Characterization and Validation of a New 3D Printing Ink for Reducing Therapeutic Gap in Pediatrics through Individualized Medicines
Abstract
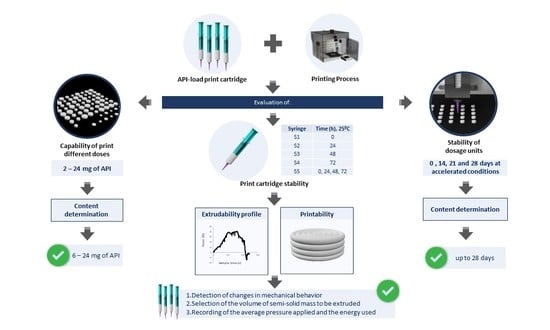
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Three-Dimensional Printing Ink Preparation
2.3. Design of the Printlets and Setting the Printing Parameters
2.3.1. Design of the 3D Shapes
2.3.2. Slic3r Profiles (Printing Settings)
2.4. Rheological Characterization of the Printing Ink
2.5. Stability Evaluation of the Printing Ink
2.5.1. Extrudability Analysis of the Printing Ink
2.5.2. Printability of the Printing Ink
2.6. Stability Evaluation of the Printlets
2.7. Evaluation of the Ink and Process Capability to Print Different Doses
2.8. In-Line Process Control
2.8.1. Extrusion Pressure Control
2.8.2. Computer Vision
3. Results and Discussions
3.1. Rheological Characteristics of the Printing Ink
3.2. Stability Evaluation of the Printing Ink
3.2.1. Extrudability Analysis
3.2.2. Printability of the Feedstock
3.3. Stability Evaluation of Printlets
3.4. Evaluation of the Ink and Process Capability to Print Different Doses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Aita, I.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-Printing with Precise Layer-Wise Dose Adjustments for Paediatric use via Pressure-Assisted Microsyringe Printing. Eur. J. Pharm. Biopharm. 2020, 157, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S. Assessment of Pharmacist’s Knowledge and Perception toward 3D Printing Technology as a Dispensing Method for Personalized Medicine and the Readiness for Implementation. Pharmacy 2021, 9, 68. [Google Scholar] [CrossRef]
- Goh, O.; Goh, W.J.; Lim, S.H.; Hoo, G.; Liew, R.; Ng, T. Preferences of Healthcare Professionals on 3D-Printed Tablets: A Pilot Study. Pharmaceutics 2022, 14, 1521. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Duran Piñeiro, G.; Giraldez Montero, J.M.; Lamas Diaz, M.J.; Gonzalez Barcia, M.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.; et al. Automated Therapy Preparation of Isoleucine Formulations using 3D Printing for the Treatment of MSUD: First Single-Centre, Prospective, Crossover Study in Patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef] [PubMed]
- Gabinete de Prensa del Hospital Universitario Vall d’Hebron. Vall D’Hebron Elaborará Con Una Impresora 3D Medicamentos Para Niños Y Niñas. Available online: https://www.vallhebron.com/es/actualidad/noticias/vall-dhebron-elaborara-con-una-impresora-3d-medicamentos-para-ninos-y-ninas (accessed on 11 April 2023).
