Timing of Administration: For Commonly-Prescribed Medicines in Australia
Abstract
:1. Introduction
Aim
2. Experimental Section
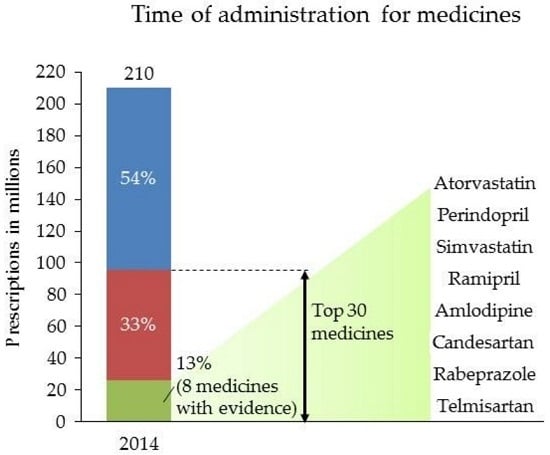
2.1. Operationalising Commonly-Prescribed Medicines
2.2. Literature Search and Analysis
2.3. Analysis of Consumer Medicine Information and Australian Approved Product Information
3. Results and Discussion
3.1. Statins
3.2. Antihypertensive Medications
3.3. Proton Pump Inhibitors
3.4. Anticholinergics
3.5. Discussion
3.6. Limitations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaur, G.; Gan, Y.-L.; Phillips, C.; Wong, K.; Saini, B. Chronotherapy in practice: The perspective of the community pharmacist. Int. J. Clin. Pharm. 2016, 38, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B. The clinical relevance of chronopharmacology in therapeutics. Pharmacol. Res. 1996, 33, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B. Chronopharmacology and controlled drug release. Expert Opin. Drug Deliv. 2005, 2, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B. The importance of biological rhythms in drug treatment of hypertension and sex-dependent modifications. Chronophysiol. Ther. 2012, 2, 9–18. [Google Scholar] [CrossRef]
- Schulz, P.; Steimer, T. Neurobiology of circadian systems. CNS Drugs 2009, 23 (Suppl. 2), 3–13. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; la Fleur, S.E.; Wortel, J.; Van Heyningen, C.; Zuiddam, L.; Mettenleiter, T.C.; Kalsbeek, A.; Nagai, K.; Niijima, A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003, 464, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Willich, S.N.; Linderer, T.; Wegscheider, K.; Leizorovicz, A.; Alamercery, I.; Schröder, R. Increased morning incidence of myocardial infarction in the ISAM study: Absence with prior beta-adrenergic blockade. ISAM study group. Circulation 1989, 80, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Lemmer, B.; Reinberg, A.E. Chronobiology and chronotherapy of allergic rhinitis and bronchial asthma. Adv. Drug Deliv. Rev. 2007, 59, 852–882. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Reinberg, A.; Labrecque, G. Twenty-four hour pattern in symptom intensity of viral and allergic rhinitis: Treatment implications. J. Allergy Clin. Immunol. 1995, 95, 1084–1096. [Google Scholar] [CrossRef]
- Mormont, M.C.; Lévi, F. Circadian-system alterations during cancer processes: A review. Int. J. Cancer 1997, 70, 241–247. [Google Scholar] [CrossRef]
- Quyyumi, A.A. Circadian rhythms in cardiovascular disease. Am. Heart J. 1990, 120, 726–733. [Google Scholar] [CrossRef]
- Bellamy, N.; Sothern, R.B.; Campbell, J.; Buchanan, W.W. Rhythmic variations in pain, stiffness, and manual dexterity in hand osteoarthritis. Ann. Rheum. Dis. 2002, 61, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Hart, F.D.; Taylor, R.T.; Huskisson, E.C. Pain at night. Lancet 1970, 1, 881–884. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Portaluppi, F.; Manfredini, R.; Hermida, R.C.; Tiseo, R.; Sackett-Lundeen, L.L.; Haus, E.L. Diurnal and twenty-four hour patterning of human diseases: Cardiac, vascular, and respiratory diseases, conditions, and syndromes. Sleep Med. Rev. 2015, 21, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Portaluppi, F.; Manfredini, R.; Hermida, R.C.; Tiseo, R.; Sackett-Lundeen, L.L.; Haus, E.L. Diurnal and twenty-four hour patterning of human diseases: Acute and chronic common and uncommon medical conditions. Sleep Med. Rev. 2015, 21, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Ohdo, S. Chronopharmacology focused on biological clock. Drug Metab. Pharmacokinet. 2007, 22, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Peppas, N.A. Chronobiology, drug delivery, and chronotherapeutics. Adv. Drug Deliv. Rev. 2007, 59, 828–851. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Siegel, R.A.; Haus, E.; Hermida, R.; Portaluppi, F. Biological rhythms, drug delivery, and chronotherapeutics. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R.A., Rathbone, M.J., Eds.; Springer: New York, NY, USA, 2012; pp. 359–443. [Google Scholar]
