Chitosan-Based Nano-Embedded Microparticles: Impact of Nanogel Composition on Physicochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
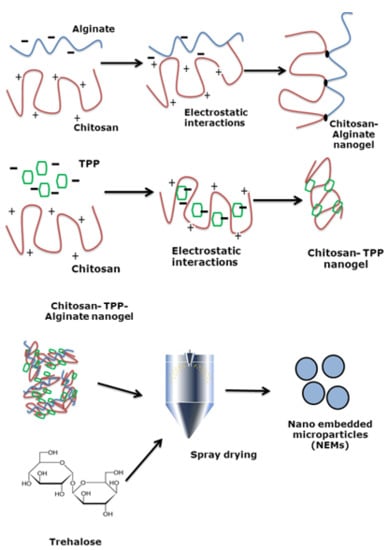
2.2. Nanogel Preparation
2.3. Nanogels Size and Zeta Potential Measurements
2.4. NEMs Preparation by Spray-Drying
2.5. Aerodynamic Diameter of NEMs
2.6. Scanning Electron Microscopy
2.7. Transmission Electron Microscopy
2.8. X-ray Powder Diffraction (XRPD)
2.9. Thermogravimetric Analysis
2.10. Statistics
3. Results
3.1. Nanogel Size Varied Depending on Composition and Concentration
3.2. Influence of Inlet Temperature on the Redispersibility of NEMs
3.3. Nanogel Composition and Matrix Excipient Impacted Redispersibility
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DLS | dynamic light scattering |
| MMAD | mass median aerodynamic diameter |
| NEMs | nano-embedded microparticles |
| PDI | polydispersity |
| SEM | scanning electron microscopy |
| TGA | thermogravimetric analysis |
| TPP | sodium tri-penta phosphate |
| wt % | weight percent |
References
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Billone, P.S.; Mullett, W.M. Nanomedicine in action: An overview of cancer nanomedicine on the market and in clinical trials. J. Nanomater. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 302–315. [Google Scholar] [CrossRef]
- Ventola, C.L. The nanomedicine revolution: Part 1: Emerging concepts. P T. 2012, 37, 512–525. [Google Scholar] [PubMed]
- Timko, B.P.; Whitehead, K.; Gao, W.; Kohane, D.S.; Farokhzad, O.; Anderson, D. Advances in drug delivery. Annu. Rev. Mater. Res. 2011, 41, 1–20. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Cho, Y.W.; Park, K. Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery. Adv. Drug Deliv. Rev. 2013, 65, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Neubert, R.H.H. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur. J. Pharm. Biopharm. 2011, 77, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Azarmi, S.; Roa, W.H.; Löbenberg, R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv. Drug Deliv. Rev. 2008, 60, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.; Ruge, C.A.; Beck-Broichsitter, M. Preparation of nanoscale pulmonary drug delivery formulations by spray drying. Adv. Exp. Med. Biol. 2014, 811, 183–206. [Google Scholar] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based nanoparticles: An overview of biomedical applications. J. Controll. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.D.; Müller, R.H. Lipid nanoparticles for parenteral delivery of actives. Eur. J. Pharm. Biopharm. 2009, 71, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, S.C.; Demeester, J.; Hennink, W.E. Cationic polymer based gene delivery systems. Pharm. Res. 2000, 17, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Park, T.G.; Kim, S.H. Self-assembled and nanostructured siRNA delivery systems. Pharm. Res. 2011, 28, 2072–2085. [Google Scholar] [CrossRef] [PubMed]
- Water, J.J.; Kim, Y.; Maltesen, M.J.; Franzyk, H.; Foged, C.; Nielsen, H.M. Hyaluronic acid-based nanogels produced by microfluidics-facilitated self-assembly improves the safety profile of the cationic host defense peptide novicidin. Pharm. Res. 2015, 32, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Schutz, C.A.; Juillerat-Jeanneret, L.; Kauper, P.; Wandrey, C. Cell response to the exposure to chitosan-TPP//alginate nanogels. Biomacromolecules 2011, 12, 4153–4161. [Google Scholar] [CrossRef] [PubMed]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesaro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Sanhai, W.R.; Sakamoto, J.H.; Canady, R.; Ferrari, M. Seven challenges for nanomedicine. Nat. Nanotechnol. 