Outcome of Ivermectin in Cancer Treatment: An Experience in Loja-Ecuador
Abstract
:1. Introduction
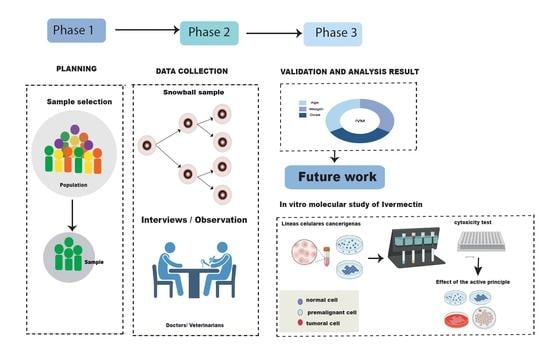
2. Materials and Methods
2.1. Sample Planning and Selection
Initial Requirements: In This Phase the Type of Sampling, Evaluation Metrics and the Instruments Preparation were Planned
2.2. Data Collection
2.2.1. Survey of Participants with Cancer
2.2.2. Interview with Medical Oncologists
2.3. Data Analysis and Interpretation
Confidentiality of Information
3. Results
Perception and Medical Opinion about the Possible Drug Effects on the Patient’s Health
4. Discussion
5. Conclusions
6. Future work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Code | Category | Subcategory | Frequency |
|---|---|---|---|
| 1 | Key aspects of the medicine | IVM is a veterinary antiparasitic drug extrapolated to humans as an anticancer treatment. Doesn’t known | 4 2 |
| 2 | Diagnosis and treatment of cancer | Preventive examinations, biopsy, endoscopy, screening, mammography, colposcopy, prostate antigen, etc. Treatment through radiotherapy, chemotherapy, surgery, etc. | 5 |
| 3 | Harmful effects of the medicine | Affects other organs of the body, causes local pain, hematoma, sciatic nerve puncture, anaphylactic effect, allergic reaction, vomiting, etc. It blocks the necrosis activities of cancer cells. It has minimal side effects; it is neither neurotoxic nor hepatocytic. | 2 2 1 |
| 4 | Medical recommendations | They do not recommend the use of these drugs since there are no scientific studies on the subject. Medical awareness is fundamental through campaigns, screening, preventive control, promoting attendance to specialized medical lefts such as SOLCA, IESS (Ecuadorian Institute of Social Security), public hospitals, etc. The patient first needs to take the treatment suggested by his doctor. | 6 5 1 |
| 5 | Molecular research | It is important to perform an in vitro molecular study on the molecular composition of these drugs. No, it is considered important. | 4 2 |
References
- Dagenais, G.R.; Leong, D.P.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Gupta, R.; Diaz, R.; Avezum, A.; Oliveira, G.B.; Wielgosz, A.; et al. Variations in com-mon diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): A prospective cohort study. Lancet 2020, 395, 785–794. [Google Scholar] [CrossRef]
- Instituto Nacional del Cancer. Genética del Cancer. 2017. Available online: https://www.cancer.gov/espanol/cancer/causas-prevencion/genetica (accessed on 12 October 2017).