- Ohdo, S. Changes in toxicity and effectiveness with timing of drug administration: Implications for drug safety. Drug Saf. 2003, 26, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Erkekoglu, P.; Baydar, T. Chronopharmacodynamics of drugs in toxicological aspects: A short review for clinical pharmacists and pharmacy practitioners. J. Res. Pharm. Pract. 2012, 1, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Erkekoglu, P.; Baydar, T. Chronopharmacokinetics of drugs in toxicological aspects: A short review for pharmacy practitioners. J. Res. Pharm. Pract. 2012, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bruguerolle, B.; Boulamery, A.; Simon, N. Biological rhythms: A neglected factor of variability in pharmacokinetic studies. J. Pharm. Sci. 2008, 97, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B.; Bruguerolle, B. Chronopharmacokinetics—Are they clinically relevant? Clin. Pharmacokinet. 1994, 26, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Bruguerolle, B. Chronopharmacokinetics. Current status. Clin. Pharmacokinet. 1998, 35, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Paschos, G.K.; Baggs, J.E.; Hogenesch, J.B.; FitzGerald, G.A. The role of clock genes in pharmacology. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B.; Nold, G. Circadian changes in estimated hepatic blood flow in healthy subjects. Br. J. Clin. Pharmacol. 1991, 32, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Reinberg, A.; Smolensky, M.; Labrecque, G. Annual Review of Chronopharmacology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 2. [Google Scholar]
- Ohdo, S. Chronotherapeutic strategy: Rhythm monitoring, manipulation and disruption. Adv. Drug Deliv. Rev. 2010, 62, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Ohdo, S.; Wang, D.S.; Koyanagi, S.; Takane, H.; Inoue, K.; Aramaki, H.; Yukawa, E.; Higuchi, S. Basis for dosing time-dependent changes in the antiviral activity of interferon-alpha in mice. J. Pharmacol. Exp. Ther. 2000, 294, 488–493. [Google Scholar] [PubMed]
- Nainwal, N. Chronotherapeutics—A chronopharmaceutical approach to drug delivery in the treatment of asthma. J. Control. Release 2012, 163, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B. Chronobiology and chronopharmacology of hypertension. In Blood Pressure Monitoring in Cardiovascular Medicine and Therapeutics; Springer: New York, NY, USA, 2007; pp. 413–435. [Google Scholar]
- Hermida, R.C.; Ayala, D.E.; Fernández, J.R.; Portaluppi, F.; Fabbian, F.; Smolensky, M.H. Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am. J. Hypertens. 2011, 24, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Hermida, R.C.; Ayala, D.E.; Tiseo, R.; Portaluppi, F. Administration-time-dependent effects of blood pressure-lowering medications: Basis for the chronotherapy of hypertension. Blood Press. Monit. 2010, 15, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Hermida, R.C.; Ayala, D.E.; Portaluppi, F. Bedtime hypertension chronotherapy: Concepts and patient outcomes. Curr. Pharm. Des. 2015, 21, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Focan, C.; Karaboué, A.; de la Valette, V.; Focan-Henrard, D.; Baron, B.; Kreutz, F.; Giacchetti, S. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Bruguerolle, B.; Labrecque, G. Rhythmic pattern in pain and their chronotherapy. Adv. Drug Deliv. Rev. 2007, 59, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.A.; Evans, C.V.; Burda, B.U.; Margolis, K.L.; O’Connor, E.; Whitlock, E.P. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: A systematic review for the US preventive services task force. Ann. Intern. Med. 2015, 162, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Head, G.A.; McGrath, B.P.; Mihailidou, A.S.; Nelson, M.R.; Schlaich, M.P.; Stowasser, M.; Mangoni, A.A.; Cowley, D.; Brown, M.A.; Ruta, L.-A. Ambulatory blood pressure monitoring in Australia: 2011 consensus position statement. J. Hypertens. 2012, 30, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H. Compliance to prescription medications entails respect for treatment timing. Chronobiol. Int. 2002, 19, 502–505. [Google Scholar] [PubMed]
- Medicare. Pharmaceutical Benefits Scheme. Available online: http://www.medicareaustralia.gov.au/provider/pbs/ (accessed on 1 March 2014).
