Contribution of the Hair Follicular Pathway to Total Skin Permeation of Topically Applied and Exposed Chemicals
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Determination of n-Octanol/Buffer Coefficient
2.3. Animals
2.4. Preparation of Skin Membrane
2.5. Hair Follicle-Plugging Process
2.6. Preparation of Applied Solution
2.7. In Vitro Skin Permeation Experiments
2.8. Determination of FD-4 and FL
2.9. Determination of Drugs
2.10. Analysis of Permeation Parameters
2.11. Statistical Analysis
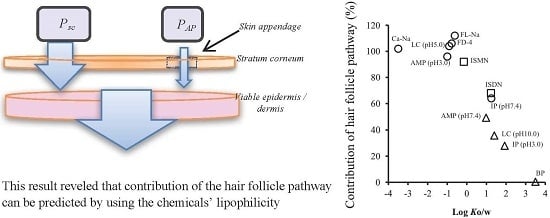
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Naik, A.; Kalia, Y.; Guy, R. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Pappas, A. Epidermal surface lipids. Dermato-endocrinology 2009, 1, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Mitsutake, S.; Tsuji, K.; Kihara, A.; Igarashi, Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie 2009, 91, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, NY, USA, 1979. [Google Scholar]
- Scheuplein, R.J. Mechanism of percutaneous absorption. II. Transient diffusion and the relative importance of various routes of skin penetration. J. Investig. Dermatol. 1967, 48, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. J. Control. Release 2003, 86, 69–92. [Google Scholar] [CrossRef]
- Wosicka, H.; Cal, K. Targeting to the hair follicles: Current status and potential. J. Dermatol. Sci. 2010, 57, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Todo, H.; Kimura, E.; Yasuno, H.; Tokudome, Y.; Hashimoto, F.; Ikarashi, Y.; Sugibayashi, K. Permeation pathway of macromolecules and nanospheres through skin. Biol. Pharm. Bull. 2010, 33, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.J.; Maibach, H.I. Regional variation in percutaneous penetration of 14C cortisol in man. J. Investig. Dermatol. 1967, 48, 181–183. [Google Scholar]
- Maibach, H.I.; Feldman, R.J.; Milby, T.H.; Serat, W.F. Regional variation in percutaneous penetration in man. Arch. Environ. Health 1971, 23, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Hueber, F.; Wepierre, J.; Schaefer, H. Role of transepidermal and transfollicular routes in percutaneous absorption of hydrocortisone and testosterone: In vivo study in the hairless rat. Skin Pharmacol. Physiol. 1992, 5, 99–107. [Google Scholar] [CrossRef]
- Grice, J.E.; Ciotti, S.; Weiner, N.; Lockwood, P.; Cross, S.E.; Roberts, M.S. Relative uptake of minoxidil into appendages and stratum corneum and permeation through human skin in vitro. J. Pharm. Sci. 2010, 299, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Tampucci, S.; Burgalassi, S.; Chetoni, P.; Lenzi, C.; Pirone, A.; Mailland, F.; Caserini, M.; Monti, D. Topical formulations containing finasteride. Part II: Determination of finasteride penetration into hair follicles using the differential stripping technique. J. Pharm. Sci. 2014, 103, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, T.; Shiraki, T.; Okajima, K.; Tanino, T.; Iwaki, M.; Wada, T. Transfollicular drug delivery: Penetration of drugs through human scalp skin and comparison of penetration between scalp and abdominal skins in vitro. J. Drug Target. 