Drug-Eluting Nasal Implants: Formulation, Characterization, Clinical Applications and Challenges
Abstract
:1. Introduction
1.1. Anatomy and Physiology of the Nose and Paranasal Sinuses

1.2. Current Strategies to Deliver Drugs to the Nasal Sinuses and the Need for Improvement
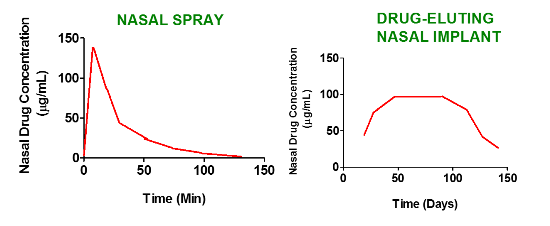
1.3. Drug Eluting Stents and Implants: Definition and Nomenclature

2. Formulation and Development of Nasal Implants
2.1. Development of Implants and the Need for Biodegradable/Bioabsorbable Impants
2.2. Formulation Considerations, Biodegradable Materials and Drug Candidates for Formulating Nasal Drug-Eluting Stents


2.3. Formulation Methods for Biodegradable Nasal Implants
2.4. Methods for Developing Nano/Microparticles/Polymer Composites for Drug-Eluting Implants
3. Characterization of Drug-Eluting Nasal Implants
4. Clinical Applications of Drug-Eluting Nasal Implants
4.1. Drug Delivery Applications of Middle Meatus Implants
4.1.1. Propel™ Sinus Implant

4.1.2. Relieva Stratus™ Microflow Spacer

4.1.3. Sinu-Foam™ Spacer
5. Challenges and Future Considerations
| FDA Approved Nasal Stents/Implants | Advantages | Limitations |
|---|---|---|
| Propel™ sinus implant | Reduces inflammation associated with CRS and promotes wound healing; Implant is made of PLGA (a biodegradable polymer) and is the first and the only FDA approved biodegradable implant for the treatment of CRS; Does not require a second surgical procedure to remove the implant; Releases the drug slowly and continuously for over a month | Not approved for use outside the USA; Not suitable for patients with intolerance to mometasone furoate; Mometasone dose is quite low (370 µg) and may be ineffective in patients with advanced stages of CRS |
| Relieva stratus™ MicroFlow spacer | Potential to reduce chronic inflammation. Minimal invasive approach for targeted local delivery of therapeutic agents slowly and continuously to the site of action | Can be implanted only for 14–28 days; Requires a second surgical procedure for implant removal; FDA-approved for use only with saline |
| Sinu-Foam™ spacer | Promotes wound healing and reduces chronic inflammation of sinus and nasal mucosa | Clinical utility remains in doubt due to variable outcomes |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Watelet, J.B.; van Cauwenberge, P. Applied anatomy and physiology of the nose and paranasal sinuses. Allergy 1999, 54, 14–25. [Google Scholar] [CrossRef]
- Van Cauwenberge, P.; Sys, L.; de Belder, T.; Watelet, J.B. Anatomy and physiology of the nose and the paranasal sinuses. Immunol. Allergy Clin. N. Am. 2004, 24, 1–17. [Google Scholar] [CrossRef]
- Jones, N. The nose and paranasal sinuses physiology and anatomy. Adv. Drug Deliv. Rev. 2001, 51, 5–19. [Google Scholar] [CrossRef]
- Singh, A. Paranasal Sinus Anatomy. Available online: http://emedicine.medscape.com/article/1899145-overview (accessed on 9 January 2014).