- Expenditure and Prescriptions Twelve Months to 30 June 2014. Available online: http://www.pbs.gov.au/info/statistics/expenditure-and-prescriptions-30-06-2014 (accessed on 1 December 2015).
- WHO. Model Lists of Essential Medicines. Available online: http://www.who.int/medicines/publications/essentialmedicines/en/index.html (accessed on 8 February 2016).
- Bulsara, C.E.; McKenzie, A. The quality of medication information in Australia: The need for more clinical expertise and accountability. Med. J. Aust. 2009, 191, 189. [Google Scholar] [PubMed]
- Koo, M.M.; Krass, I.; Aslani, P. Factors influencing consumer use of written drug information. Ann. Pharmacother. 2003, 37, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.D. Should clinical software be regulated? Med. J. Aust. 2007, 186, 607–608. [Google Scholar] [PubMed]
- Raymond, C.; Cho, L.; Rocco, M.; Hazen, S.L. New guidelines for reduction of blood cholesterol: Was it worth the wait? Clevel. Clin. J. Med. 2014, 81, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Boggia, J.; Li, Y.; Thijs, L.; Hansen, T.W.; Kikuya, M.; Björklund-Bodegård, K.; Richart, T.; Ohkubo, T.; Kuznetsova, T.; Torp-Pedersen, C.; et al. Prognostic accuracy of day versus night ambulatory blood pressure: A cohort study. Lancet 2007, 370, 1219–1229. [Google Scholar] [CrossRef]
- Ozaydin, M.; Dede, O.; Dogan, A.; Aslan, S.M.; Altinbas, A.; Ozturk, M.; Varol, E.; Turker, Y. Effects of morning versus evening intake of atorvastatin on major cardiac event and restenosis rates in patients undergoing first elective percutaneous coronary intervention. Am. J. Cardiol. 2006, 97, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Yoshida, S.; Nakaya, N.; Hata, Y.; Goto, Y. Comparison between morning and evening doses of simvastatin in hyperlipidemic subjects. A double-blind comparative study. Arterioscler. Thromb. Vasc. Biol. 1991, 11, 816–826. [Google Scholar] [CrossRef]
- Wallace, A.; Chinn, D.; Rubin, G. Taking simvastatin in the morning compared with in the evening: Randomised controlled trial. BMJ 2003, 327, 788. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.M.; Torsvik, H.; Falch, D.; Christophersen, B.; Skardal, R.; Gullestad, L. Effect of morning versus evening intake of simvastatin on the serum cholesterol level in patients with coronary artery disease. Am. J. Cardiol. 2002, 90, 784–786. [Google Scholar] [CrossRef]
- Tharavanij, T.; Wongtanakarn, S.; Lerdvuthisopon, N.; Teeraaunkul, S.; Youngsriphithak, P.; Sritipsukho, P. Lipid lowering efficacy between morning and evening simvastatin treatment: A randomized double-blind study. J. Med. Assoc. Thai. 2010, 93 (Suppl. 7), S109–S113. [Google Scholar] [PubMed]
- Plakogiannis, R.; Cohen, H.; Taft, D. Effects of morning versus evening administration of atorvastatin in patients with hyperlipidemia. Am. J. Health Syst. Pharm. 2005, 62, 2491–2494. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, M.K.; Seo, H.S.; Hyun, M.S.; Han, K.R.; Cho, S.W.; Kim, Y.K.; Park, S.H. Efficacy and safety of morning versus evening dose of controlled-release simvastatin tablets in patients with hyperlipidemia: A randomized, double-blind, multicenter phase III trial. Clin. Ther. 2013, 35, 1350–1360 e1. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.J.; Kim, H.J.; Jo, S.K.; Kim, S.G.; Song, Y.R.; Chung, W.; Han, K.H.; Lee, C.H.; Hwang, Y.H.; Oh, K.H. Comparison of the efficacy and safety profile of morning administration of controlled-release simvastatin versus evening administration of immediate-release simvastatin in chronic kidney disease patients with dyslipidemia. Clin. Ther. 2014, 36, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Kim, S.H.; Kim, J.K.; Ko, S.H.; Ko, J.E.; Park, S.J.; Park, M.G.; Lee, J.H.; Hyon, M.S. Comparison of effects of morning versus evening administration of ezetimibe/simvastatin on serum cholesterol in patients with primary hypercholesterolemia. Ann. Pharmacother. 2011, 45, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Plakogiannis, R.; Cohen, H. Optimal low-density lipoprotein cholesterol lowering—Morning versus evening statin administration. Ann. Pharmacother. 2007, 41, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.; Anderson, A.; Jones, E. The effect on 24 h blood pressure control of an angiotensin converting enzyme inhibitor (perindopril) administered in the morning or at night. J. Hypertens. 