- Jiménez-Gaona, Y.; Rodríguez-Álvarez, M.J.; Lakshminarayanan, V. Deep-Learning-Based Computer-Aided Systems for Breast Cancer Imaging: A Critical Review. Appl. Sci. 2020, 10, 8298. [Google Scholar] [CrossRef]
- Peralta, A.; Benach, J.; Borrell, C.; Espinel-Flores, V.; Cash-Gibson, L.; Queiroz, B.L.; Marí-Dell’Olmo, M. Evaluation of the mortality registry in Ecuador (2001–2013)—Social and geographical inequalities in completeness and quality. Popul. Health Metr. 2019, 17, 1–12. [Google Scholar] [CrossRef]
- Cordero, F.C.; Ayala, P.C.; Maldonado, J.Y.; Montenegro, W.T. Trends in cancer incidence and mortality over three decades in Quito-Ecuador. Colomb. Med. 2018, 49, 35–41. [Google Scholar] [CrossRef]
- Forman, D.; Sierra, M.S. Cancer in Central and South America: Introduction. Cancer Epidemiol. 2016, 44, S3–S10. [Google Scholar] [CrossRef] [Green Version]
- Cueva, P.; Yépez, J. Epidemiología del Cáncer en Quito, 2006; Sociedad de Lucha contra el Cáncer-Registro Nacional de Tumores: Quito, Ecuador, 2014. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Sierra, M.S.; Cueva, P.; Bravo, L.E.; Forman, D. Stomach cancer burden in Central and South America. Cancer Epidemiol. 2016, 44, S62–S73. [Google Scholar] [CrossRef] [Green Version]
- Yunga, E.; Garrido, H. (Eds.) Incidencia del Cáncer en Loja: Registro de Tumores 1997-2003; Cámara Ecuatoriana del Libro—Núcleo de Pichincha: Loja, Ecuador, 2006; ISBN 9789978455296. [Google Scholar]
- Stein, K. The long term effects of cancer and cancer treatment: Research at the american cancer society. In VI European Conference on Cured and Chronic Cancer Patients; Edisciences: Siracusa, Italy, 2016; p. 81. [Google Scholar]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Choudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative stress and antioxidants in disease and cancer: A review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, K.; Cheng, L.; Zhu, H.; Xu, T. Progress in understanding the molecular mechanisms underlying the antitumour effects of ivermectin. Drug Des. Dev. Ther. 2020, 14, 285. [Google Scholar] [CrossRef] [Green Version]
- Juarez, M.; Schcolnik-Cabrera, A.; Dueñas-Gonzalez, A. The multitargeted drug ivermectin: From an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 2018, 8, 317. [Google Scholar]
- Li, N.; Zhan, X. Anti-parasite drug ivermectin can suppress ovarian cancer by regulating lncRNA-EIF4A3-mRNA axes. EPMA J. 2020, 11, 289–309. [Google Scholar] [CrossRef]
- Wang, K.; Gao, W.; Dou, Q.; Chen, H.; Li, Q.; Nice, E.C.; Huang, C. Ivermectin induces PAK1-mediated cytostatic autophagy in breast cancer. Autophagy 2016, 12, 2498–2499. [Google Scholar] [CrossRef]
- Acosta, L.A.U. Factores Asociados a la Automedicación con Antiparasitarios en la Población Infantil Entre 0 y 10 Años del Area Urbana y Rural de Pelileo. Bachelor’s Thesis, Universidad Técnica de Ambato, Ambato, Ecuador, 2014. [Google Scholar]
- Marroquín, E.C.; Iglesia, N.M.; Cobos, L.P. Adecuación de la prescripción farmacéutica en personas de 65 años o más en centros de salud docentes de Cáceres. Rev. Española Salud Pública 2012, 86, 419–434. [Google Scholar]
- Ōmura, S.; Crump, A. Ivermectin: Panacea for resource-poor communities? Trends Parasitol. 2014, 30, 445–455. [Google Scholar] [CrossRef]
- Campbell, W.C. An introduction to the avermectins. N. Z. Vet. J. 1981, 29, 174–178. [Google Scholar] [CrossRef]