- Gupta, A.; Mercurio, E.; Bielamowicz, S. Endoscopic inferior turbinate reduction: An outcomes analysis. Laryngoscope 2001, 111, 1957–1959. [Google Scholar] [CrossRef]
- Tan, B.K.; Zirkle, W.; Chandra, R.K.; Lin, D.; Conley, D.B.; Peters, A.T.; Grammer, L.C.; Schleimer, R.P.; Kern, R.C. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2011, 1, 88–94. [Google Scholar]
- Al Badaai, Y.; Samaha, M. Outcome of endoscopic sinus surgery for chronic rhinosinusitis patients: A Canadian experience. J. Laryngol. Otol. 2010, 124, 1095–1099. [Google Scholar] [CrossRef]
- Chan, Y.; Kuhn, F.A. An update on the classifications, diagnosis, and treatment of rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2009, 17, 204–208. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Alobid, I.; Mullol, J. Controversies in the treatment of chronic rhinosinusitis. Expert Rev. Respir. Med. 2010, 4, 463–477. [Google Scholar] [CrossRef]
- Jarvis, D.; Newson, R.; Lotvall, J.; Hastan, D.; Tomassen, P.; Keil, T.; Gjomarkaj, M.; Forsberg, B.; Gunnbjornsdottir, M.; Minov, J.; et al. Asthma in adults and its association with chronic rhinosinusitis: The GA2LEN survey in Europe. Allergy 2012, 67, 91–98. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Otto, B.A.; Pant, H. When surgery, antibiotics, and steroids fail to resolve chronic rhinosinusitis. Immunol. Allergy Clin. N. Am. 2009, 29, 719–732. [Google Scholar] [CrossRef]
- Sedaghat, A.R.; Gray, S.T.; Wilke, C.O.; Caradonna, D.S. Risk factors for development of chronic rhinosinusitis in patients with allergic rhinitis. Int. Forum Allergy Rhinol. 2012, 2, 370–375. [Google Scholar] [CrossRef]
- Côté, D.W.; Wright, E.D. Triamcinolone-impregnated nasal dressing following endoscopic sinus surgery: A randomized, double-blind, placebo-controlled study. Laryngoscope 2010, 120, 1269–1273. [Google Scholar]
- Albu, S. Novel drug-delivery systems for patients with chronic rhinosinusitis. Drug Des. Devel. Ther. 2012, 6, 125–132. [Google Scholar] [CrossRef]
- Durand, M.; le Guellec, S.; Pourchez, J.; Dubois, F.; Aubert, G.; Chantrel, G.; Vecellio, L.; Hupin, C.; de Gersem, R.; Reychler, G.; et al. Sonic aerosol therapy to target maxillary sinuses. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Chronic Sinusitis. Available online: http://www.patient.co.uk/health/Sinusitis-Chronic.htm (accessed on 30 November 2013).
- Lin, D.C.; Chandra, R.K.; Tan, B.K.; Zirkle, W.; Conley, D.B.; Grammer, L.C.; Kern, R.C.; Schleimer, R.P.; Peters, A.T. Association between severity of asthma and degree of chronic rhinosinusitis. Am. J. Rhinol. Allergy 2011, 25, 205–208. [Google Scholar] [CrossRef]
- MedlinePlus. Stent. Available online: http://www.nlm.nih.gov/medlineplus/ency/article/002303.htm (accessed on 18 March 2014).
- US Food and Drug Administration. Medical Devices. Available online: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/InvestigationalDeviceExemptionIDE/ucm046698.htm (accessed on 17 March 2014).
- Zilberman, M.; Kraitzer, A.; Grinberg, O.; Elsner, J.J. Drug-eluting medical implants. Handb. Exp. Pharmacol. 2010, 197, 299–341. [Google Scholar] [CrossRef]
- Park, J.B.; Lakes, R.S. Soft Tissue Replacement-II: Blood Interfacing Implants. In Biomaterials an Introductionx, 3rd ed.; Springer: New York, NY, USA, 2007; pp. 331–367. [Google Scholar]
- Biomedical Implants. Available online: http://www.bmecentral.com/implants.html (accessed on 24 December 2013).
- Stanford, C.M. Application of oral implants to the general dental practice. J. Am. Dent. Assoc. 2005, 136, 1092–1100. [Google Scholar] [CrossRef]
- National Institute of Deafness and Other Communication Disorders. Cochlear Implants. Available online: http://www.nidcd.nih.gov/health/hearing/pages/coch.aspx (accessed on 18 July 2013).
- SciTechStory. Body Implants. Available online: http://scitechstory.com/impact-areas/body-implants/ (accessed on 30 November 2013).
- Miller, A. Retinal implant system delivers limited sight to some blind people. CMAJ 2013, 185, E659–E660. [Google Scholar] [CrossRef]
- Sclafani, A.P. Nasal Implants. Available online: http://emedicine.medscape.com/article/843111-overview (accessed on 18 October 2013).