2002, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Lieb, L.M.; Liimatta, A.P.; Bryan, R.N.; Brown, B.D.; Krueger, G.G. Description of the intrafollicular delivery of large molecular weight molecules to follicles of human scalp skin in vitro. J. Pharm. Sci. 1997, 86, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Grice, J.E.; Lademann, J.; Otberg, N.; Trauer, S.; Patzelt, A.; Roberts, M.S. Hair follicles contribute significantly to penetration through human skin only at times soon after application as a solvent deposited solid in man. Br. J. Clin. Pharmacol. 2011, 72, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Essa, E.A.; Bonner, M.C.; Barry, B.W. Human skin sandwich for assessing shunt route penetration during passive and iontophoretic drug and liposome delivery. J. Pharm. Pharmacol. 2002, 54, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Frum, Y.; Bonner, M.C.; Eccleston, G.M.; Meidan, V.M. The influence of drug partition coefficient on follicular penetration: In vitro human skin studies. Eur. J. Pharm. Sci. 2007, 30, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Tampucci, S.; Burgalassi, S.; Chetoni, P.; Lenzi, C.; Pirone, A.; Mailland, F. Topical formulations containing finasteride. Part I: In vitro permeation/penetration study and in vivo pharmacokinetics in hairless rat. J. Pharm. Sci. 2014, 103, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Horita, D.; Yoshimoto, M.; Todo, H.; Sugibayashi, K. Analysis of hair follicle penetration of lidocaine and fluoresce in isothiocyanate-dextran 4 kDa using hair follicle-plugging method. Drug Dev. Ind. Pharm. 2014, 40, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Otberg, N.; Patzelt, A.; Rasulev, U.; Hagemeister, T.; Linscheid, M.; Sinkgraven, R.; Sterry, W.; Lademann, J. The role of hair follicles in the percutaneous absorption of caffeine. Br. J. Clin. Pharmacol. 2008, 65, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Trauer, S.; Patzelt, A.; Otberg, N.; Knorr, F.; Rozycki, C.; Balizs, G.; Büttemeyer, R.; Linscheid, M.; Liebsch, M.; Lademann, J. Permeation of topically applied caffeine through human skin—A comparison of in vivo and in vitro data. Br. J. Clin. Pharmacol. 2009, 68, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Takeuchi, H.; Ishida, M.; Todo, H.; Urano, H.; Sugibayashi, K. Influence of skin thickness on the in vitro permeabilities of drugs through Sprague-Dawley rat or Yucatan micropig skin. Biol. Pharm. Bull. 2011, 35, 192–202. [Google Scholar] [CrossRef]
- Yamada, K.; Yamashita, J.; Todo, H.; Miyamoto, K.; Hashimoto, S.; Tokudome, Y.; Hashimoto, F.; Sugibayashi, K. Preparation and evaluation of liquid-crystal formulations with skin-permeation-enhancing abilities for entrapped drugs. J. Oleo. Sci. 2011, 60, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sugibayashi, K.; Hayashi, T.; Matsumoto, K.; Hasegawa, T. Utility of a three-dimensional cultured human skin model as a tool to evaluate the simultaneous diffusion and metabolism of ethyl nicotinate in skin. Drug Metab. Pharmacokinet. 2004, 19, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.; Coleman, J.; Evans, D.; Hawes, C. Internalisation of fluorescein isothiocyanate and fluorescein isothiocyanate-dextran by suspension-cultured plant cells. J. Cell Sci. 1990, 96, 721–730. [Google Scholar]