2008, 3, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.A.; Anyarambhatla, G.; MA, L.; Ugwu, S.; Xuan, T.; Sardone, T.; Ahmad, I. Development and characterization of a novel Cremophor® EL free liposome-based paclitaxel (LEP-ETU) formulation. Eur. J. Pharm. Biopharm. 2005, 59, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Dua, J.S.; Rana, A.C.; Bhandari, A.K. Liposome: Methods of preparation and applications. Int. J. Pharm. Stud. Res. 2012, 3, 14–20. [Google Scholar]
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49. [Google Scholar]
- Bohr, A.; Water, J.; Beck-Broichsitter, M.; Yang, M. Nanoembedded microparticles for stabilization and delivery of drug-loaded nanoparticles. Curr. Pharm. Des. 2015, 21, 5829–5844. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Peng, Z.; She, F.H.; Kong, L.X. Microencapsulation of nanoparticles with enhanced drug loading for pH-sensitive oral drug delivery for the treatment of colon cancer. J. Appl. Polym. Sci. 2013, 129, 714–720. [Google Scholar] [CrossRef]
- Al-Qadi, S.; Grenha, A.; Carrión-Recio, D.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J. Control Release 2012, 157, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; El-Sherbiny, I.; Smyth, H. Swellable ciprofloxacin-loaded nano-in-micro hydrogel particles for local lung drug delivery. AAPS PharmSciTech 2014, 15, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.P.; Ferreira Lopes, C.D.; Duarte Moreno, P.M.; Varela-Moreira, A.; Alonso, M.J.; Pêgo, A.P. Translating chitosan to clinical delivery of nucleic acid-based drugs. MRS Bull. 2014, 39, 60–70. [Google Scholar] [CrossRef]
- Saneja, A.; Nehate, C.; Alam, N.; Gupta, P. Recent advances in chitosan-based nanomedicines for cancer chemotherapy. In Chitin and Chitosan for Regenerative Medicine SE-9; Dutta, P.K., Ed.; Springer: New Delhi, India, 2016. [Google Scholar]
- López-León, T.; Carvalho, E.L.S.; Seijo, B.; Ortega-Vinuesa, J.L.; Bastos-González, D. Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. J. Colloid Interface Sci. 2005, 283, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Hu, J.; Park, H.; Lee, M. Chitosan-Based nanoparticles as a sustained protein release carrier for tissue engineering applications. J. Biomed. Mater. Res. Part A 2012, 100A, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Remuñán-López, C.; Carvalho, E.L.; Seijo, B. Microspheres containing lipid/chitosan nanoparticles complexes for pulmonary delivery of therapeutic proteins. Eur. J. Pharm. Biopharm. 2008, 69, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadi, S.; Grenha, A.; Remuñán-López, C. Microspheres loaded with polysaccharide nanoparticles for pulmonary delivery: Preparation, structure and surface analysis. Carbohydr. Polym. 2011, 86, 25–34. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Serra, C.; Remuñán-López, C. Chitosan nanoparticle-loaded mannitol microspheres: Structure and surface characterization. Biomacromolecules 2007, 8, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, L.; Cai, S.; Liu, Z.; Zhu, Y.; Xue, W.; Zhang, Y. Novel alginate coated hydrophobically modified chitosan polyelectrolyte complex for the delivery of BSA. J. Mater. Sci. Mater. Med. 2013, 24, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, X.; Shi, S.; Zheng, X.; Guo, G.; Wei, Y. Preparation of alginate coated chitosan microparticles for vaccine delivery. BMC Biotechnol. 2008, 8. [Google Scholar] [CrossRef] [PubMed]
