Effects of Ammonification–Steam Explosion Pretreatment on the Production of True Protein from Rice Straw during Solid-State Fermentation
Abstract
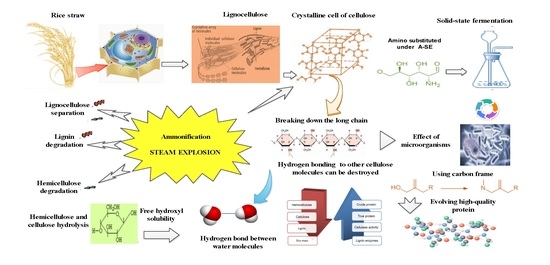
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Pretreatment Methods
2.2.1. Ammonification
2.2.2. Steam Explosion
2.3. Solid-State Fermentation
2.3.1. Microorganisms and Cultures
2.3.2. Fermentation
3. Analytical Methods
3.1. Determination of Lignocellulose Content and Degradation Rate
3.2. Determination of Enzymatic Activity
3.3. Determination of Chemical Composition
3.4. Statistical Analyses
4. Results and Discussion
4.1. Effects of Pretreatment on Lignocellulosic Compositions
4.2. Dynamic Changes in Enzyme Activity
Cellulase Activity
4.3. Lignin Enzymes
4.4. Nutrient Utilization
4.5. Proteins and Amino Acids
4.5.1. Protein Production
4.5.2. Amino Acid Compositions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Øverland, M.; Skrede, A. Yeast derived from lignocellulosic biomass as a sustainable feed resource for use in aquaculture. J. Sci. Food Agric. 2017, 97, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing factors on single-cell protein production by submerged fermentation: A review. Electron. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- Botella, C.; Hernandez, J.E.; Webb, C. Dry weight model, capacitance and metabolic data as indicators of fungal biomass growth in solid state fermentation. Food Bioprod. 2019, 114, 144–153. [Google Scholar] [CrossRef]
- Li, Y.; Peng, X.; Chen, H. Comparative characterization of proteins secreted by Neurospora sitophila in solid-state and submerged fermentation. J. Biosci. Bioeng. 2013, 116, 493–498. [Google Scholar] [CrossRef]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Anim. Nutr. 2019, 5, 319–330. [Google Scholar] [CrossRef]
- Du, X.; Li, B.; Chen, K.; Zhao, C.; Xu, L.; Yang, Z.; Sun, Q.; Chandio, F.; Wu, G. Rice straw addition and biological inoculation promote the maturation of aerobic compost of rice straw biogas residue. Biomass Convers. Biorefin. 2021, 11, 1885–1896. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, K.; Liu, P.; Han, H.; Zhao, S.; Kakade, A.; Khan, A.; Du, D.; Li, X. Lignin depolymerization and utilization by bacteria. Bioresour. Technol. 2018, 269, 557–566. [Google Scholar] [CrossRef]
- Li, B.; Chen, K.; Gao, X.; Zhao, C.; Shao, Q.; Sun, Q.; Li, H. The use of steam explosion to increase the nutrition available from rice straw. Biotechnol. Appl. Biochem. 2015, 62, 823–832. [Google Scholar] [CrossRef]
- Zhao, C.; Cao, Y.; Ma, Z.; Shao, Q. Optimization of liquid ammonia pretreatment conditions for maximizing sugar release from giant reed (Arundo donax L.). Biomass Bioenergy 2017, 98, 61–69. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, F.; Fang, Y.; Zhou, D.; Wang, S.; Wu, D.; Wang, L.; Zhong, R. High-potency white-rot fungal strains and duration of fermentation to optimize corn straw as ruminant feed. Bioresour. Technol. 2020, 312, 123512. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Castro, E. Protein extraction from agri-food residues for integration in biorefinery: Potential techniques and current status. Bioresour. Technol. 2019, 280, 459–477. [Google Scholar] [CrossRef]
