Highly Effective Non-Viral Antitumor Gene Therapy System Comprised of Biocompatible Small Plasmid Complex Particles Consisting of pDNA, Anionic Polysaccharide, and Fully Deprotected Linear Polyethylenimine
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Mice
2.2. ζ-Potential and Size Measurement
2.3. In Vitro Transfection
2.3.1. Preparation of pDNA Complex for in Vitro Transfection
2.3.2. In Vitro Transfection
2.4. In Vivo Imaging
2.4.1. Preparation of Plasmid Complex for in Vivo Imaging
2.4.2. Image Acquisition
2.5. Treatment of Cancer
2.5.1. Preparation of Plasmid Complex for in Vivo Transfection
2.5.2. Evaluation of the Therapeutic Effect of the pDNA Complexes
3. Results and Discussion
3.1. Effect of Mixing Ratio of DNA and PEI “Max”

3.2. Effect of Dextran Addition on Rehydration Property



3.3. Effect of Mixing Ratio of HA (or CS)

3.4. Effect of Salt Strength and pH of the Rehydrating Solution

3.5. Effect of Salt Strength and pH of the Complex Preparation Conditions

3.6. In Vivo Imaging

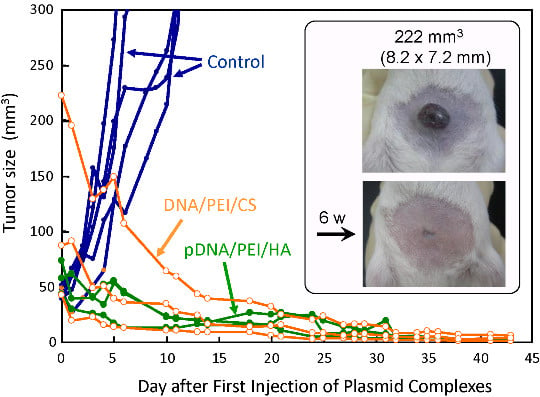
3.7. Antitumor Efficacy of the Small pDNA/(PEI “Max”)/HA (or CS) Ternary Complex in the Tumor Model Mice

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glover, D.J.; Lipps, H.J.; Jans, D.A. Towards safe, non-viral therapeutic gene expression in humans. Nat. Rev. Genet. 2005, 6, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Morille, M.; Passirani, C.; Vonarbourg, A.; Clavreul, A.; Benoit, J.P. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials 2008, 29, 3477–3496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finsinger, D.; Remy, J.S.; Erbacher, P.; Koch, C.; Plank, C. Protective copolymers for nonviral gene vectors: Synthesis, vector characterization and application in gene delivery. Gene Ther. 2000, 7, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Dauty, E.; Behr, J.P.; Remy, J.S. Development of plasmid and oligonucleotide nanometric particles. Gene Ther. 2002, 9, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Iida-Tanaka, N.; Koyama, Y. Efficient in vivo gene transfection by stable DNA/PEI complexes coated by hyaluronic acid. J. Drug Target. 2008, 16, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Trubetskoy, V.S.; Loomis, A.; Slattum, P.M.; Hagstrom, J.E.; Budker, V.G.; Wolff, J.A. Caged DNA does not aggregate in high ionic strength solutions. Bioconjug. Chem. 1999, 10, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Yamada, E.; Ito, T.; Mizutani, Y.; Yamaoka, T. Sugar-Containing Polyanions as a Self-Assembled Coating of Plasmid/Polycation Complexes for Receptor-Mediated Gene Delivery. Macromol. Biosci. 2002, 2, 251–256. [Google Scholar]
- Koyama, Y.; Ito, T.; Matsumoto, H.; Tanioka, A.; Okuda, T.; Yamaura, N.; Aoyagi, H.; Niidome, T. Novel poly(ethylene glycol) derivatives with carboxylic acid pendant groups: Synthesis and their protection and enhancing effect on non-viral gene transfection systems. J. Biomater. Sci. Polym. Ed. 2003, 14, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Yamashita, M.; Iida-Tanaka, N.; Ito, T. Enhancement of transcriptional activity of DNA complexes by amphoteric PEG derivative. Biomacromolecules 2006, 7, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Iida-Tanaka, N.; Niidome, T.; Kawano, T.; Kubo, K.; Yoshikawa, K.; Sato, T.; Yang, Z.; Koyama, Y. Hyaluronic acid and its derivative as a multi-functional gene expression enhancer: Protection from non-specific interactions, adhesion to targeted cells, and transcriptional activation. J. Control. Release 2006, 30, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Yoshihara, C.; Hamada, K.; Koyama, Y. DNA/polyethylenimine/hyaluronic acid small complex particles and tumor suppression in mice. Biomaterials 2010, 31, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Koyama, Y.; Otsuka, M. Analysis of the surface structure of DNA/polycation/hyaluronic acid ternary complex by Raman microscopy. J. Pharm. Biomed. Anal. 2010, 51, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Iwasaki, F.; Takizawa, T.; Yanagie, H.; Niidome, T.; Yamada, E.; Ito, T.; Koyama, Y. Novel receptor-mediated gene delivery system comprising plasmid/protamine/sugar-containing polyanion ternary complex. Biomaterials 2004, 25, 3267–3273. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyama, Y.; Sugiura, K.; Yoshihara, C.; Inaba, T.; Ito, T. Highly Effective Non-Viral Antitumor Gene Therapy System Comprised of Biocompatible Small Plasmid Complex Particles Consisting of pDNA, Anionic Polysaccharide, and Fully Deprotected Linear Polyethylenimine. Pharmaceutics 2015, 7, 152-164. https://doi.org/10.3390/pharmaceutics7030152
Koyama Y, Sugiura K, Yoshihara C, Inaba T, Ito T. Highly Effective Non-Viral Antitumor Gene Therapy System Comprised of Biocompatible Small Plasmid Complex Particles Consisting of pDNA, Anionic Polysaccharide, and Fully Deprotected Linear Polyethylenimine. Pharmaceutics. 2015; 7(3):152-164. https://doi.org/10.3390/pharmaceutics7030152
Chicago/Turabian StyleKoyama, Yoshiyuki, Kikuya Sugiura, Chieko Yoshihara, Toshio Inaba, and Tomoko Ito. 2015. "Highly Effective Non-Viral Antitumor Gene Therapy System Comprised of Biocompatible Small Plasmid Complex Particles Consisting of pDNA, Anionic Polysaccharide, and Fully Deprotected Linear Polyethylenimine" Pharmaceutics 7, no. 3: 152-164. https://doi.org/10.3390/pharmaceutics7030152