1997, 15, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E. Chronotherapy with the angiotensin-converting enzyme inhibitor ramipril in essential hypertension: Improved blood pressure control with bedtime dosing. Hypertension 2009, 54, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E.; Fernández, J.R.; Calvo, C. Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension 2007, 50, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Pechère-Bertschi, A.; Nussberger, J.; Decosterd, L.; Armagnac, C.; Sissmann, J.; Bouroudian, M.; Brunner, H.R.; Burnier, M. Renal response to the angiotensin II receptor subtype 1 antagonist irbesartan versus enalapril in hypertensive patients. J. Hypertens. 1998, 16, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Shimizu, M.; Hoshide, S.; Shimada, K.; Kario, K. A bedtime dose of ARB was better than a morning dose in improving baroreflex sensitivity and urinary albumin excretion—The J-TOP study. Clin. Exp. Hypertens. 2012, 34, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Jia, M.; Ran, H.; Tang, H.; Zhang, Y.; Zhang, J.; Wang, X.; Wang, H.; Yang, C.; Zeng, C. Fixed-combination of amlodipine and diuretic chronotherapy in the treatment of essential hypertension: Improved blood pressure control with bedtime dosing—A multicenter, open-label randomized study. Hypertens. Res. 2011, 34, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Nakamura, T.; Matsubara, H. The bedtime administration ameliorates blood pressure variability and reduces urinary albumin excretion in amlodipine-olmesartan combination therapy. Clin. Exp. Hypertens. 2010, 32, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E.; Fontao, M.J.; Mojón, A.; Fernández, J.R. Chronotherapy with valsartan/amlodipine fixed combination: Improved blood pressure control of essential hypertension with bedtime dosing. Chronobiol. Int. 2010, 27, 1287–1303. [Google Scholar] [CrossRef] [PubMed]
- Kasiakogias, A.; Tsioufis, C.; Thomopoulos, C.; Andrikou, I.; Aragiannis, D.; Dimitriadis, K.; Tsiachris, D.; Bilo, G.; Sideris, S.; Filis, K.; et al. Evening versus morning dosing of antihypertensive drugs in hypertensive patients with sleep apnoea: A cross-over study. J. Hypertens. 2015, 33, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, D.P.; Verho, M.; Botes, J.H.; Erasmus, T.P.; Luus, H.G. 24-hour blood pressure control with ramipril: Comparison of once-daily morning and evening administration. Curr. Ther. Res. Clin. Exp. 1995, 56, 1298–1306. [Google Scholar] [CrossRef]
- Nold, G.; Strobel, G.; Lemmer, B. Morning versus evening amlodipine treatment: Effect on circadian blood pressure profile in essential hypertensive patients. Blood Press. Monit. 1998, 3, 17–25. [Google Scholar] [PubMed]
- Mengden, T.; Binswanger, B.; Spuhler, T.; Weisser, B.; Vetter, W. The use of self-measured blood pressure determinations in assessing dynamics of drug compliance in a study with amlodipine once a day, morning versus evening. J. Hypertens. 1993, 11, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.G.; Chen, J.Z.; Zhu, J.H.; Yao, X.Y. Differential effects of morning or evening dosing of amlodipine on circadian blood pressure and heart rate. Cardiovasc. Drugs Ther. 2003, 17, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Asmar, R.; Gosse, P.; Quere, S.; Achouba, A. Efficacy of morning and evening dosing of amlodipine/valsartan combination in hypertensive patients uncontrolled by 5 mg of amlodipine. Blood Press. Monit. 2011, 16, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Fujimura, A.; Tateishi, T.; Ohashi, K.; Ebihara, A. Differences of chronopharmacokinetic profiles between propranolol and atenolol in hypertensive subjects. J. Clin. Pharmacol. 1993, 33, 756–761. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014, 37, S14–S80. [Google Scholar]
- Pehlivanov, N.D.; Olyaee, M.; Sarosiek, I.; McCallum, R.W. Comparison of morning and evening administration of rabeprazole for gastro-oesophageal reflux and nocturnal gastric acid breakthrough in patients with reflux disease: A double-blind, cross-over study. Aliment. Pharmacol. Ther. 2003, 18, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Hendel, J.; Hendel, L.; Aggestrup, S. Morning or evening dosage of omeprazole for gastro-oesophageal reflux disease? Aliment. Pharmacol. Ther. 1995, 9, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Highlights of prescribing information—ACIPHEX. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020973s035204736s005lbl.pdf (accessed on 13 December 2015).