- Soltan, Y.A.; Natel, A.S.; Araujo, R.C.; Morsy, A.S.; Abdalla, A.L. Progressive adaptation of sheep to a microencapsulated blend of essential oils: Ruminal fermentation, methane emission, nutrient digestibility, and microbial protein synthesis. Anim. Feed Sci. Technol. 2018, 237, 8–18. [Google Scholar] [CrossRef]
- Somda, M.K.; Nikiema, M.; Keita, I.; Mogmenga, I.; Kouhounde, S.H.S.; Dabire, Y.; Coulibaly, W.H.; Traore, A.S. Production of single cell protein (SCP) and essentials amino acids from Candida utilis FMJ12 by solid state fermentation using mango waste supplemented with nitrogen sources. Afr. J. Biotechnol. 2018, 17, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Ding, L.; Pan, Z.; King, D.; Hadiatullah, H.; Fan, Z. Proteinase and glycoside hydrolase production is enhanced in solid-state fermentation by manipulating the carbon and nitrogen fluxes in Aspergillus oryzae. Food Chem. 2019, 271, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Single cell protein production: A review. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 251–262. [Google Scholar]
- Jalasutram, V.; Kataram, S.; Gandu, B.; Anupoju, G.R. Single cell protein production from digested and undigested poultry litter by Candida utilis: Optimization of process parameters using response surface methodology. Clean Technol. Environ. Policy 2013, 15, 265–273. [Google Scholar] [CrossRef]
- Tengerdy, R.P.; Szakacs, G. Bioconversion of lignocellulose in solid substrate fermentation. Biochem. Eng. J. 2003, 13, 169–179. [Google Scholar] [CrossRef]
- Buerth, C.; Tielker, D.; Ernst, J.F. Candida utilis and Cyberlindnera (Pichia) jadinii: Yeast relatives with expanding applications. Appl. Microbiol. Biotechnol. 2016, 100, 6981–6990. [Google Scholar] [CrossRef]
- Li, B.; Chen, K.; Gao, X.; Zhao, C.; Shao, Q.; Sun, Q.; Li, H. Influence of steam explosion on rice straw fiber content. J. Biobased Mater. Bioenergy 2015, 9, 596–608. [Google Scholar] [CrossRef]
- Delabona, P.D.S.; Farinas, C.S.; Silva, M.R.D.; Azzoni, S.F.; Pradella, J.G.D.C. Use of a new Trichoderma harzianum strain isolated from the amazon rainforest with pretreated sugar cane bagasse for on-site cellulase production. Bioresour. Technol. 2013, 107, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Industrial derivative of tallow: A promising renewable substrate for microbial lipid, single-cell protein and lipase production by Yarrowia lipolytica. Electron. J. Biotechnol. 2017, 10, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Chundawat, S.P.S.; Vismeh, R.; Sharma, L.N.; Humpula, J.F.; Sousa, L.D.; Chambliss, C.K.; Jones, A.D.; Balan, V.; Dale, B. Multifaceted characterization of cell wall decomposition products formed during ammonia fiber expansion (AFEX) and dilute acid based pretreatments. Bioresour. Technol. 2010, 101, 8429–8438. [Google Scholar] [CrossRef]
- Laser, M.; Schulman, D.; Allen, S.G.; Lichwa, J.; Antal-Jr, M.J.; Lynd, L.R. A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresour. Technol. 2002, 81, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Xu, F.; Sun, R.C.; Fowler, P.; Baird, M.S. Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr. Res. 2005, 340, 97–106. [Google Scholar] [CrossRef]