- Sinusitis-Treatment. Available online: http://www.nhs.uk/Conditions/Sinusitis/Pages/Treatment.aspx (accessed on 23 December 2013).
- Steroid Nasal Sprays. Available online: http://www.patient.co.uk/health/steroid-nasal-sprays (accessed on 14 January 2013).
- König, A.; Schiele, T.M.; Rieber, J.; Theisen, K.; Mudra, H.; Klauss, V. Influence of stent design and deployment technique on neointima formation and vascular remodeling. Z. Kardiol. 2002, 91, 98–102. [Google Scholar] [CrossRef]
- Waksman, R. Biodegradable stents: They do their job and disappear. J. Invasive Cardiol. 2006, 18, 70–74. [Google Scholar]
- Cutlip, D.; Abbott, J.D. Drug-Eluting Compared to Bare Metal Intracoronary Stents. Available online: http://www.uptodate.com/contents/drug-eluting-compared-to-bare-metal-intracoronary-stents (accessed on 19 October 2013).
- Virmani, R.; Farb, A.; Guagliumi, G.; Kolodgie, F.D. Drug-eluting stents: Caution and concerns for long-term outcome. Coron. Artery Dis. 2004, 15, 313–318. [Google Scholar] [CrossRef]
- Hickey, T.; Kreutzer, D.; Burgess, D.J.; Moussy, F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials 2002, 23, 1649–1656. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Sura, R.; Papadimitrakopoulos, F.; Burgess, D.J. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. Int. J. Pharm. 2010, 384, 78–86. [Google Scholar] [CrossRef]
- Margolis, J.R. The excel stent: A good DES, but can we really stop clopidogrel after 6 months? JACC Cardiovasc. Interv. 2009, 2, 310–311. [Google Scholar] [CrossRef]
- Uurto, I.; Mikkonen, J.; Parkkinen, J.; Keski-Nisula, L.; Nevalainen, T.; Kellomäki, M.; Törmälä, P.; Salenius, J.P. Drug-eluting biodegradable poly-d/l-lactic acid vascular stents: An experimental pilot study. J. Endovasc. Ther. 2005, 12, 371–379. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Lai, S.K.; Suk, J.S.; Pace, A.; Wang, Y.Y.; Yang, M.; Mert, O.; Chen, J.; Kim, J.; Hanes, J. Drug carrier nanoparticles that penetrate human chronic rhinosinusitis mucus. Biomaterials 2011, 32, 6285–6290. [Google Scholar]
- Med-Tech Innovation. Drug-Eluting Bioresorbable Stents. Available online: http://www.med-techinnovation.com/Articles/articles/article/25 (accessed on 10 July 2013).
- Gao, S.Q.; Maeda, T.; Okano, K.; Palczewski, K. A microparticle/hydrogel combination drug-delivery system for sustained release of retinoids. Invest. Ophthalmol. Vis. Sci. 2012, 53, 6314–6323. [Google Scholar] [CrossRef]
- Schramm, C.; Spitzer, M.S.; Henke-Fahle, S.; Steinmetz, G.; Januschowski, K.; Heiduschka, P.; Geis-Gerstorfer, J.; Biedermann, T.; Bartz-Schmidt, K.U.; Szurman, P. The cross-linked biopolymer hyaluronic acid as an artificial vitreous substitute. Invest. Ophthalmol. Vis. Sci. 2012, 53, 613–621. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.T.; Zu, Z.H.; Ju, R.K.; Guo, M.Y.; Wang, X.M.; Xu, Q.Y.; Cui, F.Z. Combination of hyaluronic acid/hydrogel scaffold and PLGA microspheres for supporting survival of neural stem cells. Pharm. Res. 2011, 28, 1406–1414. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Zhu, C.; Zhang, Y.; Fu, C.; Yang, B.; Tao, L.; Wei, Y. Nonionic polymer cross-linked chitosan hydrogel: preparation and bioevaluation. J. Biomater. Sci. Polym. Ed. 2013, 24, 1564–1574. [Google Scholar] [CrossRef]
- Mayo Clinic. Chronic Sinusitis. Available online: http://www.mayoclinic.org/diseases-conditions/chronic-sinusitis/basics/treatment/con-20022039 (accessed on 10 August 2013).