- Calverley, P.M.A.; Lee, A.; Towse, L.; van Noord, J.; Witek, T.J.; Kelsen, S. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax 2003, 58, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Enas, E.A.; Kuruvila, A.; Khanna, P.; Pitchumoni, C.S.; Mohan, V. Benefits & risks of statin therapy for primary prevention of cardiovascular disease in Asian Indians—A population with the highest risk of premature coronary artery disease & diabetes. Indian J. Med. Res. 2013, 138, 461–491. [Google Scholar] [PubMed]
- Singh, R.; Sharma, P.K.; Malviya, R. Review on chronotherapeutics—A new remedy in the treatment of various diseases. Eur. J. Biol. Sci. 2010, 2, 67–76. [Google Scholar]
- Zhu, L.L.; Zhou, Q.; Yan, X.F.; Zeng, S. Optimal time to take once-daily oral medications in clinical practice. Int. J. Clin. Pract. 2008, 62, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K. Isoprenoids as mediators of the biological effects of statins. J. Clin. Investig. 2002, 110, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, P.; Smith, D.; Mehta, A.; Ganda, O.; Handelsman, Y.; Rodbard, H.; Shepherd, M.; Seibel, J. American association of clinical endocrinologists’ guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr. Pract. 2012, 18, 269–293. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, B. Circadian rhythms and drug delivery. J. Control. Release 1991, 16, 63–74. [Google Scholar] [CrossRef]
- Lemmer, B. Relevance for chronopharmacology in practical medicine. Semin. Perinatol. 2000, 24, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Smolensky, M.H.; Ayala, D.E.; Fernández, J.R.; Moyá, A.; Crespo, J.J.; Mojón, A.; Ríos, M.T.; Fabbian, F.; Portaluppi, F. Abnormalities in chronic kidney disease of ambulatory blood pressure 24 h patterning and normalization by bedtime hypertension chronotherapy. Nephrol. Dial. Transplant. 2014, 29, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, W.J.; Gong, W.Y.; Zhang, J.; Tang, H.; Peng, H.; Zhang, Q.Z.; Ye, Z.C.; Lou, T. High prevalence of isolated nocturnal hypertension in chinese patients with chronic kidney disease. J. Am. Heart Assoc. 2015, 4, e002025. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.W.; Li, Y.; Boggia, J.; Thijs, L.; Richart, T.; Staessen, J.A. Predictive role of the nighttime blood pressure. Hypertension 2011, 57, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar] [PubMed]
- Svensson, P.; de Faire, U.; Sleight, P.; Yusuf, S.; Östergren, J. Comparative effects of ramipril on ambulatory and office blood pressures a HOPE substudy. Hypertension 2001, 38, e28–e32. [Google Scholar] [CrossRef] [PubMed]
- Hermida, R.C.; Ayala, D.E.; Mojón, A.; Fernández, J.R. Influence of circadian time of hypertension treatment on cardiovascular risk: Results of the MAPEC study. Chronobiol. Int. 2010, 27, 1629–1651. [Google Scholar] [CrossRef] [PubMed]
- Stranges, P.M.; Drew, A.M.; Rafferty, P.; Shuster, J.E.; Brooks, A.D. Treatment of hypertension with chronotherapy: Is it time of drug administration? Ann. Pharmacother. 2015, 49, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M. Biopharmaceutics and Clinical Pharmacokinetics, 4th ed.; Lea & Febiger: Philadelphia, PA, USA, 1991; p. 406. [Google Scholar]
