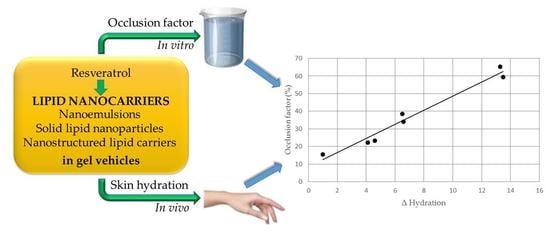
Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipid Nanocarriers Preparation
2.3. Lipid Nanocarriers Characterization
2.3.1. Transmission Electron Microscopy
2.3.2. Size and Zeta Potential Determination
2.3.3. Differential Scanning Calorimetry Analyses
2.3.4. Stability Tests
2.4. Gel Preparation
2.5. Occlusion Factor Determination
2.6. In Vivo Evaluation of Skin Hydration
3. Results and Discussion
3.1. Lipid Nanocarriers Characterization
3.2. Determination of In Vitro Occlusion Factor
3.3. In Vivo Evaluation of Gel Formulations
Author Contributions
Conflicts of Interest
References
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanism of formation and structure of microemulsions by electron microscopy. J. Phys. Chem. 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Radtke, M.; Müller, R.H. Nanostructured lipid carriers: The new generation of lipid drug carriers. New Drugs 2001, 2, 48–52. [Google Scholar]
- Nair, R.; Arun Kumar, K.S.; Vishnu Priya, K.; Sevukarajan, M. Recent advances in solid lipid nanoparticle based drug delivery systems. J. Biomed. Sci. Res. 2011, 3, 368–384. [Google Scholar]
- Montenegro, L. Nanocarriers for skin delivery of cosmetic antioxidants. J. Pharm. Pharmacogn. Res. 2014, 2, 73–92. [Google Scholar]
- Souto, E.B.; Müller, R.H. Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. Handb. Exp. Pharmacol. 2010, 197, 115–141. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Wissing, S.A.; Lippacher, A.; Müller, R.H. Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J. Cosmet. Sci. 2001, 52, 313–324. [Google Scholar] [PubMed]
- Wissing, S.A.; Müller, R.H. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int. J. Pharm. 2002, 242, 377–379. [Google Scholar] [CrossRef]
- Loo, C.H.; Basri, M.; Ismail, R.; Lau, H.L.N.; Tejo, B.A.; Kanthimathi, M.S.; Hassan, H.A.; Choo, Y.M. Effect of compositions in nanostructured lipid carriers (NLC) on skin hydration and occlusion. Int. J. Nanomed. 2013, 8, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, E.; Müller, RH. Cosmetic features and applications of lipid nanoparticles (SLN, NLC). Int. J. Cosmet. Sci. 2008, 30, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Sparr, E.; Millecamps, D.; Isoir, M.; Burnier, V.; Larsson, A.; Cabane, B. Controlling the hydration of the skin though the application of occluding barrier creams. J. R. Soc. Interface 2013, 10, 20120788. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Maibach, H.I. Effects of skin occlusion on percutaneous absorption: An overview. Skin Pharmacol. Appl. Skin Physiol. 2001, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Bouwstra, J.A.; Pirot, F.; Falson, F. Skin hydration—A key determinant in topical absorption. In Dermatologic, Cosmeceutic, and Cosmetic Development: Therapeutic and Novel Approaches, 1st ed.; Walters, K.A., Roberts, M.S., Eds.; Informa Healthcare USA Inc.: New York, NY, USA, 2008; Volume 2, pp. 115–128. ISBN 978-08-4-937589-7. [Google Scholar]
- Yousef, S.; Mohammed, Y.; Namjoshi, S.; Grice, J.; Sakran, W.; Roberts, M. Mechanistic evaluation of hydration effects on the human epidermal permeation of salicylate esters. AAPS J. 2017, 19, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Hamishehkar, H.; Same, S.; Adibkia, K.; Zarza, K.; Shokri, J.; Taghaee, M.; Kouhsoltani, M. A comparative histological study on the skin occlusion performance of a cream made of solid lipid nanoparticles and Vaseline. Res. Pharm. Sci. 2015, 10, 378–387. [Google Scholar] [PubMed]
- Bhat, K.P.L.; Kosmeder, J.W., II; Pezzuto, J.M. Biological effects of resveratrol. Antioxid. Redox Signal. 2001, 3, 1041–1064. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, M.; Philippe, C.; Mukhtara, H.; Ahmada, N. The grape antioxidant resveratrol for skin disorders: Promise, prospects, and challenges. Arch. Biochem. Biophys. 2011, 508, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.A. Anti-aging properties of resveratrol: Review and report of a potent new antioxidant skin care formulation. J. Cosmet. Dermatol. 2008, 7, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Gokce, E.H.; Korkmaz, E.; Dellera, E.; Sandri, G.; Bonferoni, M.C.; Ozer, O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: Evaluation of antioxidant potential for dermal applications. Int. J. Nanomed. 