- Lapitsky, Y. Ionically crosslinked polyelectrolyte nanocarriers: Recent advances and open problems. Curr. Opin. Colloid Interface Sci. 2014, 19, 122–130. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hu, J.; Pan, X.; Yao, P.; Jiang, M. Stable and pH-sensitive nanogels prepared by self-assembly of chitosan and ovalbumin. Langmuir 2006, 22, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Brunel, F.; Véron, L.; Ladavière, C.; David, L.; Domard, A.; Delair, T. Synthesis and structural characterization of chitosan nanogels. Langmuir 2009, 25, 8935–8943. [Google Scholar] [CrossRef] [PubMed]
- Lebhardt, T.; Roesler, S.; Uusitalo, H.P.; Kissel, T. Surfactant-free redispersible nanoparticles in fast-dissolving composite microcarriers for dry-powder inhalation. Eur. J. Pharm. Biopharm. 2011, 78, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.; Boetker, J.; Rades, T.; Rantanen, J.; Yang, M.; Bøtker, J. Application of spray-drying and electrospraying/electospinning for poorly watersoluble drugs: A particle engineering approach. Curr. Pharm. Des. 2014, 20, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.; Sheskey, P.; Cook, W.; Fenton, M. Handbook of Pharmaceutical Excipients; Pharmaceutical Press: London, UK, 2012. [Google Scholar]





| Sample Abbreviation | Chitosan Concentration (mg/mL) | Chitosan:(Alginate + TPP) Ratio (w/w) | TPP Concentration (mg/mL) | Alginate Concentration (mg/mL) |
|---|---|---|---|---|
| CT1 | 1 | 3:1 | 1.5 | - |
| CT2 | 0.5 | 3:1 | 0.75 | - |
| CTA | 0.5 | 3:1 | 0.60 | 0.15 |
| CA | 0.5 | 3:1 | - | 0.75 |
| Inlet Temperature (°C) | Moisture Content (%) | Particle Size (nm) | MMAD (µm) | |
|---|---|---|---|---|
| Before Spray-Drying | After Re-Dispersion | |||
| 100 | 11.1 | 233 ± 0.04 | 372 ± 0.03 | 7.92 ± 0.06 |
| 120 | 10.5 | 233 ± 0.04 | 236 ± 0.09 | 7.38 ± 0.10 |
| 150 | 9.7 | 233 ± 0.04 | 328 ± 0.02 | 7.61 ± 0.08 |
| 190 | 10.5 | 233 ± 0.04 | 315 ± 0.09 | 8.06 ± 0.06 |
| Samples | Moisture Content (%) | Particle Size (nm) | MMAD (µm) | |
|---|---|---|---|---|
| Before Spray-Drying | After Re-Dispersion | |||
| CT1 | 4.3 | 230 ± 0.07 | >2000 | 8.23 ± 0.08 |
| CT2 | 3.5 | 230 ± 0.05 | >2000 | 7.54 ± 0.07 |
| CTA | 3.4 | 240 ± 0.07 | >2000 | 6.68 ± 0.05 |
| CA | 3.3 | 540 ± 0.09 | >2000 | 7.00 ± 0.0.4 |
| Samples | Moisture Content (%) | Particle Size (nm) | MMAD (µm) | |
|---|---|---|---|---|
| Before Spray-Drying | After Re-Dispersion | |||
| CT1 | 6.0 | 230 ± 0.07 | 402 ± 0.05 | 7.50 ± 0.04 |
| CT2 | 8.3 | 230 ± 0.05 | 406 ± 0.04 | 10.30 ± 0.06 |
| CTA | 7.2 | 240 ± 0.07 | 280 ± 0.07 | 6.75 ± 0.05 |
| CA | 11.7 | 540 ± 0.09 | 790 ± 0.10 | 8.90 ± 0.06 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, P.; Water, J.J.; Bohr, A.; Rantanen, J. Chitosan-Based Nano-Embedded Microparticles: Impact of Nanogel Composition on Physicochemical Properties. Pharmaceutics 2017, 9, 1. https://doi.org/10.3390/pharmaceutics9010001
Islam P, Water JJ, Bohr A, Rantanen J. Chitosan-Based Nano-Embedded Microparticles: Impact of Nanogel Composition on Physicochemical Properties. Pharmaceutics. 2017; 9(1):1. https://doi.org/10.3390/pharmaceutics9010001
Chicago/Turabian StyleIslam, Paromita, Jorrit J. Water, Adam Bohr, and Jukka Rantanen. 2017. "Chitosan-Based Nano-Embedded Microparticles: Impact of Nanogel Composition on Physicochemical Properties" Pharmaceutics 9, no. 1: 1. https://doi.org/10.3390/pharmaceutics9010001