Human Growth Hormone Delivery with a Microneedle Transdermal System: Preclinical Formulation, Stability, Delivery and PK of Therapeutically Relevant Doses
Abstract
:1. Introduction

2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Diafiltration and Concentration
2.2.2. Lyophilization
2.2.3. Size Exclusion Chromatography (SEC)
2.2.4. Recombinant Human Growth Hormone (rhGH) Purity Determination
2.2.5. rhGH Content Quantification
2.2.6. Rheology
2.2.7. Scanning Electron Microscopy (SEM)
2.2.8. Contact Angle Measurement
2.2.9. Microneedle Array Coating and Packaging
2.2.10. Stability Experiments on Packaged Drug-Coated Microneedle Delivery Systems
2.3. Preclinical Pharmacokinetic Studies
2.3.1. Animal Model and Delivery Preparation
2.3.2. Zosano Pharma (ZP)-hGH Patch Application, Sub-Cutaneous (SC) and Intravenous (IV) Dosing
2.3.3. Pharmacokinetic Studies (PK)
2.3.4. Statistical Analysis
3. Results and Discussion
3.1. Formulation Characterization, Microneedle Coating and Stability



3.2. rhGH-Coated Microneedle Delivery Performance and Release Kinetics

3.3. Pharmacokinetic Data
 0.5 mg and
0.5 mg and  1 mg); Norditropin® SC injection
1 mg); Norditropin® SC injection  0.4 mg,
0.4 mg,  0.8 mg and
0.8 mg and  1.4 mg).
1.4 mg).
 0.5 mg and
0.5 mg and  1 mg); Norditropin® SC injection
1 mg); Norditropin® SC injection  0.4 mg,
0.4 mg,  0.8 mg and
0.8 mg and  1.4 mg).
1.4 mg).
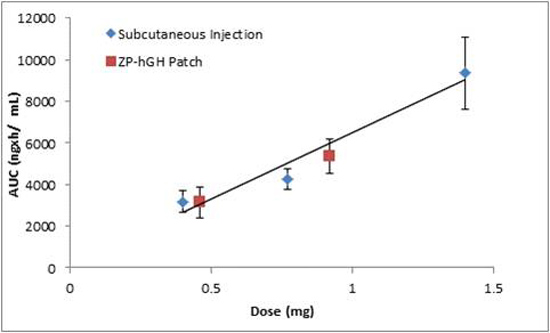
 ) vs. Norditropin® SC injection over the range of 0.4 to 1.4 mg (
) vs. Norditropin® SC injection over the range of 0.4 to 1.4 mg (  ).
).
 ) vs. Norditropin® SC injection over the range of 0.4 to 1.4 mg (
) vs. Norditropin® SC injection over the range of 0.4 to 1.4 mg (  ).
).
| PK parameters | IV | ZP-hGH | SC Norditropin | |||
|---|---|---|---|---|---|---|
| Dose (mg) | 0.05 | 0.5 | 1 | 0.4 | 0.8 | 1.4 |
| Cmax (ng/mL) | 4,766 ± 421 | 1,185 ± 207 | 2,799 ± 223 | 967 ± 133 | 1,418 ± 187 | 3,193 ± 613 |
| Tmax (min) | 1 | 30 | 30 | 120 | 60 | 120 |
| T1/2 (min) | 25 ± 2.5 | 67 ± 13 | 73 ± 12 | 151 ± 38 | 291 ± 34 | 362 ± 19 |
| AUCt (ng × h/mL) | 1,105 ± 145 | 2,774 ± 730 | 5,352 ± 924 | 3,171 ± 530 | 4,270 ± 513 | 9,352 ± 1753 |
| Absolute Bioavailability (%) * | Reference | 27 ± 6 | 26 ± 4 | 36 ± 5 | 24 ± 2 | 30 ± 4 |
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Singh, R.; Singh, S.; Lillard, J.W., Jr. Past, present and future technologies for oral delivery of therapeutic proteins. J. Pharm. Sci. 2008, 97, 2497–2523. [Google Scholar] [CrossRef]
- Park, K.; Kwon, I.C.; Park, K. Oral protein delivery: Current status and future prospects. React. Funct. Polym. 2011, 71, 280–287. [Google Scholar] [CrossRef]
- Florence, A.T.; Attwood, D. Physicochemical Principles of Pharmacy, 3rd ed.; Macmillan Press: London, UK, 1998; pp. 403–447. [Google Scholar]
- Cornaz, A.L.; Buri, P. Nasal mucosa as an absorption barrier. Eur. J. Pharm. Biopharm. 1994, 40, 261–270. [Google Scholar]
- Wall, D.A. Pulmonary absorption of peptides and proteins. Pharm. Technol. 1995, 2, 1–20. [Google Scholar]
- Kligman, A.M. Topical pharmacology and toxicology of dimethylsulfoxide. J. Am. Med. Assoc. 1965, 193, 796–804. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Summer, S.; Heinrich, A.; Bragagna, T.; Boehler, C.; Kalia, Y.N. Minimally invasive delivery of peptides and proteins across the skin using P.L.E.A.S.E.® technology. Available online: http://abstracts.aaps.org/SecureView/AAPSJournal/innwg2arlenhmg12c1zw.pdf (accessed on 7 February 2014).
