Rapid Effects of BCI-Based Attention Training on Functional Brain Connectivity in Poststroke Patients: A Pilot Resting-State fMRI Study
Abstract
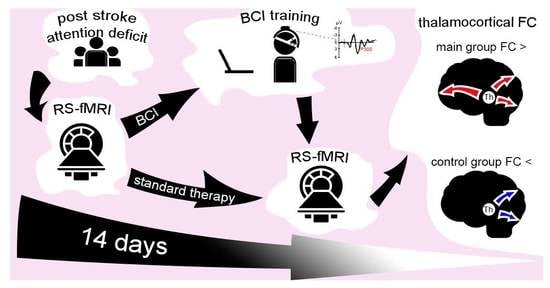
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Assessment
2.3. BCI Training Protocol
2.4. Resting-State fMRI Data Acquisition, Preprocessing, and Connectivity Analysis
3. Results
3.1. Neuropsychological Assessment
3.2. Seed-Based Functional Connectivity Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aam, S.; Einstad, M.S.; Munthe-Kaas, R.; Lydersen, S.; Ihle-Hansen, H.; Knapskog, A.B.; Ellekjær, H.; Seljeseth, Y.; Saltvedt, I. Post-Stroke Cognitive Impairment—Impact of Follow-Up Time and Stroke Subtype on Severity and Cognitive Profile: The Nor-COAST Study. Front. Neurol. 2020, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Hurford, R.; Charidimou, A.; Fox, Z.; Cipolotti, L.; Werring, D.J. Domain-Specific Trends in Cognitive Impairment after Acute Ischaemic Stroke. J. Neurol. 2013, 260, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Goh, S.J.A.; Quek, S.Y.; Phillips, R.; Guan, C.; Cheung, Y.B.; Feng, L.; Teng, S.S.W.; Wang, C.C.; Chin, Z.Y.; et al. A Brain-Computer Interface Based Cognitive Training System for Healthy Elderly: A Randomized Control Pilot Study for Usability and Preliminary Efficacy. PLoS ONE 2013, 8, e79419. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.G.; Poh, X.W.W.; Fung, S.S.D.; Guan, C.; Bautista, D.; Cheung, Y.B.; Zhang, H.; Yeo, S.N.; Krishnan, R.; Lee, T.S. A Randomized Controlled Trial of a Brain-Computer Interface Based Attention Training Program for ADHD. PLoS ONE 2019, 14, e216225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Ye, Y.; Li, X.; Wang, R. Development of a Neuro-Feedback Game Based on Motor Imagery EEG. Multimed. Tools Appl. 2018, 77, 15929–15949. [Google Scholar] [CrossRef]
- Ang, K.K.; Chua, K.S.G.; Phua, K.S.; Wang, C.; Chin, Z.Y.; Kuah, C.W.K.; Low, W.; Guan, C. A Randomized Controlled Trial of EEG-Based Motor Imagery Brain-Computer Interface Robotic Rehabilitation for Stroke. Clin. EEG Neurosci. 2015, 46, 310–320. [Google Scholar] [CrossRef]
- Angelakis, E.; Stathopoulou, S.; Frymiare, J.L.; Green, D.L.; Lubar, J.F.; Kounios, J. EEG Neurofeedback: A Brief Overview and an Example of Peak Alpha Frequency Training for Cognitive Enhancement in the Elderly. Clin. Neuropsychol. 2007, 21, 110–129. [Google Scholar] [CrossRef]
- Gruzelier, J.H. EEG-Neurofeedback for Optimising Performance. I: A Review of Cognitive and Affective Outcome in Healthy Participants. Neurosci. Biobehav. Rev. 2014, 44, 124–141. [Google Scholar] [CrossRef]
- Vernon, D.; Egner, T.; Cooper, N.; Compton, T.; Neilands, C.; Sheri, A.; Gruzelier, J. The Effect of Training Distinct Neurofeedback Protocols on Aspects of Cognitive Performance. Int. J. Psychophysiol. 2003, 47, 75–85. [Google Scholar] [CrossRef]
- Wang, J.R.; Hsieh, S. Neurofeedback Training Improves Attention and Working Memory Performance. Clin. Neurophysiol. 2013, 124, 2406–2420. [Google Scholar] [CrossRef]
- Klingberg, T. Training and Plasticity of Working Memory. Trends Cogn. Sci. 2010, 14, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Jonides, J. How Does Practice Makes Perfect? Nat. Neurosci. 2004, 7, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An Integrative Theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleih, S.C.; Gottschal, L.; Teichlein, E.; Weilbach, F.X. Toward a P300 Based Brain-Computer Interface for Aphasia Rehabilitation after Stroke: Presentation of Theoretical Considerations and a Pilot Feasibility Study. Front. Hum. Neurosci. 2016, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Ganin, I.P.; Kim, S.A.; Liburkina, S.P.; Galkina, N.V.; Luzhin, A.O.; Mayorova, L.A.; Malyukova, N.G.; Shklovsky, V.M.; Kaplan, A.Y. Text Typing in Patients with Post-Stroke Afasia in the P300 Brain-Computer Interface Based “Neurochat” Complex. Zhurnal Vyss. Nervn. Deyatelnosti Im. I.P. Pavlov. 2020, 70, 435–445. [Google Scholar] [CrossRef]