- Koopman, M.G.; Koomen, G.C.; Krediet, R.T.; de Moor, E.A.; Hoek, F.J.; Arisz, L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin. Sci. 1989, 77, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Pillay, V.; Choonara, Y.E.; du Toit, L.C. Drug delivery technologies for chronotherapeutic applications. Pharm. Dev. Technol. 2009, 14, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, B.V.; Rotolo, S.; Roth, H.L. Circadian rhythm and sleep influences on digestive physiology and disorders. Chronophysiol. Ther. 2014, 4, 67–77. [Google Scholar] [CrossRef]
- Chokroverty, S. Sleep Disorders Medicine: Basic Science, Technical Considerations, and Clinical Aspects, 3rd ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2013; p. 750. [Google Scholar]
- Stockigt, J.R. Barriers in the quest for quality drug information: Salutary lessons from TGA-approved sources for thyroid-related medications. Med. J. Aust. 2007, 186, 76–79. [Google Scholar] [PubMed]
- Fujimura, A. [Chronotherapy--present and future]. Nihon Rinsho 2013, 71, 2097–2101. [Google Scholar] [PubMed]
- Durrington, H.J.; Farrow, S.; Ray, D. Recent advances in chronotherapy for the management of asthma. Chronophysiol. Ther. 2014, 4, 125–135. [Google Scholar] [CrossRef]
- Hassan, A.; Haefeli, W.E. Appropriateness of timing of drug administration in electronic prescriptions. Pharm. World Sci. 2010, 32, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H. Knowledge and attitudes of American physicians and public about medical chronobiology and chronotherapeutics. Findings of two 1996 gallup surveys. Chronobiol. Int. 1998, 15, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Saba, M.; Phillips, C.L.; Wong, K.; Saini, B. Education intervention on chronotherapy for final-year pharmacy students. Pharmacy 2015, 3, 269–283. [Google Scholar] [CrossRef]
- De Giorgi, A.; Menegatti, A.M.; Fabbian, F.; Portaluppi, F.; Manfredini, R. Circadian rhythms and medical diseases: Does it matter when drugs are taken? Eur. J. Intern. Med. 2013, 24, 698–706. [Google Scholar] [CrossRef] [PubMed]

| Rank | Drug | Rank | Drug |
|---|---|---|---|
| 1 | Atorvastatin | 16 | Amoxicillin |
| 2 | Rosuvastatin | 17 | Ramipril |
| 3 | Esomeprazole | 18 | Paracetamol + Codeine |
| 4 | Paracetamol | 19 | Amlodipine |
| 5 | Pantoprazole | 20 | Irbesartan + Hydrochlorothiazide |
| 6 | Perindopril | 21 | Venlafaxine |
| 7 | Metformin | 22 | Clopidogrel |
| 8 | Fluticasone + Salmeterol | 23 | Omeprazole |
| 9 | Irbesartan | 24 | Amoxicillin + Clavulanic Acid |
| 10 | Simvastatin | 25 | Candesartan |
| 11 | Salbutamol | 26 | Rabeprazole |
| 12 | Atenolol | 27 | Telmisartan † |
| 13 | Cephalexin | 28 | Tiotropium |
| 14 | Oxycodone | 29 | Tramadol † |
| 15 | Warfarin | 30 | Desvenlafaxine * |
| Study | Participants (Sample Size) Age in Years | Medicine (Dose) | Study Design (Study Duration) | Suggested Time |
|---|---|---|---|---|
| Ozaydin et al. [48] | Hyperlipidemic patients (n = 152, 118 male) Age: 59 ± 5 | Atorvastatin (40 mg followed by 10 mg) | Prospective randomised study Morning/evening (12 months) | Evening |
| Plakogiannis et al. [53] | Hyperlipidemic patients taking Atorvastatin 40 mg (n = 64 males) Age: 58.5 ± 7.8 (evening group), 57.8 ± 7.8 (morning group) | Atorvastatin (40 mg) | Comparative study, Morning (before noon)/evening (after 1800 h and before midnight) (4 weeks) | Morning/evening |
| Saito et al. [49] | Hyperlipidemic patients (n = 150, 33 males) Age: 18–65 | Simvastatin (2.5 mg and 5 mg) | Double-blind, randomised, placebo-controlled study Morning/evening (12 weeks) | Evening |