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and Investigation of Novel Gastro-Floating Tablets with 3D Extrusion-Based Printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef]
- Díaz-Torres, E.; Rodríguez-Pombo, L.; Ong, J.J.; Basit, A.W.; Santoveña-Estévez, A.; Fariña, J.B.; Alvarez-Lorenzo, C.; Goyanes, A. Integrating Pressure Sensor Control into Semi-Solid Extrusion 3D Printing to Optimize Medicine Manufacturing. Int. J. Pharm. X 2022, 4, 100133. [Google Scholar] [CrossRef]
- Panraksa, P.; Qi, S.; Udomsom, S.; Tipduangta, P.; Rachtanapun, P.; Jantanasakulwong, K.; Jantrawut, P. Characterization of Hydrophilic Polymers as a Syringe Extrusion 3D Printing Material for Orodispersible Film. Polymers 2021, 13, 3454. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.; Alayoubi, A.; Asfari, S.; Coburn, J.; Ghammraoui, B.; Aqueel, S.; Cruz, C.N.; Ashraf, M. Development of Mechanistic Models to Identify Critical Formulation and Process Variables of Pastes for 3D Printing of Modified Release Tablets. Int. J. Pharm. 2019, 555, 109–123. [Google Scholar] [CrossRef]
- Conceicao, J.; Farto-Vaamonde, X.; Goyanes, A.; Adeoye, O.; Concheiro, A.; Cabral-Marques, H.; Sousa Lobo, J.M.; Alvarez-Lorenzo, C. Hydroxypropyl-Beta-Cyclodextrin-Based Fast Dissolving Carbamazepine Printlets Prepared by Semisolid Extrusion 3D Printing. Carbohydr. Polym. 2019, 221, 55–62. [Google Scholar] [CrossRef] [PubMed]
- González, K.; Larraza, I.; Berra, G.; Eceiza, A.; Gabilondo, N. 3D Printing of Customized all-Starch Tablets with Combined Release Kinetics. Int. J. Pharm. 2022, 622, 121872. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.; Alayoubi, A.; Coburn, J.; Asfari, S.; Ghammraoui, B.; Cruz, C.N.; Ashraf, M. Extrudability Analysis of Drug Loaded Pastes for 3D Printing of Modified Release Tablets. Int. J. Pharm. 2019, 554, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Comité de Medicamentos de la Asociación Española de Pediatría. Hidroclorotiazida. Available online: https://www.aeped.es/pediamecum/generatepdf/api?n=83795 (accessed on 11 April 2023).
- World Health Organization. Weight-for-Age Percentiles. Available online: https://www.who.int/tools/child-growth-standards/standards/weight-for-age (accessed on 11 April 2023).
- Callede, N.; Masciotti, T.; Casettari, L.; Loosveldt, N.; Goole, J. Development and Evaluation of a 3D Printing Protocol to Produce Zolpidem-Containing Printlets, as Compounding Preparation, by the Pressurized-Assisted Microsyringes Technique. Int. J. Pharm. 2022, 621, 121756. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia. 2.9.6. Uniformity of Content of Single-Dose Preparations, 11th ed.; Council of Europe, Ed.; Council of Europe: Strasbourg, France, 2023. [Google Scholar]
- European Pharmacopoeia. 2.9.40. Uniformity of Dosage Units, 11th ed.; Council of Europe, Ed.; Council of Europe: Strasbourg, France, 2023. [Google Scholar]
- European Pharmacopoeia. 5.25. Process Analytical Technology, 11th ed.; Council of Europe, Ed.; Council of Europe: Strasbourg, France, 2023. [Google Scholar]
- Trenfield, S.J.; Xu, X.; Goyanes, A.; Rowland, M.; Wilsdon, D.; Gaisford, S.; Basit, A.W. Releasing Fast and Slow: Non-Destructive Prediction of Density and Drug Release from SLS 3D Printed Tablets using NIR Spectroscopy. Int. J. Pharm. X 2023, 5, 100148. [Google Scholar] [CrossRef] [PubMed]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the Progress of Hydrogel-Based 3D Printing: Correlating Rheological Properties with Printing Behaviour. Int. J. Pharm. 2022, 615, 121506. [Google Scholar] [CrossRef]
- Ribeiro, A.; Blokzijl, M.M.; Levato, R.; Visser, C.W.; Castilho, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Assessing Bioink Shape Fidelity to Aid Material Development in 3D Bioprinting. Biofabrication 2017, 10, 014102. [Google Scholar] [CrossRef]
- Cai, F.; Heid, S.; Boccaccini, A.R. Potential of Laponite® Incorporated Oxidized Alginate-gelatin (ADA-GEL) Composite Hydrogels for Extrusion-based 3D Printing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1090–1104. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D Printed Pharmaceuticals: From Hype to Real-World Clinical Applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Gonzalez, J.; Magarinos-Trivino, M.; Diaz-Torres, E.; Caceres-Perez, A.R.; Santovena-Estevez, A.; Farina, J.B. Individualized Orodispersible Pediatric Dosage Forms obtained by Molding and Semi-Solid Extrusion by 3D Printing: A Comparative Study for Hydrochlorothiazide. J. Drug Deliv. Sci. Technol. 2021, 66, 102884. [Google Scholar] [CrossRef]
- ICH. The International Conference on Harmonisation. Validation of Analytical Procedures: Text and Methodology Q2 (R1). Available online: https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf (accessed on 11 April 2023).