- Heger, M.; Salles, I.I.; Van Vuure, W.; Deckmyn, H.; Beek, J.F. Fluorescent labeling of platelets with polyanionic fluorescein derivatives. Anal. Quant. Cytol. Histol. 2009, 31, 227–232. [Google Scholar] [PubMed]
- Cázares-Delgadillo, J.; Naik, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Skin permeation enhancement by sucrose esters: a pH-dependent phenomenon. Int. J. Pharm. 2005, 297, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kolthoff, I.M.; Stenger, V.A. Volumetric Analysis; Interscience Publishers: New York, NY, USA, 1942. [Google Scholar]
- Domańska, U.; Pobudkowska, A.; Pelczarska, A.; Gierycz, P. pKa and solubility of drugs in water, ethanol, and 1-octanol. J. Phys. Chem. B. 2009, 113, 8941–8947. [Google Scholar] [CrossRef] [PubMed]
- Błędzkaa, D.; Gryglikb, D.; Millera, S.J. Photodegradation of butylparaben in aqueous solutions by 254 nm irradiation. J. Photochem. Photobiol. A 2009, 203, 131–136. [Google Scholar] [CrossRef]
- Ahmed, S.; Imai, T.; Otagiri, M. Evaluation of stereoselective transdermal transport and concurrent cutaneous hydrolysis of several ester prodrugs of propranolol: Mechanism of stereoselective permeation. Pharm. Res. 1996, 13, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Hashida, M.; Okamoto, H.; Sezaki, H. Analysis of drug penetration through skin considering donor concentration decrease. J. Pharmacobiodyn. 1988, 11, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Hashida, M.; Sezaki, H. Structure-activity relationship of 1-alkyl- or 1-alkenylazacycloalkanone derivatives as percutaneous penetration enhancers. J. Pharm. Sci. 1988, 77, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Oda, T.; Sugibayashi, K.; Morimoto, Y. Estimation of blood concentration of drugs after topical application from in vitro skin permeation data. I. Prediction by convolution and confirmation by deconvolution. Chem. Pharm. Bull. 1988, 36, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.R.; Stone, K.J.; Boissy, Y.L. Hydration disrupts human stratum corneum ultrastructure. J. Investig. Dermatol. 2003, 120, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Patzelt, A.; Richter, H.; Buettemeyer, R.; Huber, H.J.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. Differential stripping demonstrates a significant reduction of the hair follicle reservoir in vitro compared to in vivo. Eur. J. Pharm. Biopharm. 2008, 70, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Knorr, F.; Patzelt, A.; Richter, H.; Schanzer, S.; Sterry, W.; Lademann, J. Approach towards developing a novel procedure to selectively quantify topically applied substances in the hair follicles of the model tissue porcine ear skin. Exp. Dermatol. 2013, 22, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Raber, A.S.; Mittal, A.; Schäfer, J.; Bakowsky, U.; Reichrath, J.; Vogt, T.; Schaefer, U.F.; Hansen, S.; Lehr, C.M. Quantification of nanoparticle uptake into hair follicles in pig ear and human forearm. J. Control. Release 2014, 179, 25–32. [Google Scholar] [CrossRef] [PubMed]





| Model Drug | Molecular Weight (MW) | Log Ko/w (pH) | pKa |
|---|---|---|---|
| FD-4 | ca. 4000 | −0.77 (7.4) (e) | 6.7 (h) |
| Ca-Na | 644.5 | −3.50 (7.4) (f) | 5.5 (i) |