- Campbell, W.C.; Fisher, M.H.; Stapley, E.O.; Albers-Schönberg, G.; Jacob, T.A. Ivermectin: A potent new antiparasitic agent. Science 1983, 221, 823–828. [Google Scholar] [CrossRef]
- Kobylinski, K.C.; Foy, B.D.; Richardson, J.H. Ivermectin inhibits the sporogony of Plasmodium falciparum in Anopheles gambiae. Malar. J. 2012, 11, 381. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.E.; Vilchèze, C.; Ng, C.; Jacobs, W.R., Jr.; Ramón-García, S.; Thompson, C.J. Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrug-resistant clinical strains. Antimicrob. Agents Chemother. 2013, 57, 1040–1046. [Google Scholar] [CrossRef] [Green Version]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; de Lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef] [Green Version]
- Sharmeen, S.; Skrtic, M.; Sukhai, M.A.; Hurren, R.; Gronda, M.; Wang, X.; Fonseca, S.B.; Sun, H.; Wood, T.E.; Ward, R.; et al. The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood J. Am. Soc. Hematol. 2010, 116, 3593–3603. [Google Scholar] [CrossRef] [Green Version]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Lv, C.; Liu, W.; Wang, B.; Dang, R.; Qiu, L.; Ren, J.; Yan, C.; Yang, Z.; Wang, X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antivir. Res. 2018, 159, 55–62. [Google Scholar] [CrossRef]
- Dou, Q.; Chen, H.N.; Wang, K.; Yuan, K.; Lei, Y.; Li, K.; Lan, J.; Chen, Y.; Huang, Z.; Xie, N.; et al. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Cancer Res. 2016, 76, 4457–4469. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Hu, X.; Wang, Y.; Yao, X.; Zhang, W.; Yu, C.; Cheng, F.; Li, J.; Fang, Q. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol. Res. 2021, 163, 105207. [Google Scholar] [CrossRef]
- Ashraf, S.; Prichard, R. Ivermectin exhibits potent anti-mitotic activity. Vet. Parasitol. 2016, 226, 1–4. [Google Scholar] [CrossRef]
- Draganov, D.; Han, Z.; Rana, A.; Bennett, N.; Irvine, D.J.; Lee, P.P. Ivermectin converts cold tumors hot and synergizes with immune checkpoint blockade for treatment of breast cancer. NPJ Breast Cancer 2021, 7, 22. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, T.; Zhu, Z.; Hong, F.; Xu, Y.; Zhang, X.; Xu, X.; Ma, A. Ivermectin augments the in vitro and in vivo efficacy of cisplatin in epithelial ovarian cancer by suppressing Akt/mTOR signaling. Am. J. Med. Sci. 2020, 359, 123–129. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, P.; Sun, Y.J.; Wu, Y.J. Ivermectin reverses the drug resistance in cancer cells through EGFR/ERK/Akt/NF-κB pathway. J. Exp. Clin. Cancer Res. 2019, 38, 265. [Google Scholar] [CrossRef]
- Zhou, S.; Wu, H.; Ning, W.; Wu, X.; Xu, X.; Ma, Y.; Li, X.; Hu, J.; Wang, C.; Wang, J. Ivermectin has New Application in Inhibiting Colorectal Cancer Cell Growth. Front. Pharmacol. 2021, 2021, 2145. [Google Scholar] [CrossRef]
- Driniaev, V.A.; Mosin, V.A.; Krugliak, E.B.; Sterlina, T.C.; Novik, T.; Ermakova, N.V.; Kublik, L.N.; Levitman, M.K.; Shaposhnikova, V.V.; Korystov, I.N. Modification of antitumor effect of vincristine by natural avermectins. Antibiot. Chemoterapy 2004, 49, 3–5. [Google Scholar]
- Crump, A. Ivermectin: Enigmatic multifaceted ‘wonder’ drug conti-nues to surprise and exceed expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Diazgranados-Sanchez, J.A.; Barrios-Arrázola, G.; Costa, J.L.; Burbano-Pabon, J.; Pinzón-Bedoya, J. Ivermectin as a therapeutic alternative in neurocysticercosis that is resistant to conventional pharmacological treatment. Rev. De Neurol. 2008, 46, 671–674. [Google Scholar]
