Anti-Apoptotic Gene Delivery with cyclo-(d-Trp-Tyr) Peptide Nanotube via Eye Drop Following Corneal Epithelial Debridement
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of cyclo-(d-Trp-Tyr) Peptide Nanotubes
2.2. Plasmid DNA
2.3. Plasmid DNA Labeling
2.4. The Formulation of Plasmid/PNTs Complexes
2.5. Characterization of CAP3 pRFP-C-RS/PNTs
2.5.1. Scanning Electron Microscope Imaging
2.5.2. Atomic Force Microscope Imaging
2.5.3. Fluorescence Microscope Imaging
2.5.4. Size and Zeta Potential Measurement
2.5.5. Fluorescence Measurement
2.5.6. Stability of CAP3 pRFP-C-RS /PNTs with DNase I
2.6. Animals Used for in Vivo Gene Delivery
2.7. Epithelial Debridement in Mouse Cornea and Eye Drop Gene Delivery to Epithelial-Defective Cornea
2.8. Distribution of CAP3 pRFP-C-R/PNTs in Mouse Epithelial-Defective Cornea
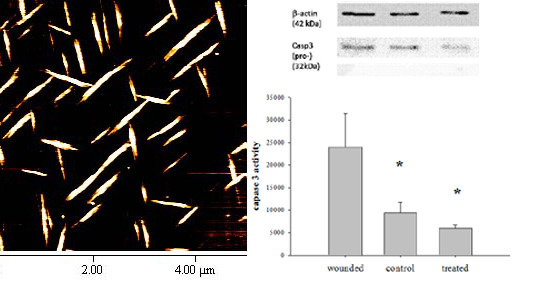
2.9. Determination of Caspase 3 Activity
2.10. Western Blotting Analysis
3. Results and Discussion
3.1. Characterization of CAP3 pRFP-C-RS/PNTs


| Formulation | DLS Size (μm) a | Microscope b | ζ-potential (mV) c | ||
|---|---|---|---|---|---|
| Width (μm) | Length (μm) | ||||
| P d | 0.068 ± 0.003 | – | – | −47.1 | ±7.7 |
| PNTs e | 2.2 ± 0.5 | 0.29 ± 0.08 | 1.8 ± 0.6 | −37.6 | ±2.8 |
| P/PNTs f | 1.7 ± 0.6 | 0.26 ± 0.06 | 1.4 ±0.8 | −74.3 | ±1.3 |


