK/EU Water Standards [51,52] | NSW Government [49] | USEPA Reclaimed Water Standard for Water Closet Flushing [53] |
|---|---|---|---|---|
| Water quality | ||||
| pH | 6–9 | 6–9 | 5.5–7.5 | 6–9 (monitor 1/month) |
| TSS | <30 mg/L | <30 mg/L | 30 mg/L | |
| BOD | <30 mg/L | <30 mg/L | 20 mg/L | <10 Monitor 1/week |
| Turbidity | 0.1 NTU | <2 NTU continuous monitor | ||
| Pathogen criteria | ||||
| TC | 2.2 cfu/100 mL | 2 cfu/100 mL | 10 cfu/100 mL | |
| FC | ≤200 cfu/100 mL | ≤200 cfu/100 mL | No FC/100 mL |
| Products | pH | EC |
|---|---|---|
| Water + Shampoo | 5.78 | 5.02 |
| Water + Mouthwash | 5.12 | 0.09 |
| Water + Toothpaste | 9.54 | 1.38 |
| Water + Body wash | 4.36 | >6 |
| Water + Laundry soap | 10.72 | >6 |
| C | S1 | S2 | S3 | S4 | |
|---|---|---|---|---|---|
| C | - | 1 | 0.25 | 1 | 1 |
| S1 | 1 | - | 0 | 1 | 0 |
| S2 | 0.25 | 0 | - | 0 | −0.25 |
| S3 | 1 | 1 | 0 | - | 0.5 |
| S4 | 1 | 0 | −0.25 | 0.5 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadir, G.; Pino, V.; Brambilla, A.; Alonso-Marroquin, F. Staircase Wetlands for the Treatment of Greywater and the Effect of Greywater on Soil Microbes. Sustainability 2023, 15, 6102. https://doi.org/10.3390/su15076102
Qadir G, Pino V, Brambilla A, Alonso-Marroquin F. Staircase Wetlands for the Treatment of Greywater and the Effect of Greywater on Soil Microbes. Sustainability. 2023; 15(7):6102. https://doi.org/10.3390/su15076102
Chicago/Turabian StyleQadir, Ghulam, Vanessa Pino, Arianna Brambilla, and Fernando Alonso-Marroquin. 2023. "Staircase Wetlands for the Treatment of Greywater and the Effect of Greywater on Soil Microbes" Sustainability 15, no. 7: 6102. https://doi.org/10.3390/su15076102