Evaluation of Rapidly Disintegrating Vaginal Tablets of Tenofovir, Emtricitabine and Their Combination for HIV-1 Prevention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Vaginal Tablets and Gel
2.3. In Vitro Permeability
| Description | TFV Tablets | FTC Tablets | TFV/FTC Tablets |
|---|---|---|---|
| White to off-White, Round, Flat Faced Tablet | |||
| Drug Dose | 10 mg TFV | 10 mg FTC | 10 mg TFV/10 mg FTC |
| Assay (% Label Dose) | 97.2% | 102.3% | 101.9% TFV/100.8% FTC |
| Total Mass | ~125 mg | ~125 mg | ~125 mg |
| Diameter | 8 mm | 8 mm | 9 mm |
| Disintegration Time a | 60–75 s | 60–75 s | 60–75 s |
| Moisture Content | 2.6% | 1.7% | 2.2% |
| Hardness (N) | 28 | 97 | 14 |
| Friability (%) | 0.5% | 0.1% | 0.4% |
2.4. Pharmacokinetics in Rabbits

2.5. Bioanalysis
2.6. Statistics
3. Results
3.1. Physicochemical Characterization
3.2. In Vitro Permeability

3.3. In Vivo Pharmacokinetics—Study 1

| Formulation | Tmax (h) | Cmax (ng/mL) | AUC0–24 h (ng·h/mL) |
|---|---|---|---|
| TFV Tablet (intact) | 0.70 ± 0.27 a | 297 ± 199 | 858 ± 342 |
| TFV Tablet (pre-dissolved) | 1.20 ± 1.57 | 694 ± 879 | 778 ± 802 |
| TFV 1% Gel | 0.60 ± 0.22 | 2352 ± 2300 | 3615 ± 2558 |
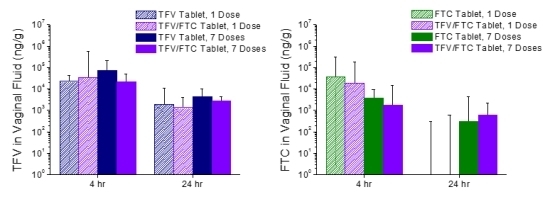
3.4. In Vivo Pharmacokinetics—Study 2

| Formulation | Tmax (h) | Cmax (ng/mL) | AUC0–24 h (ng·h/mL) |
|---|---|---|---|
| Single Dose | |||
| TFV Tablet | 5.83 ± 9.36 a | 139 ± 91.9 | 1458 ± 555 |
| TFV/FTC Tablet | 0.667 ± 0.258 | 249 ± 150 | 1304 ± 416 |
| Seven Doses | |||
| TFV Tablet | 2.17 ± 2.86 | 242 ± 123 | 1534 ± 489 |
| TFV/FTC Tablet | 0.917 ± 0.204 | 235 ± 231 | 849 ± 553 |
| Formulation | Tmax (h) | Cmax (ng/mL) | AUC0–24 h (ng·h/mL) |
|---|---|---|---|
| Single Dose | |||
| FTC Tablet | 2.17 ± 0.983 a | 567 ± 185 | 2992 ± 1027 |
| TFV/FTC Tablet | 1.50 ± 1.22 | 407 ± 142 | 2361 ± 692 |
| Seven Doses | |||
| FTC Tablet | 1.33 ± 0.516 | 403 ± 123 | 2822 ± 170 |
| TFV/FTC Tablet | 1.0 ± 0.0 | 279 ± 223 | 1854 ± 971 |


