Preparation of Spray-Dried Soy Isoflavone-Loaded Gelatin Microspheres for Enhancement of Dissolution: Formulation, Characterization and in Vitro Evaluation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Spray Drying Conditions
2.3. Characterization
2.3.1. Yield, Morphology and Particle Size Analysis
2.3.2. Encapsulation Efficiency and Drug Content
2.3.3. X-ray Diffraction
2.4. Quantification of SI in SE and Microparticle Formulations
2.5. Determination of DAI and GEN in Vitro Release from Formulations
2.6. Mathematical Modeling
2.6.1. Drug-Release Kinetics
2.6.2. Release Profile Comparison
2.7. Statistical Analysis
3. Results and Discussion

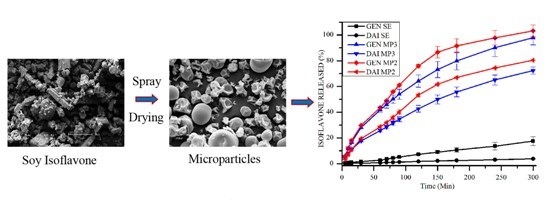
3.1. Yield, Morphology and Particle Size


3.2. Encapsulation Efficiency (EE) and Drug Content
| SI | MP3 | MP2 | ||
|---|---|---|---|---|
| DC (mg/g) | EE (%) | DC (mg/g) | EE (%) | |
| DAI | 90.93 ± 1.67 | 105.67 | 121.23 ± 0.27 | 97.47 |
| GEN | 11.33 ± 0.85 | 95.21 | 15.11 ± 1.39 | 90.22 |
| Total | 102.26 ± 2.52 | 136.34 ± 1.66 | ||
3.3. Powder X-ray Diffraction (XRD)

3.4. Determination of in Vitro DAI and GEN Release from SE and Microparticle Formulations

3.5. Mathematical Modeling
3.6. Drug Release Kinetics
| SI | Test | Zero-Order | First-Order | Higuchi | Hixson–Crowell | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r2 | K0 (mg/min) | r2 | K1 (min−1) | r2 | KH (mg/min½) | r2 | KHC (mg1/3/min) | r2 | n | ||
| DAI | MP2 | 0.929 | 0.273 | 0.991 | −0.006 | 0.986 | 5.478 | 0.978 | 0.007 | 0.993 | 0.802 |
| MP3 | 0.959 | 0.239 | 0.998 | −0.004 | 0.995 | 4.74 | 0.992 | 0.006 | 0.995 | 0.698 | |
| GEN | MP2 | 0.874 | 0.334 | 0.984 | −0.012 | 0.976 | 6.87 | 0.995 | 0.014 | 0.989 | 0.773 |
| MP3 | 0.916 | 0.325 | 0.981 | −0.011 | 0.995 | 6.60 | 0.999 | 0.011 | 0.992 | 0.740 | |
3.7. Comparison of Profile Release
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, N.C.; Jeon, B.J.; Ahn, J.; Kwak, H.S. In vitro study of microencapsulated isoflavone and beta-galactosidase. J. Agric. Food Chem. 2006, 54, 2582–2586. [Google Scholar] [CrossRef]
- Kim, E.H.; Ro, H.M.; Kim, S.L.; Kim, H.S.; Chung, I.M. Analysis of Isoflavone, Phenolic, Soyasapogenol, and Tocopherol Compounds in Soybean [Glycine max (L.) Merrill] Germplasms of Different Seed Weights and Origins. J. Agric. Food Chem. 2012, 60, 6045–6055. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Solid-phase extraction of soy isoflavones. J. Chromatogr. A 2005, 1076, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, K.; Furuta, T.; Yokokawa, A.; Ogura, K.; Hiratsuka, A.; Ishii, K. Plasma profiling of intact isoflavone metabolites by high-performance liquid chromatography and mass spectrometric identification of flavone glycosides daidzin and genistin in human plasma after administration of kinako. Drug Metab. Dispos. 2008, 36, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Vafeiadou, K.; Hallund, J.; Bugel, S.; Koebnick, C.; Reimann, M.; Ferrari, M.; Branca, F.; Talbot, D.; Dadd, T.; et al. Soy-isoflavone-enriched foods and inflammatory biomarkers of cardiovascular disease risk in postmenopausal women: Interactions with genotype and equol production. Am. J. Clin. Nutr. 2005, 82, 1260–1268. [Google Scholar] [PubMed]