| Wallace et al. [50] | Hyperlipidemic patients (n = 57, 27 males) Age: 44–82 | Simvastatin (10 mg, 20 mg) | Randomised study Morning/evening (8 weeks) | Evening |
| Lund et al. [51] | Coronary Artery Disease patients (n = 25, 18 males), Age: 66 ± 11 | Simvastatin (10, 20 and 40 mg) | Randomised crossover study Morning/evening (12 weeks) | Evening |
| Tharanvanij et al. [52] | Dyslipidaemia subjects (n = 52) Age: 18–70 | Simvastatin (10 mg) | Randomised, double-blind, controlled study, Morning (0600–1000 h)/evening (1900–2200 h) (12 weeks) | Evening |
| Kim et al. [54] | Dyslipidaemia Korean subjects (n = 132, 55 males) Age: 58.7± 8.3 (MG) 58.5 ± 9.5 (EG) | Simvastatin controlled release (20 mg) | Prospective, randomised, double-blind, placebo controlled, multicentre study Morning/evening (8 weeks) | Morning/evening |
| Yong et al. [55] | Dyslipidaemia patients with chronic kidney disease (n = 122, 57 males), Age: 20–75 | Simvastatin Controlled release (CR) and immediate-release (IR) (20 mg) | Prospective, randomised, multicentre, double-blind study Morning (CR)/evening (IR) (8 weeks) | Morning/evening |
| Yoon et al. [56] | Primary hypercholesterolemia patients (n = 145, 101 males) Age: 18+ | Ezetimibe/simvastatin (10 mg/20 mg) | Multicentre, open label, randomised, 2-sequence, 2 period crossover study Morning/evening (6 weeks) | Morning/evening |
| Reference | Participants (Sample Size) Age in Years | Medicine (Dose) | Study Design (Study Duration) | Suggested Time |
|---|---|---|---|---|
| Morgan et al. [58] | Essential hypertensive patients (n = 18 males) Age: 33–85 | Perindopril (4 mg) | Randomised crossover study, Morning (0900 h)/nighttime (2100 h) (4 weeks) | Nighttime |
| Hermida et al. [59] | Uncomplicated essential hypertensive patients (n = 115, 52 males) Age: 46.7 ± 11.2 | Ramipril (5 mg) | Multicentre, PROBE, parallel group study Awakening/bedtime (6 weeks) | Bedtime |
| Myburgh et al. [67] | Mild to moderate essential hypertensive patients (n = 39, 35 males) Age—24–73 | Ramipril (2.5 mg) | Open, randomised, crossover study Morning (0800 h)/Evening (2000 h) (4 weeks) | Morning/evening |
| Hermida et al. [60] | Grade 1 or 2 essential hypertensive patients (n = 215, 114 males) Age: 46.4 ± 12.0 | Telmisartan (80 mg) | PROBE, parallel group study, Morning/bedtime (12 weeks) | Bedtime |
| Pechere-Bertschi et al. [61] | Mild to moderate essential hypertensive patients (n = 10) Age: 35–70 | Irbesartan (100 mg) | Randomised, double-blind, double-dummy, crossover study Morning/evening (12 weeks) | Morning/evening |
| Eguchi et al. [62] | Patients having at least one antihypertensive medicine or been unmedicated (n = 109, 70 males) Age: 58.3 ± 11.3 | Candesartan (4 mg, 8 mg) | Randomised study Morning/bedtime (6 months) | Bedtime |
| Nold et al. [68] | Mild to moderate essential hypertensive patients (n = 12, 7 males) | Amlodipine (5 mg, 10 mg) | Open label, randomised, crossover study, Morning (0800 h)/evening (2000 h) (3 weeks) | Morning/evening |
| Mengden et al. [69] | Mild to moderate hypertensive patients (n = 20) | Amlodipine (5 mg) | Randomised, placebo-controlled open-label, crossover study Morning/evening (8 weeks) | Morning/evening |