4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Voelker, R. Women shoulder growing HIV/AIDS burden. JAMA 2005, 293, 281–282. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Global report: Unaids report on the global aids epidemic 2012. In Joint United Nations Programme on Hiv Aids (Unaids); Joint United Nations Programme on HIV/AIDs: Geneva, Switzerland, 2012. [Google Scholar]
- Abdool Karim, Q.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.M.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, J.M.; Ramjee, G.; Nair, G.; Palanee, T.; Mkhize, B.; Nakabbiito, C.; Taljaard, M.; Piper, J.; Gomez Feliciano, K.; Chirenje, M. Pre-exposure prophylaxis for HIV in women: Daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the voice study (MTN-003). In Proceedings of the 2013 Conference on Retroviruses and Opportunistic Infections, Atlanta, GA, USA, 3–6 March 2013; Volume 26LB.
- Van Damme, L.; Corneli, A.; Ahmed, K.; Agot, K.; Lombaard, J.; Kapiga, S.; Malahleha, M.; Owino, F.; Manongi, R.; Onyango, J.; et al. Preexposure prophylaxis for HIV infection among african women. N. Engl. J. Med. 2012, 367, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Walensky, R.P.; Park, J.-E.; Wood, R.; Freedberg, K.A.; Scott, C.A.; Bekker, L.-G.; Losina, E.; Mayer, K.H.; Seage, G.R.; Paltiel, A.D. The cost-effectiveness of pre-exposure prophylaxis for HIV infection in south african women. Clin. Infect. Dis. 2012, 54, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.R. Pharmaceutical development of microbicide drug products. Pharm. Dev. Technol. 2010, 15, 562–581. [Google Scholar] [CrossRef]
- Garg, S.; Goldman, D.; Krumme, M.; Rohan, L.C.; Smoot, S.; Friend, D.R. Advances in development, scale-up and manufacturing of microbicide gels, films, and tablets. Antivir. Res. 2010, 88, S19–S29. [Google Scholar] [CrossRef] [PubMed]
- Romano, J.; Malcolm, R.K.; Garg, S.; Rohan, L.C.; Kaptur, P.E. Microbicide delivery: Formulation technologies and strategies. Curr. Opin. HIV AIDS 2008, 3, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.M.; Mitchnick, L.B.; Risha, P.; Muungo, L.T.M.; Norick, P.M. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among african women. J. Women’s Health 2011, 20, 1207–1214. [Google Scholar] [CrossRef]
- Van der Straten, A.; Montgomery, E.; Cheng, H.; Wegner, L.; Masenga, G.; von Mollendorf, C.; Bekker, L.; Ganesh, S.; Young, K.; Romano, J.; et al. High acceptability of a vaginal ring intended as a microbicide delivery method for HIV prevention in african women. AIDS Behav. 2012, 16, 1775–1786. [Google Scholar]
- Frezieres, R.G.; Walsh, T.; Kilbourne-Brook, M.; Coffey, P.S. Couples’ acceptability of the silcs diaphragm for microbicide delivery. Contraception 2012, 85, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, N.S.; Joshi, S.N.; Navlakha, S.N.; Katti, U.R.; Mehendale, S.M. Acceptability of praneem polyherbal vaginal tablet among HIV uninfected women & their male partners in pune, india—Phase I study. Indian J. Med. Res. 2006, 123, 547–552. [Google Scholar] [PubMed]
- Joshi, S.N.; Dutta, S.; Kumar, B.K.; Katti, U.; Kulkarni, S.; Risbud, A.; Mehendale, S. Expanded safety study of praneem polyherbal vaginal tablet among HIV-uninfected women in pune, India: A phase II clinical trial report. Sex. Transm. Infect. 2008, 84, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Woolfson, A.D.; Umrethia, M.L.; Kett, V.L.; Malcolm, R.K. Freeze-dried, mucoadhesive system for vaginal delivery of the hiv microbicide, dapivirine: Optimisation by an artificial neural network. Int. J. Pharm. 2010, 388, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, L.E.; Clark, M.R.; Friend, D.; Mitchell, J.; Bachman, S.; Deyounks, F.; Garber, D.; McNicholl, J.; Hendry, M.; Smith, J. Preliminary pharmacokinetic, pharmacodynamic, and safety analysis of vaginal tenofovir tablets in pigtail macaques. In Proceedings of 30th Annual Symposium for Nonhuman Primate Models for AIDS, Abstract 77. San Antonio, TX, USA, 24–27 October 2012.