- European Pharmacopoeia. Uniformity of Mass of Single-Dose Preparations, 11th ed.; Council of Europe, Ed.; Council of Europe: Strasbourg, France, 2023. [Google Scholar]
- ICH. The International Conference on Harmonisation. Stability Testing of New Drug Substances and Products Q1A (R2). Available online: https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf (accessed on 11 April 2023).
- Amorim, P.A.; d’Ávila, M.A.; Anand, R.; Moldenaers, P.; Van Puyvelde, P.; Bloemen, V. Insights on Shear Rheology of Inks for Extrusion-Based 3D Bioprinting. Bioprinting 2021, 22, e00129. [Google Scholar] [CrossRef]
- Wang, Q.J.; Chung, Y. Encyclopedia of Tribology; Springer: New York, NY, USA, 2013. [Google Scholar]
- Shahbazi, M.; Jäger, H. Current Status in the Utilization of Biobased Polymers for 3D Printing Process: A Systematic Review of the Materials, Processes, and Challenges. ACS Appl. Bio Mater. 2021, 4, 325–369. [Google Scholar] [CrossRef]
- Townsend, J.M.; Beck, E.C.; Gehrke, S.H.; Berkland, C.J.; Detamore, M.S. Flow Behavior Prior to Crosslinking: The Need for Precursor Rheology for Placement of Hydrogels in Medical Applications and for 3D Bioprinting. Prog. Polym. Sci. 2019, 91, 126–140. [Google Scholar] [CrossRef]
- Mouser, V.H.M.; Melchels, F.P.W.; Visser, J.; Dhert, W.J.A.; Gawlitta, D.; Malda, J. Yield Stress Determines Bioprintability of Hydrogels Based on Gelatin-Methacryloyl and Gellan Gum for Cartilage Bioprinting. Biofabrication 2016, 8, 35003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, H.A. A Review of the Slip (Wall Depletion) of Polymer Solutions, Emulsions and Particle Suspensions in Viscometers: Its Cause, Character, and Cure. J. Non-Newton. Fluid Mech. 1995, 56, 221–251. [Google Scholar] [CrossRef]
- Desai, P.M.; Liew, C.V.; Heng, P.W.S. Review of Disintegrants and the Disintegration Phenomena. J. Pharm. Sci. 2016, 105, 2545–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zupancic, A.; Lapasin, R.; Kristoffersson, A. Influence of Particle Concentration on Rheological Properties of Aqueous A-Al2O3 Suspensions. J. Eur. Ceram. Soc. 1998, 18, 467–477. [Google Scholar] [CrossRef]
- Ojile, J.E.; Macfarlane, C.B.; Selkirk, A.B. Drug Distribution during Massing and its Effect on Dose Uniformity in Granules. Int. J. Pharm. 1982, 10, 99–107. [Google Scholar] [CrossRef]
- Ramesh, S.; Harrysson, O.L.A.; Rao, P.K.; Tamayol, A.; Cormier, D.R.; Zhang, Y.; Rivero, I.V. Extrusion Bioprinting: Recent Progress, Challenges, and Future Opportunities. Bioprinting 2021, 21, e00116. [Google Scholar] [CrossRef]
- Liu, Z.; Bhandari, B.; Prakash, S.; Mantihal, S.; Zhang, M. Linking Rheology and Printability of a Multicomponent Gel System of Carrageenan-Xanthan-Starch in Extrusion Based Additive Manufacturing. Food Hydrocoll. 2019, 87, 413–424. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D Printing of Hydrogels: Rational Design Strategies and Emerging Biomedical Applications. Mater. Sci. Eng. R Rep. A Rev. J. 2020, 140, 100543. [Google Scholar] [CrossRef]