- Zhu, M.; Li, Y.; Zhou, Z. Antibiotic ivermectin preferentially targets renal cancer through inducing mitochondrial dysfunction and oxidative damage. Biochem. Biophys. Res. Commun. 2017, 492, 373–378. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.; Sun, Q.; Liu, B. Anthelmintic drug ivermectin inhibits angiogenesis, growth and survival of glioblastoma through inducing mitochondrial dysfunction and oxidative stress. Biochem. Biophys. Res. Commun. 2016, 480, 415–421. [Google Scholar] [CrossRef]
- Drinyaev, V.A.; Mosin, V.A.; Kruglyak, E.B.; Novik, T.S.; Sterlina, T.S.; Ermakova, N.V.; Kublik, L.N.; Levitman, M.K.; Shaposhnikova, V.V.; Korystov, Y.N. Antitumor effect of avermectins. Eur. J. Pharmacol. 2004, 501, 19–23. [Google Scholar] [CrossRef]
- Hashimoto, H.; Sudo, T.; Maruta, H.; Nishimura, R. The direct PAK1 inhibitor, TAT-PAK18, blocks preferentially the growth of human ovarian cancer cell lines in which PAK1 is abnormally activated by autophosphorylation at Thr 423. Drug Discov. Ther. 2010, 4, 1–4. [Google Scholar]
- Melotti, A.; Mas, C.; Kuciak, M.; Lorente-Trigos, A.; Borges, I.; Altaba, A.R. The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol. Med. 2014, 6, 1263–1278. [Google Scholar] [CrossRef]
- Khan, M.S.I.; Khan, M.S.I.; Debnath, C.R.; Nath, P.N.; Al Mahtab, M.; Nabeka, H.; Matsuda, S.; Akbar, S.M.F. Ivermectin treatment may improve the prognosis of patients with COVID-19. Arch. Bronconeumol. 2020, 56, 828. [Google Scholar] [CrossRef]
- Okumuş, N.; Demirtürk, N.; Çetinkaya, R.A.; Güner, R.; Avcı, Y.; Orhan, S.; Konya, P.; Şaylan, B.; Karalezli, A.; Yamanel, L.; et al. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect. Dis. 2021, 21, 411. [Google Scholar] [CrossRef]
- Ahmed, S.; Karim, M.M.; Ross, A.G.; Hossain, M.S.; Clemens, J.D.; Sumiya, M.K.; Phru, C.S.; Rahman, M.; Zaman, K.; Somani, J.; et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 2021, 103, 214–216. [Google Scholar] [CrossRef]
- Pott-Junior, H.; Paoliello, M.M.B.; de Queiroz Constantino Miguel, A.; da Cunha, A.F.; de Melo Freire, C.C.; da Silva de Av, L.R.; Roscani, M.G.; De Sousa dos Santos, S.; Chach, S.G.F. Use of ivermectin in the treatment of Covid-19: A pilot trial. Toxicol. Rep. 2021, 8, 505–510. [Google Scholar] [CrossRef]



| Reference | Type of Study | Description | Objective | Subtype of Cancer |
|---|---|---|---|---|
| Draganov et al. [32] | In Vivo | The IVM effects were study using a 4T1 mouse model of TNBC. The results indicate that IVM has dual immunomodulatory and ICD-inducing effects in breast cancer | To evaluate animal line cells | Breast cancer |
| Dou et al. [29] | In Vivo and In Vitro | The authors report a role for IVM-induced autophagy in breast cancer cells. The results provide a molecular basis for the use of IVM to inhibit the proliferation of breast cancer cells. | To evaluate cellular PAK 1 line | Breast cancer |
| Zhang et al. [33] | In Vivo and In Vitro | The molecular mechanism and effects of IVM alone and its combination with cisplatin on growth and survival were examined. Results indicate that IVM significantly augmented the inhibitory effect of cisplatin on ovarian cancer cells in a dose-dependent manner. | To evaluate cultured ovarian cancer cells and a xenograft mouse model, focusing on Akt/mTOR signaling | Ovarian cancer |