3.2. Stability of CAP3 pRFP-C-RS/PNTs with DNase I

3.3. In Vivo Eye Drop Gene Transfer in Cornea Area

3.4. Gene Delivery via Eye Drop to Mouse Cornea after Epithelial Debridement


Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mohan, R.R.; Hutcheon, A.E.K.; Choi, R.; Hong, J.; Lee, J.; Mohan, R.R.; Ambrósio, R.A.; Zieske, J.D.; Wilson, S.E. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res. 2003, 76, 71–87. [Google Scholar] [CrossRef]
- Esquenazi, S.; Mendoza, A. Two-year follow-up of laser in situ keratomileusis for hyperopia. J. Refract. Surg. 1999, 15, 648–652. [Google Scholar] [PubMed]
- Helena, M.C.; Baerveldt, F.; Kim, W.-J.; Wilson, S.E. Keratocyte apoptosis after corneal surgery. Investig. Ophthalmol. Vis. Sci. 1998, 39, 276–283. [Google Scholar]
- Dua, H.S.; King, A.J.; Joseph, A. A new classification of ocular surface burns. Br. J. Ophthalmol. 2001, 85, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Sun, X.; Li, J.; Cui, M.; Tan-Allen, K.; Bonanno, J.A. Hypoxia preconditioning protects corneal stromal cells against induced apoptosis. Exp. Eye Res. 2006, 82, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Song, I.K.; Joo, C.K. Morphological and functional changes in the rat cornea with an ethanol-mediated epithelial flap. Investig. Ophthalmol. Vis. Sci. 2004, 45, 423–428. [Google Scholar] [CrossRef]
- Kim, W.J.; Rabinowitz, Y.S.; Meisler, D.M.; Wilson, S.E. Keratocyte apoptosis associated with keratoconus. Exp. Eye Res. 1999, 69, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; He, Y.G.; Weng, J.; Li, Q.; McDowall, A.W.; Vital, M.; Chwang, E.L. Epithelial injury induces keratocyte apoptosis: Hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization an wound healing. Exp. Eye Res. 1996, 62, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Burns, A.R.; Smith, C.W. Two waves of neutrophil emigration in response to corneal epithelial abrasion: Distinct adhesion molecule requirements. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Shiraishi, A.; Saika, S.; Liu, C.Y.; Funderburgh, J.L.; Kao, C.W.C.; Converse, R.L.; Kao, W.W.-Y. Role of lumican in the corneal epithelium during wound healing. J. Biol. Chem. 2000, 275, 2607–2612. [Google Scholar] [CrossRef] [PubMed]
- Zieske, J.D.; Guimaraes, S.R.; Hutcheon, A.E. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp. Eye Res. 2001, 72, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sah, W.J.; Park, C.K.; Hahn, T.W.; Kim, M.S. Myopic regression after photorefractive keratectomy. Ophthalmic Surg. Lasers 1996, 27, S435–S439. [Google Scholar] [PubMed]
- Netto, M.V.; Mohan, R.R.; Sinha, S.; Sharma, A.; Dupps, W.; Wilson, S.E. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp. Eye Res. 2006, 82, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Ferrington, D.A.; Roehrich, H.; Chang, A.A.; Huang, C.W.; Maldonado, M.; Bratten, W.; Rageh, A.A.; Heuss, N.D.; Gregerson, D.S.; Nelson, E.F. Corneal wound healing is compromised by immunoproteasome deficiency. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ramaesh, T.; Ramaesh, K.; Leask, R.; Springbett, A.; Riley, S.C.; Dhillon, B.; West, J.D. Increased apoptosis and abnormal wound-healing responses in the heterozygous Pax6+/− mouse cornea. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.C.; Chang, S.F.; Kao, W.W.Y.; Liu, C.Y.; Liaw, J. Polymeric micelle gene delivery of bcl-xL via eye drop reduced corneal apoptosis following epithelial debridement. J. Control. Release 2010, 147, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, Y.C.; Wu, C.H.; Yeh, C.C.; Su, M.T.; Wu, Y.C. Preparation of fluorescent silica nanotubes and their application in gene delivery. Adv. Mater. 2005, 17, 404–407. [Google Scholar] [CrossRef]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The Effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Hedrick, J.L.; Ong, Z.Y.; Yang, C.; Ee, P.L.R.; Hammond, P.T.; Yang, Y.Y. The Effects of polymeric nanostructure shape on drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Chipot, C.; Tarek, M. Interaction of a peptide nanotube with a water-membrane interface. Phys. Biol. 2006, 3, S20–S25. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M. The heptad repeat in the largest subunit of RNA polymerase II binds by intercalating into DNA. Nature 1990, 344, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Wagenknecht, H.A.; Stemp, E.D.A.; Barton, J.K. DNA-Bound peptide radicals generated through DNA-mediated electron transport. Biochemistry 2000, 39, 5483–5491. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.R.; Granja, J.R.; Buehler, L.K. Artificial transmembrane ion channels from self-assembling peptide nanotubes. Nature 1994, 369, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.D.; Buehler, L.K.; Ghadiri, M.R. Self-assembling cyclic β3-peptide nanotubes as artificial transmembrane ion channels. J. Am. Chem. Soc. 1998, 120, 651–656. [Google Scholar] [CrossRef]