- Levin, G.; Gershonowitz, A.; Sacks, H.; Stern, M.; Sherman, A.; Rudaev, S.; Zivin, I.; Phillip, M. Transdermal delivery of human growth hormone through RF-microchannels. Pharm. Res. 2005, 22, 550–555. [Google Scholar] [CrossRef]
- Badkar, A.; Smith, A.; Eppstein, J.; Banga, A. Transdermal delivery of interferon α-2B using microporation and iontophoresis in hairless rats. Pharm. Res. 2007, 24, 1389–1395. [Google Scholar] [CrossRef]
- Han, T.; Das, D.B. Permeability enhancement for transdermal delivery of large molecule using low-frequency sonophoresis combined with microneedles. J. Pharm. Sci. 2013, 102, 3614–3622. [Google Scholar] [CrossRef]
- Badran, M.M.; Kuntsche, J.; Fahr, A. Skin penetration enhancement by a microneedle device (Dermaroller) in vitro: Dependency on needle size and applied formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef]
- Burton, S.A.; Ng, C.Y.; Simmers, R.; Moeckly, C.; Brandwein, D.; Gilbert, T.; Johnson, N.; Brown, K.; Alston, T.; Prochnow, G.; et al. Rapid intradermal delivery of liquid formulations using a hollow microstructured array. Pharm. Res. 2011, 28, 31–40. [Google Scholar] [CrossRef]
- Gupta, J.; Felner, E.I.; Prausnitz, M.R. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol. Ther. 2011, 13, 451–456. [Google Scholar] [CrossRef]
- Li, G.; Badkar, A.; Kalluri, H.; Banga, A.K. Microchannels created by sugar and metal microneedles: Characterization by microscopy, macromolecular flux and other techniques. J. Pharm. Sci. 2010, 99, 1931–1941. [Google Scholar]
- Fukushima, K.; Ise, A.; Morita, H.; Hasegawa, R.; Ito, Y.; Sugioka, N.; Takada, K. Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats. Pharm. Res. 2011, 28, 7–21. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, S.O.; Felner, E.I.; Prausnitz, M.R. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small 2011, 18, 531–539. [Google Scholar]
- Migalska, K.; Morrow, D.I.; Garland, M.J.; Thakur, R.; Woolfson, A.D.; Donnelly, R.F. Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm. Res. 2011, 28, 1919–1930. [Google Scholar] [CrossRef]
- Cosman, F.; Lane, N.E.; Bolognese, M.; Zanchetta, J.; Garcia-Hernandez, P.A.; Sees, K.; Matriano, J.A.; Gaumer, K.; Daddona, P.E. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J. Clin. Endocrinol. Metab. 2010, 95, 151–158. [Google Scholar] [CrossRef]
- Cormier, M.; Neukermans, A.P.; Block, B.; Theeuwes, F.T.; Amkraut, A. A Device for Enhancing Transdermal Agent Delivery or Sampling. Eur. Pat. 0914178 B1, 12 March 2003. [Google Scholar]
- Ameri, M.; Fan, S.C.; Maa, Y.F. Parathyroid hormone PTH (1–34) formulation that enables uniform coating on a novel transdermal microprojection delivery system. Pharm. Res. 2010, 27, 303–313. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals; NIH Publication: Bethesda, MD, USA, 1985. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Daddona, P.E.; Matriano, J.A.; Mandema, J.; Maa, Y.-F. Parathyroid hormone (1–34)-coated microneedle patch system: Clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm. Res. 2011, 28, 159–165. [Google Scholar] [CrossRef]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Ameri, M.; Daddona, P.E.; Maa, Y.-F. Demonstrated solid-state stability of parathyroid hormone PTH (1℃34) coated on a novel transdermal microneedle delivery system. Pharm. Res. 2009, 26, 2454–2463. [Google Scholar] [CrossRef]