- Hosemann, W.; Schindler, E.; Wiegrebe, E.; Göpferich, A. Innovative frontal sinus stent acting as a local drug-releasing system. Eur. Arch. Otorhinolaryngol. 2003, 260, 131–134. [Google Scholar]
- Shuwisitkul, D. Biodegradable implants with different drug release profiles. Inaugural-Dissertation, Department of biology, chemistry and pharmacy of Freie Universität: Berlin, Germany, May 2011. Available online: http://www.diss.fu-berlin.de/diss/servlets/MCRFileNodeServlet/FUDISS_derivate_000000009580/Duangrat_thesis_online.pdf?hosts= (accessed on 12 January 2014).
- Widmer, M.S.; Gupta, P.K.; Lu, L.; Meszlenyi, R.K.; Evans, G.R.; Brandt, K.; Savel, T.; Gurlek, A.; Patrick, C.W., Jr.; Mikos, A.G. Manufacture of porous biodegradable polymer conduits by an extrusion process for guided tissue regeneration. Biomaterials 1998, 19, 1945–1955. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Meyer, R.F.; Liang, Y.; Winey, K.I.; Siegel, S.J. A rapid method for creating drug implants: Translating laboratory-based methods into a scalable manufacturing process. J. Biomed. Mater. Res. Part B 2010, 93, 562–572. [Google Scholar]
- Rabin, C.; Liang, Y.; Ehrlichman, R.S.; Budhian, A.; Metzger, K.L.; Majewski-Tiedeken, C.; Winey, K.I.; Siegel, S.J. In vitro and in vivo demonstration of risperidone implants in mice. Schizophr. Res. 2008, 98, 66–78. [Google Scholar]
- Siegel, S.J.; Kahn, J.B.; Metzger, K.; Winey, K.I.; Werner, K.; Dan, N. Effect of drug type on the degradation rate of PLGA matrices. Eur. J. Pharm. Biopharm. 2006, 64, 287–293. [Google Scholar] [CrossRef]
- Fan, M.; Guo, Q.; Luo, J.; Luo, F.; Xie, P.; Tang, X.; Qian, Z. Preparation and in vitro characterization of dexamethasone-loaded poly(d,l-lactic acid) microspheres embedded in poly(ethylene glycol)-poly[(varepsilon)-caprolactone]-poly(ethylene glycol) hydrogel for orthopedic tissue engineering. J. Biomater. Appl. 2013, 28, 288–297. [Google Scholar] [CrossRef]
- Cascone, M.G.; Pot, P.M.; Lazzeri, L.; Zhu, Z. Release of dexamethasone from PLGA nanopartilces entrapped into dextran/poly(vinyl) alcohol hydrogels. J. Mater. Sci. Mater. Med. 2002, 13, 265–269. [Google Scholar] [CrossRef]
- Galeska, I.; Kim, T.K.; Patil, S.D.; Bhardwaj, U.; Chatttopadhyay, D.; Papadimitrakopoulos, F.; Burgess, D.J. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVAhydrogels. AAPS J. 2005, 7, E231–E240. [Google Scholar] [CrossRef]
- Ju, Y.M.; Yu, B.; West, L.; Moussy, Y.; Moussy, F. A dexamethasone loaded PLGA microspheres/collagen scaffold composite for implantable glucose sensors. J. Biomed. Mater. Res. Part A 2010, 93, 200–210. [Google Scholar]
- Sanginario, V.; Ginebra, M.P.; Tanner, K.E.; Planell, J.A.; Ambrosio, L. Biodegradable and semi-biodegradable composite hydrogels as bone substitutes: Morphology and mechanical characterization. J. Mater. Sci. Mater. Med. 2006, 17, 447–454. [Google Scholar] [CrossRef]
- Lee, A.G.; Arena, C.P.; Beebe, D.J.; Palecek, S.P. Development of macroporous poly(ethylene glycol) hydrogel arrays within microfluidic channels. Biomacromolecules 2010, 11, 3316–3324. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Carpi, A., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Bleier, B.S.; Paulson, D.P.; O’Malley, B.W.; Li, D.; Palmer, J.N.; Chiu, A.G.; Cohen, N.A. Chitosan glycerophosphate-based semirigid dexamethasone eluting biodegradable stent. Am. J. Rhinol. Allergy 2009, 23, 76–79. [Google Scholar] [CrossRef]