| Variable | Units | Description | Data Source |
|---|---|---|---|
| Start flow pressure (yield point) | kPa | Pressure value where the non-steady flow through the nozzle starts | Pressure–time plot |
| Max. applied pressure | kPa | Maximum applied pressure value recorded when the extrusion force is developed | Pressure–time plot |
| Steady flow pressure | kPa | Last time-sequence pressure value when the flow of semi-solid mass through the nozzle is constant | Pressure–time plot |
| Flow cessation pressure | kPa | Pressure value after the extrusion displacement of the plunger when the flow of semi-solid mass is interrupted | Pressure–time plot |
| Recoverable stress | % | ||
| Qweight, time | mg·s−1 | Flow of semi-solid mass through the nozzle as function of the test time | Weight–time plot |
| Qweight, displacement | mg·mm−1 | Flow of semi-solid mass through the nozzle as function of the test displacement | Weight–distance plot |
| AUC1 | kPa·s | Energy required to reach the steady flow of the semi-solid material through the nozzle | Pressure–time plot |
| AUC2 | kPa·s | Energy used to extrude a certain amount of semi-solid material | Pressure–time plot |
| AUC2/total weight | kPa·s·mg−1 | Energy used to extrude 1 mg of semi-solid material when the steady flow was reached | Pressure–time plot |
| Young’s modulus | kPa | Indicator of a material’s capacity to withstand length changes caused by longitudinal tension or compression. | Pressure–distance plot, slope of the linear part of the pressure–displacement curve |
| Storage Time | 0 h | 24 h | 48 h | 72 h | ||||
|---|---|---|---|---|---|---|---|---|
| Print Cartridge | S1 | S5 | S2 | S5 | S3 | S5 | S4 | S5 |
| Start flow pressure (yield point) (kPa) | 78.5 | 86.7 | 58.0 | 58.7 | 79.2 | 83.4 | 78.8 | 73.6 |
| Max. applied pressure (kPa) | 99.5 | 94.6 | 140.5 | 135.7 | 155.4 | 184.3 | 163.8 | 213.3 |
| Steady flow pressure (kPa) | 82.5 | 93.2 | 137.4 | 134.0 | 141.8 | 170.4 | 160.7 | 209.2 |
| Flow cessation pressure (kPa) | 47.6 | 58.4 | 80.4 | 78.5 | 89.3 | 98.4 | 107.3 | 110.9 |
| Recoverable stress (%) | 57.7 | 62.6 | 58.6 | 58.6 | 63.0 | 57.7 | 66.8 | 53.0 |
| Qweight, time (mg/s) | 2.08 | 2.08 | 2.07 | 2.04 | 2.16 | 2.04 | 2.00 | 2.15 |
| Qweight, displacement (mg/mm) | 415.3 | 415.3 | 414.0 | 408.5 | 432.6 | 408.4 | 400.3 | 430.3 |
| AUC1 (104 kPa·s) | 2.16 | 2.17 | 2.39 | 2.07 | 3.98 | 4.71 | 4.59 | 5.07 |
| AUC2 (104 kPa·s) | 4.12 | 3.92 | 5.78 | 6.09 | 4.31 | 4.94 | 4.91 | 6.64 |
| AUC2/total weight (kPa·s·mg−1) | 39.4 | 42.7 | 58.4 | 56.0 | 53.3 | 69.2 | 72.8 | 85.1 |
| Young’s modulus (kPa) | 52.8 | 60.3 | 90.2 | 92.5 | 51.0 | 81.6 | 47.2 | 66.4 |
| Storage Time | 0 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|
| Print Cartridge | S1 | S2 | S3 | S4 | |
| Applied pressure (kPa) (mean ± SD) | i = 1…10 | 71.6 ± 3.0 | 111.8 ± 2.5 | 119.5 ± 6.4 | 137.1 ± 11.8 |
| i = 11…100 | 88.8 ± 6.7 | 136.3 ± 16.1 | 140.1 ± 12.8 | 157.4 ± 8.6 | |