- Pereira, L.E.; Clark, M.R.; Friend, D.R.; Garber, D.A.; McNicholl, J.M.; Hendry, R.M.; Doncel, G.F.; Smith, J.M. Pharmacokinetic and safety analyses of tenofovir and tenofovir-emtricitabine vaginal tablets in pigtailed macaques. Antimicrob. Agents Chemother. 2014, 58, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.; Cosgrove Sweeney, Y.T.; Friend, D. Preclinical safety and plasma assessments of a vaginal rapid dissolving tablet containing tenofovir (macaca nemestrina model). In Proceedings of the Microbicides 2012, Syndey, Australia, 15–18 April 2012. Poster 27.
- Clark, M.R.; McCormick, T.J.; Doncel, G.; Friend, D.R. Preclinical evaluation of uc781 microbicide vaginal drug delivery. Drug Del. Transl. Res. 2011, 1, 175–182. [Google Scholar] [CrossRef]
- Kiser, P.F.; Mahalingam, A.; Fabian, J.; Smith, E.; Damian, F.R.; Peters, J.J.; Katz, D.F.; Elgendy, H.; Clark, M.R.; Friend, D.R. Design of tenofovir-uc781 combination microbicide vaginal gels. J. Pharm. Sci. 2012, 101, 1852–1864. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.R.; Friend, D.R. Pharmacokinetics and topical vaginal effects of two tenofovir gels in rabbits. AIDS Res. Hum. Retrovir. 2012, 28, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Myrick, F.; Vela, J.; Ray, A.; Borrot-Esoda, K.; Miller, M. In vitro evaluation of the anti-hiv activity and metabolic interactions of tenofovir and emtricitabine. In Proceedings of the The 3rd IAS Conference on HIV Pathogenesis and Treatment, Rio de Janeiro, Brazil, 24–27 July 2005.
- Borroto-Esoda, K.; Vela, J.E.; Myrick, F.; Ray, A.S.; Miller, M.D. In vitro evaluation of the anti-hiv activity and metabolic interactions of tenofovir and emtricitabine. Antivir. Ther. 2006, 11, 377–384. [Google Scholar] [PubMed]
- Dobard, C.; Sharma, S.; Martin, A.; Pau, C.-P.; Holder, A.; Kuklenyik, Z.; Lipscomb, J.; Hanson, D.L.; Smith, J.; Novembre, F.J.; et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J. Virol. 2012, 86, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Parikh, U.M.; Dobard, C.; Sharma, S.; Cong, M.E.; Jia, H.; Martin, A.; Pau, C.P.; Hanson, D.L.; Guenthner, P.; Smith, J.; et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J. Virol. 2009, 83, 10358–10365. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.S.; Kashuba, A.D.; Werner, L.; Karim, Q.A. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: Implications for hiv prevention in women. Lancet 2011, 378, 279–281. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, M.R.; Peet, M.M.; Davis, S.; Doncel, G.F.; Friend, D.R. Evaluation of Rapidly Disintegrating Vaginal Tablets of Tenofovir, Emtricitabine and Their Combination for HIV-1 Prevention. Pharmaceutics 2014, 6, 616-631. https://doi.org/10.3390/pharmaceutics6040616
Clark MR, Peet MM, Davis S, Doncel GF, Friend DR. Evaluation of Rapidly Disintegrating Vaginal Tablets of Tenofovir, Emtricitabine and Their Combination for HIV-1 Prevention. Pharmaceutics. 2014; 6(4):616-631. https://doi.org/10.3390/pharmaceutics6040616
Chicago/Turabian StyleClark, Meredith R., M. Melissa Peet, Sarah Davis, Gustavo F. Doncel, and David R. Friend. 2014. "Evaluation of Rapidly Disintegrating Vaginal Tablets of Tenofovir, Emtricitabine and Their Combination for HIV-1 Prevention" Pharmaceutics 6, no. 4: 616-631. https://doi.org/10.3390/pharmaceutics6040616