- López-González, J.A.; Suárez-Estrella, F.; Vargas-García, M.C.; López, M.J.; Jurado, M.M.; Moreno, J. Dynamics of bacterial microbiota during lignocellulosic waste composting: Studies upon its structure, functionality and biodiversity. Bioresour. Technol. 2015, 175, 406–416. [Google Scholar] [CrossRef]
- He, L.; Lv, H.; Wang, C.; Zhou, W.; Pian, R.; Zhang, Q.; Chen, X. Dynamics of fermentation quality, physiochemical property and enzymatic hydrolysis of high-moisture corn stover ensiled with sulfuric acid or sodium hydroxide. Bioresour. Technol. 2020, 298, 122510. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Cort, J.R.; Qian, W.; Yang, B. Lipid production from non-sugar compounds in pretreated lignocellulose hydrolysates by Rhodococcus jostii RHA1. Biomass Bioenergy 2021, 145, 105970. [Google Scholar] [CrossRef]
- Goto, T.; Zaccaron, S.; Bacher, M.; Hettegger, H.; Potthast, A.; Rosenau, T. On nitrogen fixation and “residual nitrogen content” in cellulosic pulps. Carbohydr. Polym. 2021, 253, 117235. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Tsapekos, P.; Zhu, X.; Khoshnevisan, B.; Lu, X.; Angelidaki, I. Bioconversion of wastewater to single cell protein by methanotrophic bacteria. Bioresour. Technol. 2021, 320, 124351. [Google Scholar] [CrossRef]
- Roman, K.; Barwicki, J.; Hryniewicz, M.; Szadkowska, D.; Szadkowski, J. Production of Electricity and Heat from Biomass Wastes Using a Converted Aircraft Turbine AI-20. Process 2021, 9, 364. [Google Scholar] [CrossRef]



| Sample | Hemicellulose | Cellulose | Lignin | Reducing Sugar | Total Nitrogen | Soluble Nitrogen | Crude Protein | True Protein | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| TC | 23.6 ± 0.08 a | 37.7 ± 0.11 a | 9.12 ± 0.15 c | 0.90 ± 0.05 d | 0.72 ± 0.08 c | 0.02 ± 0.01 c | 4.48 ± 0.12 c | 1.67 ± 0.03 c | 0.05 |
| TA | 20.2 ± 0.05 a | 33.3 ± 0.12 b | 9.96 ± 0.16 a | 8.46 ± 0.04 c | 1.83 ± 0.10 b | 0.75 ± 0.02 b | 11.4 ± 0.15 b | 1.92 ± 0.04 b | |
| TSE | 17.1 ± 0.07 b | 35.9 ± 0.13 b | 9.50 ± 0.16 a | 9.52 ± 0.04 a | 0.73 ± 0.11 c | 0.38 ± 0.02 c | 4.51 ± 0.09 c | 2.26 ± 0.05 a | |
| TA-SE | 15.8 ± 0.06 b | 33.3 ± 0.12 b | 9.15 ± 0.17 b | 5.49 ± 0.05 b | 2.45 ± 0.09 a | 1.43 ± 0.06 a | 15.3 ± 0.16 a | 2.43 ± 0.05 a |
| Sample | Hemicellulose | Cellulose | Lignin | Reducing Sugar | Total Nitrogen | Soluble Nitrogen | Weight Loss Ratio | p-Value |
|---|---|---|---|---|---|---|---|---|
| TC | 9.31 ± 0.11 a | 22.01 ± 0.25 a | 8.41 ± 0.23 a | 4.32 ± 0.08 a | 0.92 ± 0.08 b | 0.02 ± 0.01 d | 16.7 ± 0.3 d | 0.05 |
| TA | 5.03 ± 0.08 b | 15.80 ± 0.21 b | 7.20 ± 0.22 b | 3.21 ± 0.07 a | 1.83 ± 0.10 a | 0.75 ± 0.02 b | 25.2 ± 0.4 c | |
| TSE | 2.52 ± 0.03 c | 13.06 ± 0.19 c | 5.51 ± 0.16 b | 1.58 ± 0.03 b | 0.73 ± 0.11 b | 0.38 ± 0.02 c | 47.8 ± 0.5 b | |
| TA-SE | 0.76 ± 0.01 c | 6.85 ± 0.12 d | 4.28 ± 0.13 c | 0.03 ± 0.01 c | 2.45 ± 0.09 a | 1.43 ± 0.06 a | 64.5 ± 0.5 a |
| Crude Proteins in Dry Matter (%) | ||||||
| Sample | Fermentation Time (h) | p-Value | ||||
| 0 | 24 | 48 | 72 | 96 | ||
| TC | 4.48 ± 0.15 c | 5.36 ± 0.14 c | 6.48 ± 0.18 c | 7.16 ± 0.18 c | 3.47 ± 0.14 d | 0.05 |
| TA | 11.4 ± 0.21 b | 10.3 ± 0.25 b | 9.86 ± 0.21 b | 8.57 ± 0.19 b | 8.20± 0.18 b | |
| TSE | 4.51 ± 0.16 c | 5.32 ± 0.16 c | 6.49 ± 0.19 c | 7.25± 0.16 c | 7.51 ± 0.17 c | |
| TA-SE | 15.3 ± 0.22 a | 15.6 ± 0.28 a | 16.6 ± 0.27 a | 17.6 ± 0.28 a | 17.7 ± 0.30 a | |