| i = 1…100 | 87.1 ± 8.3 | 133.9 ± 17.0 | 137.5 ± 13.9 | 154.9 ± 11.2 | |
| Printlet weight (mg) (mean ± SD) | i = 1…10 | 30.1 ± 6.6 | 28.6 ± 6.3 | 24.4 ± 6.4 | 24.6 ± 5.8 |
| i = 11…100 | 29.0 ± 0.9 | 29.5 ± 2.0 | 30.0 ± 1.6 | 29.7 ± 3.2 | |
| i = 1…100 | 29.1 ± 2.2 | 29.4 ± 2.8 | 29.4 ± 3.1 | 29.1 ± 4.0 | |
| Printlet estimated weight * (mg) | 30.2 | 31.1 | 32.5 | 30.1 | |
| % DV, i = 11…100 | 99.6 ± 1.6 | 95.1 ± 0.6 | 99.6 ± 1.6 | 105.8 ± 0.2 | |
| Shape fidelity (mean ± SD, %), i = 11…100 | 97.8 ± 1.5 | 103.3 ± 1.5 | 101.5 ± 1.6 | 102.5 ± 2.9 | |
| Size reduction (mean ± SD, %), i = 11…100 | 44.5 ± 2.1 | 45.5 ± 1.5 | 44.3 ± 2.3 | 43.0 ± 2.8 | |
| Time (Days) | % DV | % WR |
|---|---|---|
| 0 | 99.6 ± 1.6 | 0.00 ± 0.0 |
| 3 | 102.9 ± 0.4 | 5.41 ± 0.2 |
| 7 | 99.2 ± 4.2 | 5.39 ± 0.3 |
| 14 | 104.6 ± 4.6 | 5.42 ± 0.2 |
| 21 | 104.2 ± 4.2 | 5.91 ± 0.2 |
| 28 | 99.3 ± 1.3 | 5.67 ± 0.2 |
| Dose (mg) | Weight (mean ± SD, mg) | Dose (mean ± SD, mg) | % DV (mean ± SD, %) | Shape Fidelity (mean ± SD, %) | Size Reduction (mean ± SD, %) |
|---|---|---|---|---|---|
| 2.0 | 6.4 ± 2.6 | 2.6 ± 0.9 | 129.7 ± 46.2 | 92.6 ± 6.9 | 38.2 ± 2.1 |
| 4.0 | 9.6 ± 2.7 | 3.2 ± 0.8 | 79.2 ± 20.3 | 94.0 ± 4.3 | 39.3 ± 0.9 |
| 6.0 | 15.9 ± 2.6 | 6.0 ± 0.7 | 101.5 ± 10.4 | 103.0 ± 1.9 | 44.6 ± 2.2 |
| 8.0 | 21.3 ± 2.4 | 8.2 ± 0.5 | 102.6 ± 6.2 | 99.8 ± 1.6 | 42.5 ± 1.2 |
| 10.0 | 27.6 ± 2.3 | 10.4 ± 0.5 | 104.5 ± 5.2 | 99.5 ± 1.9 | 44.1 ± 1.2 |
| 12.0 | 32.9 ± 5.5 | 11.9 ± 0.8 | 98.7 ± 6.4 | 101.0 ± 2.5 | 44.3 ± 1.9 |
| 14.0 | 37.3 ± 4.9 | 14.7 ± 0.3 | 104.6 ± 2.1 | 100.1 ± 2.1 | 44.3 ± 1.8 |
| 16.0 | 44.6 ± 1.8 | 16.1 ± 0.6 | 100.6 ± 3.8 | 99.2 ± 0.3 | 43.2 ± 1.2 |
| 18.0 | 51.2 ± 3.5 | 19.0 ± 0.7 | 105.3 ± 3.8 | 100.3 ± 2.9 | 44.1 ± 1.4 |
| 20.0 | 55.7 ± 2.6 | 20.6 ± 1.1 | 102.7 ± 5.6 | 98.4 ± 1.9 | 43.7 ± 0.8 |
| 22.0 | 62.8 ± 2.0 | 22.4 ± 1.0 | 101.6 ± 4.3 | 99.5 ± 1.4 | 44.6 ± 0.5 |
| 24.0 | 67.7 ± 2.7 | 24.3 ± 1.1 | 101.4 ± 4.8 | 101.8 ± 2.4 | 43.8 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Torres, E.; Suárez-González, J.; Monzón-Rodríguez, C.N.; Santoveña-Estévez, A.; Fariña, J.B. Characterization and Validation of a New 3D Printing Ink for Reducing Therapeutic Gap in Pediatrics through Individualized Medicines. Pharmaceutics 2023, 15, 1642. https://doi.org/10.3390/pharmaceutics15061642
Díaz-Torres E, Suárez-González J, Monzón-Rodríguez CN, Santoveña-Estévez A, Fariña JB. Characterization and Validation of a New 3D Printing Ink for Reducing Therapeutic Gap in Pediatrics through Individualized Medicines. Pharmaceutics. 2023; 15(6):1642. https://doi.org/10.3390/pharmaceutics15061642
Chicago/Turabian StyleDíaz-Torres, Eduardo, Javier Suárez-González, Cecilia N. Monzón-Rodríguez, Ana Santoveña-Estévez, and José B. Fariña. 2023. "Characterization and Validation of a New 3D Printing Ink for Reducing Therapeutic Gap in Pediatrics through Individualized Medicines" Pharmaceutics 15, no. 6: 1642. https://doi.org/10.3390/pharmaceutics15061642