- Stancanelli, R.; Mazzaglia, A.; Tommasini, S.; Calabro, M.L.; Villari, V.; Guardo, M.; Ficarra, P.; Ficarra, R. The enhancement of isoflavones water solubility by complexation with modified cyclodextrins: A spectroscopic investigation with implications in the pharmaceutical analysis. J. Pharm. Biomed. Anal. 2007, 44, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Turhan, N.O.; Bolkan, F.; Duvan, C.I.; Ardicoglu, Y. The effect of isoflavones on bone mass and bone remodelling markers in postmenopausal women. Turk. J. Med. Sci. 2008, 38, 145–152. [Google Scholar]
- Yamori, Y. Food factors for atherosclerosis prevention: Asian perspective derived from analyses of worldwide dietary biomarkers. Exp. Clin. Cardiol. 2006, 11, 94–98. [Google Scholar]
- Albertazzi, P.; Purdie, D. The nature and utility of the phytoestrogens: A review of the evidence. Maturitas 2002, 42, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Bao, B.; Ahmad, A.; Sarkar, F.H. Induction of cancer cell death by isoflavone: The role of multiple signaling pathways. Nutrients 2011, 3, 877–896. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.A.; Lozano, M.L.; Castillo, J.; Benavente-Garcia, O.; Vicente, V.; Rivera, J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J. Thromb. Haemost. 2005, 3, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Aubertin-Leheudre, M.; Lord, C.; Khalil, A.; Dionne, I.J. Isoflavones and clinical cardiovascular risk factors in obese postmenopausal women: A randomized double-blind placebo-controlled trial. J. Womens Health 2008, 17, 1363–1369. [Google Scholar] [CrossRef]
- Jeng, T.; Shih, Y.; Wu, M.; Sung, J. Comparisons of flavonoids and anti-oxidative activities in seed coat, embryonic axis and cotyledon of black soybeans. Food Chem. 2010, 123, 112–116. [Google Scholar] [CrossRef]
- Kageyama, A.; Sakakibara, H.; Zhou, W.; Yoshioka, M.; Ohsumi, M.; Shimoi, K.; Yokogoshi, H. Genistein regulated serotonergic activity in the hippocampus of ovariectomized rats under forced swimming stress. Biosci. Biotechnol. Biochem. 2010, 74, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Vargas, L.; Cshmitthenner, A.; Graham, T. Soybean flavonoid effects on and metabolism by Phytophthora sojae. Phytochemistry 1993, 32, 851–857. [Google Scholar] [CrossRef]
- Lee, M.H.; Yu, M.W.; Kao, L.; Lin, C.C. Enhancement of the encapsulation and transmembrane permeation of isoflavone-containing red clover extracts in phospholipid-based microemulsions using different extraction processes. J. Agric. Food Chem. 2009, 57, 9489–9495. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Picerno, P.; Mencherini, T.; Porta, A.; Lauro, M.R.; Russo, P.; Aquino, R.P. Technological properties and enhancement of antifungical activity of a Paeonia rockii extract encapsulated in a chitosan-based matrix. J. Food Eng. 2013, 120, 260–267. [Google Scholar] [CrossRef]
- Motlekar, N.; Khan, M.; Youan, B. Preparation and characterization of genistein containing poly(ethylene glycol) microparticles. J. Appl.Polym. Sci. 2006, 101, 2070–2078. [Google Scholar] [CrossRef]
- Chen, F.; Peng, J.; Lei, D.; Liu, J.; Zhao, G. Optimization of genistein solubilization by κ-carrageenan hydrogel using response surface methodology. Food Sci. Hum. Wellness 2013, 2, 124–131. [Google Scholar] [CrossRef]