- Perez, A.C.; Cunha Junior Ada, S.; Fialho, S.L.; Silva, L.M.; Dorgam, J.V.; Murashima Ade, A.; Silva, A.R.; Rossato, M.; Anselmo-Lima, W.T. Assessing the maxillary sinus mucosa of rabbits in the presence of biodegradable implants. Braz. J. Otorhinolaryngol. 2012, 78, 40–46. [Google Scholar] [Green Version]
- Chiu, A.G.; Antunes, M.B.; Feldman, M.; Cohen, N.A. An animal model for the study of topical medications in sinusitis. Am. J. Rhinol. 2007, 21, 5–9. [Google Scholar] [CrossRef]
- Kara, C.O. Animal models of sinusitis: Relevance to human disease. Curr. Allergy Asthma Rep. 2004, 4, 496–499. [Google Scholar] [CrossRef]
- Bednarski, K.A.; Kuhn, F.A. Stents and drug-eluting stents. Otolaryngol. Clin. N. Am. 2009, 42, 857–866. [Google Scholar] [CrossRef]
- Tan, B.K.; Lane, A.P. Endoscopic sinus surgery in the management of nasal obstruction. Otolaryngol. Clin. N. Am. 2009, 42, 227–240. [Google Scholar] [CrossRef]
- Kuhn, F.A.; Church, C.A.; Goldberg, A.N.; Levine, H.L.; Sillers, M.J.; Vaughan, W.C.; Weiss, R.L. Balloon catheter sinusotomy: One-year follow-up—Outcomes and role in functional endoscopic sinus surgery. Otolaryngol. Head Neck Surg. 2008, 139, S27–S37. [Google Scholar] [CrossRef]
- Removal of Nasal Adhesions. Surgery overview. Available online: http://www.webmd.com/a-to-z-guides/removal-of-nasal-adhesions-surgery-overview (accessed on 30 November 2013).
- Weitzel, E.K.; Wormald, P.J. A scientific review of middle meatal packing/stents. Am. J. Rhinol. 2008, 22, 302–307. [Google Scholar] [CrossRef]
- Weber, R.; Hochapfel, F.; Draf, W. Packing and stents in endonasal surgery. Rhinology 2000, 38, 49–62. [Google Scholar]
- Murr, A.H.; Smith, T.L.; Hwang, P.H.; Bhattacharyya, N.; Lanier, B.J.; Stambaugh, J.W.; Mugglin, A.S. Safety and efficacy of a novel bioabsorbable, steroid-eluting sinus stent. Int. Forum Allergy Rhinol. 2011, 1, 23–32. [Google Scholar]
- Desrosiers, M.; Evans, G.A.; Keith, P.K.; Wright, E.D.; Kaplan, A.; Bouchard, J.; Ciavarella, A.; Doyle, P.W.; Javer, A.R.; Leith, E.S.; et al. Canadian clinical practice guidelines for acute and chronic rhinosinusitis. J. Otolaryngol. Head Neck Surg. 2011, 40, S99–S193. [Google Scholar]
- Li, P.M.; Downie, D.; Hwang, P.H. Controlled steroid delivery via bioabsorbable stent: Safety and performance in a rabbit model. Am. J. Rhinol. Allergy 2009, 23, 591–596. [Google Scholar] [CrossRef]
- Kennedy, D.W. The PROPEL™ steroid-releasing bioabsorbable implant to improve outcomes of sinus surgery. Expert Rev. Respir. Med. 2012, 6, 493–498. [Google Scholar] [CrossRef]
- Catalano, P.J.; Thong, M.; Weiss, R.; Rimash, T. The MicroFlow Spacer: A Drug-Eluting stent for the Ethmoid Sinus. Indian J. Otolaryngol. Head Neck Surg. 2011, 63, 279–284. [Google Scholar] [CrossRef]
- Effect of Sinufoam-Dxamethasone Mixture on Post Endoscopic Sinus Surgery Outcomes. Available online: http://clinicaltrials.gov/show/NCT01024075 (accessed on 28 October 2013).