| FL-Na | 376.3 | −0.61 (7.4) (e) | 6.4 (e) |
| ISDN | 236.1 | 1.23 (7.4) (g) | - |
| LC | 234.3 | −0.90 (5.0) (b) 1.40 (10.0) (d) | 7.9 (j) |
| AMP | 231.3 | −1.00 (3.0) (a) 0.98 (7.4) (c) | 5.0 (k) |
| IP | 206.3 | 1.93 (3.0) (a) 1.25 (7.4) (c) | 4.9 (l) |
| BP | 194.2 | 3.50 (7.4) (c) | 8.3 (m) |
| ISMN | 191.1 | −0.15 (7.4) (g) | - |
| Skin Permeation Parameter | pH 3.0 | pH 7.4 | pH 10.0 |
|---|---|---|---|
| Q6 (μmol/cm2) # | 1.30 × 10−1 ± 1.64 × 10−2 | 1.19 × 10−1 ± 2.18 × 10−2 | 1.22 × 10−1 ± 1.11 × 10−2 |
| P (cm/s) | 2.46 × 10−6 ± 4.67 × 10−7 | 2.01 × 10−6 ± 2.93 × 10−7 | 1.86 × 10−6 ± 2.34 × 10−7 |
| tlag (h) | 1.91 ± 0.23 | 2.22 ± 0.16 | 2.02 ± 0.21 |
| DL−2 (cm−1) | 1.02 × 10−1 ± 1.25 × 10−2 | 7.56 × 10−2 ± 5.36 × 10−3 | 8.42 × 10−2 ± 8.27 × 10−3 |
| KL (cm) | 2.35 × 10−5 ± 2.74 × 10−6 | 2.62 × 10−5 ± 2.24 × 10−6 | 2.30 × 10−5 ± 5.03 × 10−6 |
| Skin Permeation Parameter at Different pH | Non-PA Plugging | PA Plugging | Ratio # | |
|---|---|---|---|---|
| pH 3.0 | Q10 (μmol/cm2) | 2.40 × 10−2 ± 7.55 × 10−3 | 1.17 × 10−2 ± 4.41 × 10−3 | 0.49 |
| P (cm/s) | 8.70 × 10−9 ± 2.38 × 10−9 | 4.73 × 10−9 ± 2.02 × 10−9 | 0.54 | |
| tlag (h) | 2.53 ± 0.50 | 2.56 ± 0.40 | 1.01 | |
| DL−2 (h−1) | 1.17 × 10−4 ± 2.32 × 10−5 | 1.19 × 10−4 ± 1.86 × 10−5 | 1.02 | |
| KL (cm) | 7.58 × 10−5 ± 2.00 × 10−5 | 3.99 × 10−5 ± 1.70 × 10−6 | 0.52 | |
| pH 7.4 | Q10 (μmol/cm2) | 3.78 × 10−2 ± 1.41 × 10−3 | 2.73 × 10−2 ± 2.11 × 10−3 | 0.72 |
| P (cm/s) | 1.61 × 10−7 ± 8.10 × 10−9 | 1.43 × 10−7 ± 1.61 × 10−8 | 0.89 | |
| tlag (h) | 3.85 ± 0.14 | 4.18 ± 0.24 | 1.09 | |
| DL−2 (h−1) | 1.78 × 10−4 ± 6.34 × 10−6 | 1.94 × 10−4 ± 1.11 × 10−5 | 1.09 | |
| KL (cm) | 9.06 × 10−4 ± 5.05 × 10−5 | 7.51 × 10−4 ± 1.05 × 10−4 | 0.83 | |
| Chemical | p Values through HF-non-Plugged Skin (×10−8 cm/s) | p Values through HF-Plugged Skin (×10−8 cm/s) | Reduction Ratio (%) |
|---|---|---|---|
| FD-4 | 0.064 ± 0.032 | 0.026 ± 0.012 | 59 |
| Ca-Na | 2.8 ± 1.2 | 1.4 ± 1.7 | 50 |
| FL-Na | 1.4 ± 0.57 | 0.62 ± 0.089 | 56 |
| ISDN | 371 ± 122 | 242 ± 65 | 35 |
| Ionized LC | 2.3 ± 0.61 | 1.1 ± 0.31 | 52 |
| Non-ionized LC | 255 ± 109 | 210 ± 49 | 18 |
| Ionized AMP | 0.88 ± 0.48 | 0.46 ± 0.42 | 48 |
| Non-ionized AMP | 17 ± 0.74 | 13 ± 0.50 | 24 |
| Non-ionized IP | 1520 ± 270 | 1300 ± 81 | 14 |
| Ionized IP | 87 ± 16 | 59 ± 5.4 | 32 |
| Non-ionized BP | 132 ± 50 | 132 ± 143 | 0 |
| ISMN | 8.9 ± 1.2 | 4.9 ± 3.6 | 45 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd, F.; Todo, H.; Yoshimoto, M.; Yusuf, E.; Sugibayashi, K. Contribution of the Hair Follicular Pathway to Total Skin Permeation of Topically Applied and Exposed Chemicals. Pharmaceutics 2016, 8, 32. https://doi.org/10.3390/pharmaceutics8040032
Mohd F, Todo H, Yoshimoto M, Yusuf E, Sugibayashi K. Contribution of the Hair Follicular Pathway to Total Skin Permeation of Topically Applied and Exposed Chemicals. Pharmaceutics. 2016; 8(4):32. https://doi.org/10.3390/pharmaceutics8040032
Chicago/Turabian StyleMohd, Fadli, Hiroaki Todo, Masato Yoshimoto, Eddy Yusuf, and Kenji Sugibayashi. 2016. "Contribution of the Hair Follicular Pathway to Total Skin Permeation of Topically Applied and Exposed Chemicals" Pharmaceutics 8, no. 4: 32. https://doi.org/10.3390/pharmaceutics8040032