- Maa, Y.-F.; Nguyen, P.A.; Hsu, S.W. Spray-drying of air-liquid interface sensitive recombinant human growth hormone. J. Pharm. Sci. 1998, 87, 152–159. [Google Scholar] [CrossRef]
- Cormier, M.; Johnson, B.; Ameri, M.; Nyam, K.; Libiran, L.; Zhang, D.; Daddona, P.E. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release 2004, 97, 503–511. [Google Scholar] [CrossRef]
- Harvey, A.J.; Kaestner, S.A.; Sutter, D.E.; Harvey, N.G.; Mikszta, J.A.; Pettis, R.J. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm. Res. 2011, 28, 107–116. [Google Scholar] [CrossRef]
- Reutens, A.T.; Veldhuis, J.D.; Hoffman, D.M.; Leung, K.C.; Ho, K.K. A highly sensitive growth hormone (GH) enzyme-linked immunosorbent assay uncovers increased contribution of a tonic mode of GH secretion in adults with organic GH deficiency. J. Clin. Endocrinol. Metab. 1996, 81, 1591–1597. [Google Scholar]
- Hartman, M.L.; Faria, A.C.; Vance, M.L.; Johnson, M.L.; Thorner, M.O.; Veldhuis, J.D. Temporal structure of in vivo growth hormone secretory events in humans. Am. J. Physiol. 1991, 260, E101–E110. [Google Scholar]
- Surya, S.; Horowitz, J.F.; Goldenberg, N.; Sakharova, A.; Harber, M.; Cornford, A.S.; Symons, K.; Barkan, A.L. The pattern of growth hormone delivery to peripheral tissues determines insulin-like growth factor-1 and lipolytic responses in obese subjects. J. Clin. Endocrinol. Metab. 2009, 94, 2828–2834. [Google Scholar] [CrossRef]
- Cersosimo, E.; Danou, F.; Persson, M.; Miles, J.M. Effects of pulsatile delivery of basal growth hormone on lipolysis in humans. Am. J. Physiol. 1996, 271, E123–E126. [Google Scholar]
- Orskov, L.; Schmitz, O.; Jørgensen, J.O.; Arnfred, J.; Abildgaard, N.; Christiansen, J.S.; Alberti, K.G.; Orskov, H. Influence of growth hormone on glucose-induced glucose uptake in normal men as assessed by the hyperglycemic clamp technique. J. Clin. Endocrinol. Metab. 1989, 68, 276–282. [Google Scholar] [CrossRef]
- Yuen, K.C.J.; Conway, G.S.; Popovic, V.; Merriam, G.R.; Bailey, T.; Hamrahian, A.H.; Biller, B.M.K.; Kipnes, M.; Moore, J.A.; Humpriss, E.; et al. A long acting human growth hormone with delayed clearance (VRS-317): Results of a double blind, placebo controlled, single ascending dose study in growth hormone deficient adults. J. Clin. Endocrinol. Metab. 2013, 98, 2595–2603. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ameri, M.; Kadkhodayan, M.; Nguyen, J.; Bravo, J.A.; Su, R.; Chan, K.; Samiee, A.; Daddona, P.E. Human Growth Hormone Delivery with a Microneedle Transdermal System: Preclinical Formulation, Stability, Delivery and PK of Therapeutically Relevant Doses. Pharmaceutics 2014, 6, 220-234. https://doi.org/10.3390/pharmaceutics6020220
Ameri M, Kadkhodayan M, Nguyen J, Bravo JA, Su R, Chan K, Samiee A, Daddona PE. Human Growth Hormone Delivery with a Microneedle Transdermal System: Preclinical Formulation, Stability, Delivery and PK of Therapeutically Relevant Doses. Pharmaceutics. 2014; 6(2):220-234. https://doi.org/10.3390/pharmaceutics6020220
Chicago/Turabian StyleAmeri, Mahmoud, Miryam Kadkhodayan, Joe Nguyen, Joseph A. Bravo, Rebeca Su, Kenneth Chan, Ahmad Samiee, and Peter E. Daddona. 2014. "Human Growth Hormone Delivery with a Microneedle Transdermal System: Preclinical Formulation, Stability, Delivery and PK of Therapeutically Relevant Doses" Pharmaceutics 6, no. 2: 220-234. https://doi.org/10.3390/pharmaceutics6020220