- Clinical Policy Bulletin: Devices for Post-Operative Use Following Endoscopic Sinus Surgery. Available online: http://www.aetna.com/cpb/medical/data/800_899/0840.html (accessed on 28 October 2013).
- Propel Steroid-Releasing Implant. Available online: http://www.intersectent.com/advantage-propel_mini.html (accessed on 1 November 2013).
- FDA Approves Propel Mini for Chronic Sinusitis. Physician’s weekly. November 2012. Available online: http://www.physiciansweekly.com/propel-mini-chronic-sinusitis/ (accessed on 1 November 2013).
- PROPEL®. Intersect ENT. Available online: http://www.intersectent.com/docs/Propel_IFU.pdf (accessed on 4 January 2014).
- Product brief. Propel (Intersect ENT, Inc.) Steroid-eluting implant for maintaining sinus patency after ethmoid sinus surgery. Available online: https://www.ecri.org/Documents/Sample_Reports/Product_Brief_Propel.pdf (accessed on 18 March 2014).
- Available online: http://www.businesswire.com/news/home/20110913007026/en/Intersect-ENT-Announces-Positive-Data-Pivotal-Study#.UyXdBfldUUg (accessed on 16 March 2014).
- ENT today. Drug-Eluting Sinus Stent Hits the Market: May help maintain patency after FESS. Available online: http://www.enttoday.org/details/article/1420005/Drug-Eluting_Sinus_Stent_Hits_the_Market_May_help_maintain_patency_after_FESS.html (accessed on 10 August 2013).
- Newsletters: New ethmoid spacer for drug delivery. Available online: https://med.uth.edu/orl/newsletter/ethmoid-spacer-drug-delivery/ (accessed on 19 May 2014).
- Premera Blue Cross. Medical Policy. Available online: https://www.premera.com/medicalpolicies/cmi_136631.htm (accessed on 16 March 2014).
- US Food and Drug Administration. Maude adverse event report: Acclarent, inc.relieva stratus microflow spacer stratus ethmoid spacer. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/detail.cfm?mdrfoi__id=1461307 (accessed on 15 January 2014).
- Acclarent. Instructions for Use. Available online: https://www.jnjgatewayifu.com/eLabelingContent/Acc/USENG/IFU005013_Rev_F_Stratus_81590.pdf (accessed on 18 March 2014).
- Shikani, A. A new middle meatal antrostomy stent for functional endoscopic sinus surgery. Laryngoscope 1994, 104, 638–641. [Google Scholar] [CrossRef]
- Chadwell, J.S.; Gustafson, L.M.; Tami, T.A. Toxic shock syndrome associated with frontal sinus stents. Otolaryngol. Head Neck Surg. 2001, 124, 573–574. [Google Scholar] [CrossRef]
- Implantable Sinus Spacers and Stents for Postoperative Use Following Endoscopic Sinus Surgery. Medical policy. Available online: https://www.bcidaho.com/providers/medical_policies/sur/mp_701134.asp (accessed on 12 August 2013).
- Wei, C.C.; Kennedy, D.W. Mometasone implant for chronic rhinosinusitis. Med. Devices 2012, 5, 75–80. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Parikh, A.; Anand, U.; Ugwu, M.C.; Feridooni, T.; Massoud, E.; Agu, R.U. Drug-Eluting Nasal Implants: Formulation, Characterization, Clinical Applications and Challenges. Pharmaceutics 2014, 6, 249-267. https://doi.org/10.3390/pharmaceutics6020249
Parikh A, Anand U, Ugwu MC, Feridooni T, Massoud E, Agu RU. Drug-Eluting Nasal Implants: Formulation, Characterization, Clinical Applications and Challenges. Pharmaceutics. 2014; 6(2):249-267. https://doi.org/10.3390/pharmaceutics6020249
Chicago/Turabian StyleParikh, Ankit, Utkarshini Anand, Malachy C. Ugwu, Tiam Feridooni, Emad Massoud, and Remigius U. Agu. 2014. "Drug-Eluting Nasal Implants: Formulation, Characterization, Clinical Applications and Challenges" Pharmaceutics 6, no. 2: 249-267. https://doi.org/10.3390/pharmaceutics6020249