- Hsieh, W.H.; Chang, S.F.; Chen, H.M.; Chen, J.H.; Liaw, J. Oral gene delivery with cyclo-(d-Trp-Tyr) peptide nanotubes. Mol. Pharm. 2012, 9, 1231–1249. [Google Scholar] [PubMed]
- Eriksson, S.; Norden, B.; Takahashi, M. Binding of DNA quenches tyrosine fluorescence of RecA without energy transfer to DNA bases. J. Biol. Chem. 1993, 268, 1805–1810. [Google Scholar] [PubMed]
- Xiao, J.; Wei, X.; Wang, Y.; Liu, C. Fluorescence resonance energy-transfer affects the determination of the affinity between ligand and proteins obtained by fluorescence quenching method. Spectrochim. Acta A 2009, 74, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Barik, A.; Priyadarsini, K.I.; Mohan, H. Fluorescence spectroscopic studies on binding of a flavonoid antioxidant quercetin to serum albumins. J. Chem. Sci. 2005, 117, 641–647. [Google Scholar] [CrossRef]
- Kumaraswamy, P.; Lakshmanan, R.; Sethuraman, S.; Krishnan, U.M. Self-assembly of peptides: Influence of substrate, pH and medium on the formation of supramolecular assemblies. Soft Matter 2011, 7, 2744–2754. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Chen, Y.; Burnett, C.; Liu, X.Y.; Downs, S.; Collins, R.D.; Hawiger, J. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J. Biol. Chem. 2004, 279, 48434–48442. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.; Goujon, D.N.; Forsyth, M. Inducing alignment of cyclic peptide nanotubes through the use of structured ionic liquids. Chem. Commun. 2013, 49, 7729–7731. [Google Scholar] [CrossRef] [PubMed]
- Shimizu1, T.; Minamikawa, H.; Kogiso, M.; Aoyagi, M.; Kameta, N.; Ding, W.; Masuda, M. Self-organized nanotube materials and their application in bioengineering. Polymer J. 2014, 46, 831–858. [Google Scholar] [CrossRef]
- Huang, R.; Qui, W.; Su, R.; Zhao, J.; He, Z. Solvent and surface controlled self-assembly of diphenylalanine peptide: From microtubes to nanofibers. Soft Matter 2011, 7, 6418–6421. [Google Scholar] [CrossRef]
- Joshi, K.B.; Verma, S. Participation of aromatic side chains in diketopiperazine ensembles. Tetrahedron Lett. 2008, 49, 4231–4234. [Google Scholar] [CrossRef]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Ruiz, C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Cheng, C.T.; Lo, V.; Chen, J.; Chen, W.C.; Lin, C.Y.; Lin, H.C.; Yang, C.H.; Sheh, L. Synthesis and DNA nicking studies of a novel cyclic peptide: Cyclo [Lys-Trp-Lys-Ahx-]. Bioorg. Med. Chem. 2001, 9, 1493–1498. [Google Scholar] [CrossRef]
- Wu, Y.; Phillips, J.A.; Liu, H.; Yang, R.; Tan, W. Carbon nanotubes protect DNA strands during cellular delivery. ACS Nano 2008, 2, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Jiban, J.; Panda, J.J.; Yandrapu, S.; Kadam, R.S.; Chauhan, V.S.; Kompella, U.B. Self-assembled phenylalanine-α,β-dehydrophenylalanine nanotubes for sustained intravitreal delivery of a multi-targeted tyrosine kinase inhibitor. J. Control. Release 2013, 172, 1151–1160. [Google Scholar]
- Ruberti, J.W.; Roy, A.S.; Roberts, C.J. Corneal biomechanics and biomaterials. Annu. Rev. Biomed. Eng. 2011, 13, 269–295. [Google Scholar] [CrossRef] [PubMed]
- Pescina, S.; Govoni, P.; Antopolsky, M.; Murtomaki, L.; Padula, C.; Santi, P.; Nicoli, S. Permeation of proteins, oligonucleotide and dextrans across ocular tissues: Experimental studies and a literature update. J. Pharm. Sci. 2015, 104, 2190–2202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sukthankar, P.; Tomich, J.M.; Conrad, G.W. Effect of the synthetic NC-1059 peptide on diffusion of riboflavin across an intact corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Kim, K.; Zhang, J.; Escalada, A.; Tunuguntla, R.; Comolli, L.R.; Allen, F.I.; Shnyrova, A.V.; Cho, K.R.; Munoz, D.; et al. Stochastic transport through carbon nanotubes in lipid bilayers and live cell membranes. Nature 2014, 514, 612–615. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Chang, S.-F.; Liaw, J. Anti-Apoptotic Gene Delivery with cyclo-(d-Trp-Tyr) Peptide Nanotube via Eye Drop Following Corneal Epithelial Debridement. Pharmaceutics 2015, 7, 122-136. https://doi.org/10.3390/pharmaceutics7030122
Lee Y-H, Chang S-F, Liaw J. Anti-Apoptotic Gene Delivery with cyclo-(d-Trp-Tyr) Peptide Nanotube via Eye Drop Following Corneal Epithelial Debridement. Pharmaceutics. 2015; 7(3):122-136. https://doi.org/10.3390/pharmaceutics7030122
Chicago/Turabian StyleLee, Yu-Hsing, Shwu-Fen Chang, and Jiahorng Liaw. 2015. "Anti-Apoptotic Gene Delivery with cyclo-(d-Trp-Tyr) Peptide Nanotube via Eye Drop Following Corneal Epithelial Debridement" Pharmaceutics 7, no. 3: 122-136. https://doi.org/10.3390/pharmaceutics7030122