- Cannava, C.; Crupi, V.; Ficarra, P.; Guardo, M.; Majolino, D.; Mazzaglia, A.; Stancanelli, R.; Venuti, V. Physico-chemical characterization of an amphiphilic cyclodextrin/genistein complex. J. Pharm. Biomed. Anal. 2010, 51, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Yatsu, F.K.; Koester, L.S.; Lula, I.; Passos, J.J.; Sinisterra, R.; Bassani, V.L. Multiple complexation of cyclodextrin with soy isoflavones present in an enriched fraction. Carbohydr. Polym. 2013, 98, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Hong, M.; Liu, C.; Pei, Y. Application of Box-Behnken design in understanding the quality of genistein self-nanoemulsified drug delivery systems and optimizing its formulation. Pharm. Dev. Technol. 2009, 14, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, N.; Ji, H.; Liu, H.; Wang, Z.; Wu, L. Eudragit nanoparticles containing genistein: Formulation, development, and bioavailability assessment. Int. J. Nanomed. 2011, 6, 2429–2435. [Google Scholar]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L.; Cardoso, M.L.; Lucchesi, M.B.; Gremiao, M.P. Gelatin microparticles containing propolis obtained by spray-drying technique: Preparation and characterization. Int. J. Pharm. 2003, 264, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Picerno, P.; Mencherini, T.; Russo, P.; Gasparri, F.; Giannini, V.; Lauro, M.R.; Puglisi, G.; Aquino, R.P. Enhanced technological and permeation properties of a microencapsulated soy isoflavones extract. J. Food Eng. 2013, 115, 298–305. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- ICH. ICH harmonized tripartite guideline validation of analytical procedures: text and methodology Q2 (R1). In Proceedings of International conference on the harmonization requirements for the registration of pharmaceuticals for human use, Geneva, Switzerland, 2005.
- Agência Nacional de Vigilância Sanitária. RE 899/2003-ANVISA; Agência Nacional de Vigilância Sanitária: Brasilia, DF, Brazil, 2003. [Google Scholar]
- The United States Pharmacopeial Convention. United States Pharmacopeia and National Formulary USP 32–NF 27; The United States Pharmacopeial Convention: Rockville, MD, USA, 2009. [Google Scholar]
- Sansone, F.; Picerno, P.; Mencherini, T.; Villecco, F.; D’Ursi, A.; Aquino, R.; Lauro, M. Flavonoid microparticles by spray drying: Influence of enhancers of the dissolution rate on properties and stability. J. Food Eng. 2011, 103, 188–196. [Google Scholar] [CrossRef]
- Fernandez-Perez, V.; Tapaidor, J.; Martin, A.; Luque de Castro, M.D. Optimization of the drying step for preparing a new commercial powdered soup. Innov. Food Sci. Emerg. Technol. 2004, 8, 361–368. [Google Scholar] [CrossRef]
- Shu, B.; Yu, W.; Zhao, Y.; Liu, X. Study on microencapsulation of lycopene by spray-drying. J. Food Eng. 2006, 76, 664–669. [Google Scholar] [CrossRef]
- Suhimi, N.; Mohammad, A. A Study on Spray Dried Gelatine: Effect of Feed Concentration. J. Appl. Sci. 2011, 11, 2431–2435. [Google Scholar] [CrossRef]
- Esposito, E.; Cervellati, F.; Menegatti, E.; Nastruzzi, C.; Cortesi, R. Spray dried Eudragit microparticles as encapsulation devices for vitamin C. Int. J. Pharm. 2002, 242, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Mencherini, T.; Picerno, P.; D’Amore, M.; Aquino, R.; Lauro, M. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Fahr, A.; Liu, X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin. Drug Deliv. 2007, 4, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.R.; Hung, C.F.; Lin, Y.K.; Fang, J.Y. In vitro and in vivo evaluation of topical delivery and potential dermal use of soy isoflavones genistein and daidzein. Int. J. Pharm. 2008, 364, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhargava, D.; Thakkar, A.; Arora, S. Drug carrier systems for solubility enhancement of BCS class II drugs: A critical review. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 217–256. [Google Scholar] [CrossRef] [PubMed]