| Jiang et al. [34] | In Vivo and In Vitro | The effects of IVM on cancer cells lines which are resistant to the chemotherapeutin drugs vincristine and adriamycin were investigate in vitro. Flow cytometry, immunohistochemistry, and immunofluorescence were used to investigate the reversal effect of IVM in vivo. Results indicated that IVM at its very low dose drastically reversed the resistance of the tumor cells to the chemotherapeutic drugs both in vitro and in vivo. | To evaluate two (HCT-8) colorectal cancer cells and (MCF-7) breast cancer cells), one hematologic tumor (K562) and two xenograft mice models | Colorectal, breast cancer and one hematologic tumor cell line |
| Zhou et al. [35] | In Vivo | The results demonstrated that ivermectin dose-dependently inhibited colorectal cancer SW480 and SW1116 cell growth, which was followed by promoting cell apoptosis and increasing Caspase-3/7 activity. | To evaluate the influence of ivermectin on CRC using CRC cell lines SW480 and SW1116 | Colorectal cancer |
| Group | Sub-Group | Frequency (N) | Percentage (%) |
|---|---|---|---|
| Gender | Male | 20 | 41.67 |
| Female | 26 | 54.17 | |
| Undefined | 2 | 4.16 | |
| Age | 20–40 | 8 | 16.7 |
| 41–60 | 27 | 56.3 | |
| 61–80 | 8 | 16.7 | |
| 81–100 | 5 | 10.3 | |
| Rural area | Catamayo | 30 | 62.5 |
| Changaimina | 1 | 2.08 | |
| Gonzanama | 3 | 6.26 | |
| Paja Blanca | 1 | 2.08 | |
| Sacapalca | 13 | 27.08 |
| Age | Weight | Dose | ||
|---|---|---|---|---|
| Age | Pearson correlation | 1 | 0.260 | −0.004 |
| Sig. (bilateral) | 0.080 | 0.982 | ||
| N | 48 | 48 | 32 | |
| Weight | Pearson correlation | 0.260 | 1 | 0.381 * |
| Sig. (bilateral) | 0.080 | 0.031 | ||
| N | 48 | 48 | 32 | |
| Dose | Pearson correlation | −0.004 | 0.381 * | 1 |
| Sig. (bilateral) | 0.982 | 0.031 | ||
| N | 32 | 32 | 32 | |
| Model | R | R Square | R Fitted Square | Standard Error |
|---|---|---|---|---|
| 1 | 0.381 a | 0.146 | 0.117 | 0.316 |
| Model | Sum of Squares | df | Root Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| 1 | Regression | 0.509 | 1 | 0.509 | 5.109 | 0.031 b |
| Residue | 2.991 | 30 | 0.100 | |||
| Total | 3.500 | 31 | ||||
| Model | Non-Standardized Coefficients | Standardized Coefficients | T | Sig. | ||
|---|---|---|---|---|---|---|
| B | Standard Error | Beta | ||||
| 1 | (Constant) | 1.433 | 0.311 | 4.605 | 0.000 | |
| Weight | 0.010 | 0.004 | 0.381 | 2.260 | 0.031 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Gaona, Y.; Vivanco-Galván, O.; Morales-Larreategui, G.; Cabrera-Bejarano, A.; Lakshminarayanan, V. Outcome of Ivermectin in Cancer Treatment: An Experience in Loja-Ecuador. Nurs. Rep. 2023, 13, 315-326. https://doi.org/10.3390/nursrep13010030
Jiménez-Gaona Y, Vivanco-Galván O, Morales-Larreategui G, Cabrera-Bejarano A, Lakshminarayanan V. Outcome of Ivermectin in Cancer Treatment: An Experience in Loja-Ecuador. Nursing Reports. 2023; 13(1):315-326. https://doi.org/10.3390/nursrep13010030
Chicago/Turabian StyleJiménez-Gaona, Yuliana, Oscar Vivanco-Galván, Gonzalo Morales-Larreategui, Andrea Cabrera-Bejarano, and Vasudevan Lakshminarayanan. 2023. "Outcome of Ivermectin in Cancer Treatment: An Experience in Loja-Ecuador" Nursing Reports 13, no. 1: 315-326. https://doi.org/10.3390/nursrep13010030