- Burton, L.; Tyson, S.F. Screening for Cognitive Impairment after Stroke: A Systematic Review of Psychometric Properties and Clinical Utility. J. Rehabil. Med. 2015, 47, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Zietemann, V.; Georgakis, M.K.; Dondaine, T.; Muller, C.; Mendyk, A.M.; Kopczak, A.; Hénon, H.; Bombois, S.; Wollenweber, F.A.; Bordet, R.; et al. Article Early Moca Predicts Long-Term Cognitive and Functional Outcome and Mortality after Stroke. Neurology 2018, 91, E1838–E1850. [Google Scholar] [CrossRef]
- Salis, C.; Kelly, H.; Code, C. Assessment and Treatment of Short-Term and Working Memory Impairments in Stroke Aphasia: A Practical Tutorial. Int. J. Lang. Commun. Disord. 2015, 50, 721–736. [Google Scholar] [CrossRef]
- Maldjian, J.A.; Laurienti, P.J.; Kraft, R.A.; Burdette, J.H. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-Based Interrogation of FMRI Data Sets. Neuroimage 2003, 19, 1233–1239. [Google Scholar] [CrossRef]
- Ardekani, B.A.; Choi, S.J.; Hossein-Zadeh, G.A.; Porjesz, B.; Tanabe, J.L.; Lim, K.O.; Bilder, R.; Helpern, J.A.; Begleiter, H. Functional Magnetic Resonance Imaging of Brain Activity in the Visual Oddball Task. Cogn. Brain Res. 2002, 14, 347–356. [Google Scholar] [CrossRef]
- Mccarthy, G.; Luby, M.; Gore, J.; Goldman-Rakic, P. Infrequent Events Transiently Activate Human Prefrontal and Parietal Cortex as Measured by Functional MRI. J. Neurophysiol. 1997, 77, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Bledowski, C.; Prvulovic, D.; Hoechstetter, K.; Scherg, M.; Wibral, M.; Goebel, R.; Linden, D.E.J. Localizing P300 Generators in Visual Target and Distractor Processing: A Combined Event-Related Potential and Functional Magnetic Resonance Imaging Study. J. Neurosci. 2004, 24, 9353–9360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, L.-Q.; Hu, Y. Localizing P300 Generators in High-Density Event-Related Potential with FMRI. Med. Sci. Monit. 2009, 15, MT47-53. [Google Scholar] [PubMed]
- Clark, V.P.; Fannon, S.; Lai, S.; Benson, R.; Bauer, L. Responses to Rare Visual Target and Distractor Stimuli Using Event- Related FMRI. J. Neurophysiol. 2000, 83, 3133–3139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirino, E.; Belger, A.; Goldman-Rakic, P.; McCarthy, G. Prefrontal Activation Evoked by Infrequent Target and Novel Stimuli in a Visual Target Detection Task: An Event-Related Functional Magnetic Resonance Imaging Study. J. Neurosci. 2000, 20, 6612–6618. [Google Scholar] [CrossRef]
- Warbrick, T.; Mobascher, A.; Brinkmeyer, J.; Musso, F.; Richter, N.; Stoecker, T.; Fink, G.R.; Shah, N.J.; Winterer, G. Single-Trial P3 Amplitude and Latency Informed Event-Related FMRI Models Yield Different BOLD Response Patterns to a Target Detection Task. Neuroimage 2009, 47, 1532–1544. [Google Scholar] [CrossRef]
- Aggleton, J.P.; O’Mara, S.M.; Vann, S.D.; Wright, N.F.; Tsanov, M.; Erichsen, J.T. Hippocampal-Anterior Thalamic Pathways for Memory: Uncovering a Network of Direct and Indirect Actions. Eur. J. Neurosci. 2010, 31, 2292–2307. [Google Scholar] [CrossRef] [Green Version]