| Qui et al. [70] | Mild to moderate essential hypertensive patients (n = 60, 44 males) Age: 21–77 | Amlodipine (5 mg) | Prospective, double-blind, randomised, crossover study, Morning (0700 h)/evening (2100 h) (12 weeks) | Morning |
| Zeng et al. [63] | Essential hypertensive (n = 80), Age: 67 ± 9.8 | Amlodipine and hydrochlorothiazide (5 and 25 mg) | Multicentre, open label randomized study Morning (0800 h)/bedtime (2200 h) (12 weeks) | Bedtime |
| Hoshino et al. [64] | Essential hypertensive patients (n = 31, 12 males) Age: 69 ± 11 | Amlodipine and olmesartan combination (2.5–10 mg and 20–40 mg) | Open-label randomised crossover study Morning/bedtime (32 weeks) | Bedtime |
| Hermida et al. [65] | Untreated uncomplicated essential hypertensive patients (n = 203, 92 males) Age: 56.7 ± 12.5 | Valsartan (V) and amlodipine (A) (160 mg (V) and 5 mg (A)/day) (medications taken single or together) | PROBE and parallel group study Morning/bedtime (12 weeks) | Bedtime |
| Asmar et al. [71] | Essential hypertensive patients with BP uncontrolled by 5 mg amlodipine (I = 463, 291 males) Age: 56 ± 10 | Amlodipine (A)/valsartan (V) combination (5 mg (A) and 160 mg) (increase to 10 mg and 160 mg after 4 weeks) | PROBE, Multicentre, study Morning/evening admin in 2 (12 weeks) | Morning/evening |
| Kasiskogias et al. [66] | Untreated essential hypertensive patients with obstructive sleep apnoea (n = 41, 32 males) Age: >30 | Amlodipine (A)/valsartan (V) combination (5/160 mg), (10/160 mg), (10/360 mg) or valsartan (160 mg) | Prospective, open label crossover study Morning/evening (16 weeks) | Evening |
| Shiga et al. [72] | Essential hypertensive patients (n = 13, 8 males) Age: 46.5 ± 8.4 | Atenolol (50 mg) | Randomised, crossover comparative study day trial (0900 h)/night trial (2100 h) (16 days) | Morning/nighttime |
| Reference | Participants (Sample Size) Age in Years | Medicine (Dose) | Study Design (Study Duration) | Suggested Time |
|---|---|---|---|---|
| Pehlivanov et al. [74] | GERD patients (n = 20, 6 males) Age: 45.4 ± 9.2 | Rabeprazole (20 mg) | Randomised, double-blinded study Morning/evening (7 days) | Evening |
| Hendel et al. [75] | GERD patients (n = 17, 7 males) Age: 22–67 | Omeprazole (40 mg) | Crossover study Morning (0800–1000 h)/evening (2100–2300 h) (28 days) | Morning/evening |
| Reference | Participants (Sample Size) Age in Years | Medicine (Dose) | Study Design (Study Duration) | Suggested Time |
|---|---|---|---|---|
| Calverley et al. [77] | COPD patients (n = 121, 71 males) Age: 65.8 ± 7.9 | Tiotropium (18 mg) dry powder device (HandiHaler) | Randomised, double-blind, placebo-controlled Morning (0900 h)/evening (2100 h) before meals (6 weeks) | Morning/evening |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, G.; Phillips, C.L.; Wong, K.; McLachlan, A.J.; Saini, B. Timing of Administration: For Commonly-Prescribed Medicines in Australia. Pharmaceutics 2016, 8, 13. https://doi.org/10.3390/pharmaceutics8020013
Kaur G, Phillips CL, Wong K, McLachlan AJ, Saini B. Timing of Administration: For Commonly-Prescribed Medicines in Australia. Pharmaceutics. 2016; 8(2):13. https://doi.org/10.3390/pharmaceutics8020013
Chicago/Turabian StyleKaur, Gagandeep, Craig L. Phillips, Keith Wong, Andrew J. McLachlan, and Bandana Saini. 2016. "Timing of Administration: For Commonly-Prescribed Medicines in Australia" Pharmaceutics 8, no. 2: 13. https://doi.org/10.3390/pharmaceutics8020013