- Brown, W. Apparatus 4 flow through cell: Some thoughts on operational characteristics. Dissolution Tecnol. 2005, 12, 28–30. [Google Scholar]
- Fotaki, N.; Reppas, C. The flow through cell methodology in the evaluation of intralumenal drug release characteristics. Dissolution Tecnol. 2005, 12, 17–21. [Google Scholar]
- Ishii, K.; Saito, Y.; Itai, S.; Takayama, K.; Nagai, T. In vitro dissolution test corresponding to in vivo dissolution of sofalcone formulations. STP Pharm. Sci. 1997, 7, 270–276. [Google Scholar]
- Coldham, N.G.; Zhang, A.Q.; Key, P.; Sauer, M.J. Absolute bioavailability of [14C] genistein in the rat; plasma pharmacokinetics of parent compound, genistein glucuronide and total radioactivity. Eur. J. Drug Metab. Pharmacokinet. 2002, 27, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Mallis, L.M.; Sarkahian, A.B.; Harris, H.A.; Zhang, M.Y.; McConnell, O.J. Determination of rat oral bioavailability of soy-derived phytoestrogens using an automated on-column extraction procedure and electrospray tandem mass spectrometry. J. Chromatogr. B 2003, 796, 71–86. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Hixson, A.W.; Crowell, J.H. Dependence of reaction velocity upon surface and agitation I-Theorical consideration. Ind. Eng. Chem. 1931, 23, 924–931. [Google Scholar] [CrossRef]
- Sitta, D.L.; Guilherme, M.R.; da Silva, E.P.; Valente, A.J.; Muniz, E.C.; Rubira, A.F. Drug release mechanisms of chemically cross-linked albumin microparticles: Effect of the matrix erosion. Colloids Surf. B 2014, 122, 404–413. [Google Scholar] [CrossRef]
- Reza, M.S.; Quadir, M.A.; Haider, S.S. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. J. Pharm. Pharm. Sci. 2003, 6, 282–291. [Google Scholar] [PubMed]
- Kavanagh, N.; Corrigan, O.I. Swelling and erosion properties of hydroxypropylmethylcellulose (Hypromellose) matrices—Influence of agitation rate and dissolution medium composition. Int. J. Pharm. 2004, 279, 141–152. [Google Scholar] [CrossRef]
- Klech, C.M.; Li, X.M. Consideration of drug load on the swelling kinetics of glassy gelatin matrices. J. Pharm. Sci. 1990, 79, 999–1004. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panizzon, G.P.; Bueno, F.G.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P. Preparation of Spray-Dried Soy Isoflavone-Loaded Gelatin Microspheres for Enhancement of Dissolution: Formulation, Characterization and in Vitro Evaluation. Pharmaceutics 2014, 6, 599-615. https://doi.org/10.3390/pharmaceutics6040599
Panizzon GP, Bueno FG, Ueda-Nakamura T, Nakamura CV, Dias Filho BP. Preparation of Spray-Dried Soy Isoflavone-Loaded Gelatin Microspheres for Enhancement of Dissolution: Formulation, Characterization and in Vitro Evaluation. Pharmaceutics. 2014; 6(4):599-615. https://doi.org/10.3390/pharmaceutics6040599
Chicago/Turabian StylePanizzon, Gean Pier, Fernanda Giacomini Bueno, Tânia Ueda-Nakamura, Celso Vataru Nakamura, and Benedito Prado Dias Filho. 2014. "Preparation of Spray-Dried Soy Isoflavone-Loaded Gelatin Microspheres for Enhancement of Dissolution: Formulation, Characterization and in Vitro Evaluation" Pharmaceutics 6, no. 4: 599-615. https://doi.org/10.3390/pharmaceutics6040599