| Crude Proteins in Liquid (%) | ||||||
| Sample | Fermentation Time (h) | p-Value | ||||
| 0 | 24 | 48 | 72 | 96 | ||
| TC | 0.03 ± 0.01 c | 2.57 ± 0.08 d | 4.69 ± 0.10 c | 6.38 ± 0.13 d | 8.91 ± 0.19 c | 0.05 |
| TA | 0.05 ± 0.02 c | 6.41 ± 0.12 c | 11.1 ± 0.15 b | 18.9 ± 0.25 b | 23.5 ± 0.34 b | |
| TSE | 0.13 ± 0.02 b | 8.56 ± 0.11 b | 11.1 ± 0.16 b | 14.3 ± 0.22 c | 15.1 ± 0.22 b | |
| TA-SE | 0.22 ± 0.03 a | 10.3 ± 0.15 a | 25.7 ± 0.26 a | 32.1 ± 0.27 a | 36.3 ± 0.28 a | |
| True Proteins in Dry Matter (%) | ||||||
| Sample | Fermentation Time (h) | p-Value | ||||
| 0 | 24 | 48 | 72 | 96 | ||
| TC | 1.66 ± 0.09 a | 1.82 ± 0.12 d | 1.94 ± 0.13 d | 2.03 ± 0.12 d | 2.15 ± 0.13 d | 0.05 |
| TA | 1.68 ± 0.11 a | 3.06 ± 0.17 b | 4.92 ± 0.21 b | 5.81 ± 0.18 b | 6.81 ± 0.23 b | |
| TSE | 1.73 ± 0.12 a | 2.87 ± 0.16 c | 3.24 ± 0.16 c | 3.80 ± 0.17 c | 4.21 ± 0.24 c | |
| TA-SE | 1.82 ± 0.15 a | 6.81 ± 0.18 a | 11.8 ± 0.24 a | 14.5 ± 0.22 a | 15.4 ± 0.28 a | |
| True Proteins in Liquid (%) | ||||||
| Sample | Fermentation Time (h) | p-Value | ||||
| 0 | 24 | 48 | 72 | 96 | ||
| TC | 0.01 ± 0.01 c | 1.65 ± 0.06 d | 3.37 ± 0.18 c | 5.68 ± 0.19 d | 7.13 ± 0.21 c | 0.05 |
| TA | 0.03 ± 0.01 b | 4.56 ± 0.23 b | 8.91 ± 0.27 b | 13.3 ± 0.26 b | 16.6 ± 0.28 b | |
| TSE | 0.03 ± 0.01 b | 3.67 ± 0.21 c | 9.05 ± 0.26 b | 11.5 ± 0.31 c | 12.8 ± 0.32 c | |
| TA-SE | 0.05 ± 0.01 a | 12.4 ± 0.30 a | 20.1 ± 0.32 a | 28. 8 ± 0.35 a | 31.3 ± 0.37 a | |
| Amino Acid Species | Content (%) | Amino Acid Species | Content (%) | p-Value | ||
|---|---|---|---|---|---|---|
| TA-SE | TC | TA-SE | TC | |||
| aspartic acid | 4.62 ± 0.15 | 0.40 ± 0.02 | isoleucine | 1.57 ± 0.07 | 0.31 ± 0.02 | 0.05 |
| threonine | 1.82 ± 0.07 | 0.22 ± 0.01 | leucine | 4.31 ± 0.11 | 0.36 ± 0.02 | |
| serine | 1.46 ± 0.05 | 0.23 ± 0.01 | tyrosine | 3.55 ± 0.12 | 0.15 ± 0.01 | |
| glutamic acid | 5.07 ± 0.18 | 0.54 ± 0.03 | phenylalanine | 2.79 ± 0.15 | 0.28 ± 0.01 | |
| glycine | 2.15 ± 0.06 | 0.26 ± 0.01 | lysine | 3.56 ± 0.11 | 0.22 ± 0.01 | |
| alanine | 0.56 ± 0.03 | 0.27 ± 0.01 | histidine | 1.20 ± 0.05 | 0.13 ± 0.01 | |
| cystine | 0.37 ± 0.02 | - | tryptophan | 0.03 ± 0.01 | - | |
| valine | 2.74 ± 0.06 | 0.33 ± 0.02 | arginine | 3.09 ± 0.05 | 0.19 ± 0.01 | |
| methionine | 1.87 ± 0.06 | 0.02 ± 0.01 | proline | 1.35 ± 0.04 | 0.38 ± 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Zhao, C.; Sun, Q.; Chen, K.; Zhao, X.; Xu, L.; Yang, Z.; Peng, H. Effects of Ammonification–Steam Explosion Pretreatment on the Production of True Protein from Rice Straw during Solid-State Fermentation. Sustainability 2023, 15, 5964. https://doi.org/10.3390/su15075964
Li B, Zhao C, Sun Q, Chen K, Zhao X, Xu L, Yang Z, Peng H. Effects of Ammonification–Steam Explosion Pretreatment on the Production of True Protein from Rice Straw during Solid-State Fermentation. Sustainability. 2023; 15(7):5964. https://doi.org/10.3390/su15075964
Chicago/Turabian StyleLi, Bin, Chao Zhao, Qian Sun, Kunjie Chen, Xiangjun Zhao, Lijun Xu, Zidong Yang, and Hehuan Peng. 2023. "Effects of Ammonification–Steam Explosion Pretreatment on the Production of True Protein from Rice Straw during Solid-State Fermentation" Sustainability 15, no. 7: 5964. https://doi.org/10.3390/su15075964