- Carrera, E.; Bogousslavsky, J. The Thalamus and Behavior: Effects of Anatomically Distinct Strokes. Neurology 2006, 66, 1817–1823. [Google Scholar] [CrossRef]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-Analytic Evidence for a Superordinate Cognitive Control Network Subserving Diverse Executive Functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Ide, J.S.; Li, C.; Shan, R. A Cerebellar Thalamic Cortical Circuit for Error-Related Cognitive Control. Neuroimage 2011, 54, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Tsapkini, K.; Vindiola, M.; Rapp, B. Patterns of Brain Reorganization Subsequent to Left Fusiform Damage: FMRI Evidence from Visual Processing of Words and Pseudowords, Faces and Objects. Neuroimage 2011, 55, 1357–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouhaz, Z.; Fleming, H.; Mitchell, A.S. Cognitive Functions and Neurodevelopmental Disorders Involving the Prefrontal Cortex and Mediodorsal Thalamus. Front. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parnaudeau, S.; Bolkan, S.S.; Kellendonk, C. The Mediodorsal Thalamus: An Essential Partner of the Prefrontal Cortex for Cognition. Biol. Psychiatry 2018, 83, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Stern, Y. What Is Cognitive Reserve? Theory and Research Application of the Reserve Concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef]
- Umarova, R.M.; Sperber, C.; Kaller, C.P.; Schmidt, C.S.M.; Urbach, H.; Klöppel, S.; Weiller, C.; Karnath, H.-O. Cognitive Reserve Impacts on Disability and Cognitive Deficits in Acute Stroke. J. Neurol. 2019, 266, 2495–2504. [Google Scholar] [CrossRef]
- Rosenich, E.; Hordacre, B.; Paquet, C.; Koblar, S.A.; Hillier, S.L. Cognitive Reserve as an Emerging Concept in Stroke Recovery. Neurorehabil. Neural Repair 2020, 34, 187–199. [Google Scholar] [CrossRef]



| Main Group (n = 7) | Control Group (n = 11) | p-Value | |
|---|---|---|---|
| Age, years | 62 (10.5) | 60 (19.5) | 0.492 |
| Female, % | 28.6 | 27.3 | 1.000 |
| Higher education, % | 71.4 | 27.3 | 0.074 |
| Disease duration, month | 1.9 (0.48) | 1.1 (1.48) | 0.132 |
| Lesion volume, cm3 | 2.14 (17.08) | 1.91 (4.51) | 0.809 |
| Time between first and second fMRI | 13 (2.25) | 10 (3.75) | 0.261 |
| Seed | Region | MNI (x, y, z) | Cluster Size | F2,15 | p-FWE |
|---|---|---|---|---|---|
| R thalamus | L cerebellum | −14 −64 −18 | 287 | 17.4 | 0.000 |
| R fusiform gyrus (occipital part) | 26 −80 −18 | ||||
| R calcarine cortex | 6 −84 6 | ||||
| L cerebellum | −22 −48 −46 | 39 | 15.1 | 0.027 | |
| Superior frontal gyrus | No significant clusters | ||||
| Middle frontal gyrus | |||||
| Inferior frontal gyrus | |||||
| Anterior cingulate cortex | |||||
| Precentral cortex | |||||
| Parietal cortex | |||||
| Posterior cingulate cortex | |||||
| Occipitotemporal cortex | |||||
| Cuneus | |||||
| Cerebellum | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayorova, L.; Kushnir, A.; Sorokina, V.; Pradhan, P.; Radutnaya, M.; Zhdanov, V.; Petrova, M.; Grechko, A. Rapid Effects of BCI-Based Attention Training on Functional Brain Connectivity in Poststroke Patients: A Pilot Resting-State fMRI Study. Neurol. Int. 2023, 15, 549-559. https://doi.org/10.3390/neurolint15020033
Mayorova L, Kushnir A, Sorokina V, Pradhan P, Radutnaya M, Zhdanov V, Petrova M, Grechko A. Rapid Effects of BCI-Based Attention Training on Functional Brain Connectivity in Poststroke Patients: A Pilot Resting-State fMRI Study. Neurology International. 2023; 15(2):549-559. https://doi.org/10.3390/neurolint15020033
Chicago/Turabian StyleMayorova, Larisa, Anastasia Kushnir, Viktoria Sorokina, Pranil Pradhan, Margarita Radutnaya, Vasiliy Zhdanov, Marina Petrova, and Andrey Grechko. 2023. "Rapid Effects of BCI-Based Attention Training on Functional Brain Connectivity in Poststroke Patients: A Pilot Resting-State fMRI Study" Neurology International 15, no. 2: 549-559. https://doi.org/10.3390/neurolint15020033