2012, 7, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Carbone, C.; Condorelli, G.; Drago, R.; Puglisi, G. Effect of oil phase lipophilicity on in vitro drug release from o/w microemulsions with low surfactant content. Drug Dev. Ind. Pharm. 2006, 32, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Campisi, A.; Sarpietro, M.G.; Carbone, C.; Acquaviva, R.; Raciti, G.; Puglisi, G. In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. Drug Dev. Ind. Pharm. 2011, 37, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Trapani, A.; Latrofa, A.; Puglisi, G. In vitro evaluation on a model of blood brain barrier of idebenone-loaded solid lipid nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Radomska-Soukharev, A. Stability of lipid excipients in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Sarpietro, M.G.; Accolla, M.L.; Puglisi, G.; Castelli, F.; Montenegro, L. Idebenone loaded solid lipid nanoparticles: Calorimetric studies on surfactant and drug loading effects. Int. J. Pharm. 2014, 471, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Pasquinucci, L.; Zappalà, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary essential oil-loaded lipid nanoparticles: In vivo topical activity from gel vehicles. Pharmaceutics 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Jenning, V.; Schäfer-Korting, M.; Cohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef]
- Fernandes, C.B.; Prabhu, P.S.; Guggulothu, D.; Patravale, V.B. Lipid nanocarriers for topical anti-cancer therapy: An update. In Lipid Nanocarriers in Cancer Diagnosis and Therapy; Souto, E.B., Ed.; iSmithers: Shrewsbury, UK, 2011; pp. 461–492. ISBN 978-1-84735-477-8. [Google Scholar]
- Bahari, L.A.S.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; a comparative literature review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations. Eur. J. Pharm Biopharm. 2004, 5, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L. Lipid-based nanoparticles as carriers for dermal delivery of antioxidants. Curr. Drug Metab. 2017, 18, 469–480. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity-in vivo study. Eur. J. Pharm. Biopharm. 2003, 56, 67–72. [Google Scholar] [CrossRef]






| Code | Ingredients | ||||
|---|---|---|---|---|---|
| Oleth-20 | GO | CP | IPM | RSV | |
| SLN | 9.0 | 5.0 | 7.0 | - | - |
| SLN1 RSV | 9.0 | 5.0 | 7.0 | - | 0.5 |
| SLN2 RSV | 9.0 | 5.0 | 7.0 | - | 0.7 |
| SLN3 RSV | 9.0 | 5.0 | 7.0 | - | 1.0 |
| NLC1 | 9.0 | 5.0 | 6.0 | 1.0 | - |
| NLC2 | 9.0 | 5.0 | 5.0 | 2.0 | - |
| NLC3 | 9.0 | 5.0 | 4.0 | 3.0 | - |
| NLC4 | 9.0 | 5.0 | 3.0 | 4.0 | - |
| NLC5 | 9.0 | 5.0 | 2.0 | 5.0 | - |
| NLC6 | 9.0 | 5.0 | 1.0 | 6.0 | - |
| NLC6 RSV | 9.0 | 5.0 | 1.0 | 6.0 | 1.0 |
| NE | 9.0 | 5.0 | - | 7.0 | - |
| NE RSV | 9.0 | 5.0 | - | 7.0 | 1.0 |
| Code | Z-Ave ± S.D. (nm) | PDI ± S.D. | ζ Potential ± S.D. (mV) |
|---|---|---|---|
| SLN | 35.76 ± 1.23 | 0.221 ± 0.003 | −1.83 ± 0.31 |
| SLN1 RSV | 36.40 ± 1.26 | 0.202 ± 0.002 | −1.99 ± 0.39 |
| SLN2 RSV | 47.14 ± 1.39 | 0.273 ± 0.005 | −2.15 ± 0.41 |
| SLN3 RSV | 45.99 ± 1.30 | 0.231 ± 0.001 | −2.09 ± 0.40 |
| NLC1 | 28.92 ± 0.66 | 0.361 ± 0.003 | −1.88 ± 0.35 |
| NLC2 | 37.43 ± 0.84 | 0.423 ± 0.002 | −2.19 ± 0.41 |
| NLC3 | 42.73 ± 1.20 | 0.443 ± 0.019 | −1.79 ± 0.55 |
| NLC4 | 43.77 ± 2.47 | 0.490 ± 0.034 | −2.03 ± 0.62 |
| NLC5 | 35.80 ± 1.31 | 0.443 ± 0.041 | −2.21 ± 0.46 |
| NLC6 | 27.24 ± 1.20 | 0.218 ± 0.006 | −2.03 ± 0.51 |
| NLC6 RSV | 26.14 ± 1.04 | 0.215 ± 0.003 | −1.98 ± 0.37 |
| NE | 30.16 ± 1.17 | 0.259 ± 0.005 | −1.78 ± 0.44 |
| NE RSV | 27.08 ± 1.36 | 0.207 ± 0.006 | −1.96 ± 0.31 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics 2017, 9, 58. https://doi.org/10.3390/pharmaceutics9040058
Montenegro L, Parenti C, Turnaturi R, Pasquinucci L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics. 2017; 9(4):58. https://doi.org/10.3390/pharmaceutics9040058
Chicago/Turabian StyleMontenegro, Lucia, Carmela Parenti, Rita Turnaturi, and Lorella Pasquinucci. 2017. "Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect" Pharmaceutics 9, no. 4: 58. https://doi.org/10.3390/pharmaceutics9040058