Influence of Polyvinylpyrrolidone Molecular Weight and Concentration on the Precipitation Inhibition of Supersaturated Solutions of Poorly Soluble Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVP Solutions
2.3. Thermogravimetric Analysis of PVP
2.4. Equilibrium Solubility of the Drugs in PVP Solutions
2.5. Quantitative Analysis
2.6. Viscosity of Solutions of PVP
2.7. Nucleation and Crystal Growth of Drugs in a Supersaturated Solution
2.8. Data Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Influence of PVP Concentration and Molecular Weight on the Solubility of the Drugs
3.2. Influence of PVP Concentration and Molecular Weight on Solution Viscosity
3.3. Supersaturation Studies
3.3.1. Influence of PVP Concentration, Solution Viscosity and Molecular Weight on the tind of the Drugs
3.3.2. Influence of PVP Concentration, Solution Viscosity and Molecular Weight on the Precipitation Rate of the Drugs
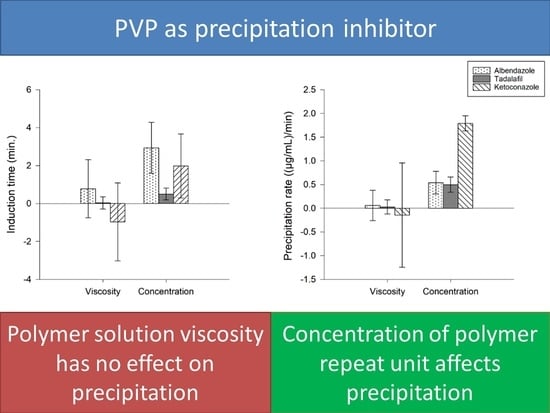
3.3.3. Multivariate Evaluation of the Influence of Concentration and Solution Viscosity on tind and Precipitation Rate of the Drugs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

Appendix C

Appendix D




References
- Price, D.J.; Ditzinger, F.; Koehl, N.J.; Jankovic, S.; Tsakiridou, G.; Nair, A.; Holm, R.; Kuentz, M.; Dressman, J.B.; Saal, C. Approaches to increase mechanistic understanding and aid in the selection of precipitation inhibitors for supersaturating formulations—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 483–509. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Blaabjerg, L.I.; Grohganz, H.; Lindenberg, E.; Löbmann, K.; Müllertz, A.; Rades, T. The influence of polymers on the supersaturation potential of poor and good glass formers. Pharmaceutics 2018, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.B.; Benameur, H.; Porter, C.J.H.; Pouton, C.W. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: A mechanistic basis for utility. J. Drug Target. 2010, 18, 704–731. [Google Scholar] [CrossRef]
- Schöpe, H.J.; Bryant, G.; Van Megen, W. Two-step crystallization kinetics in colloidal hard-sphere systems. Phys. Rev. Lett. 2006, 96, 175701. [Google Scholar] [CrossRef]
- Meng, F.; Gala, U.; Chauhan, H. Classification of solid dispersions: Correlation to (i) stability and solubility (II) preparation and characterization techniques. Drug Dev. Ind. Pharm. 2015, 41, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Palmelund, H.; Madsen, C.M.; Plum, J.; Müllertz, A.; Rades, T. Studying the Propensity of Compounds to Supersaturate: A Practical and Broadly Applicable Approach. J. Pharm. Sci. 2016, 105, 3021–3029. [Google Scholar] [CrossRef]
- Garekani, H.A.; Ford, J.L.; Rubinstein, M.H.; Rajabi-Siahboomi, A.R. Highly compressible paracetamol: I: Crystallization and characterization. Int. J. Pharm. 2000, 208, 87–99. [Google Scholar] [CrossRef]
- Pakuro, N.I.; Arest-Yakubovich, A.A.; Nakhmanovich, B.I.; Chibirova, F.K. Thermo- and pH-Sensitivity of Poly(N-Vinylpyrrolidone) in Water Media. In Polymer Phase Behavior; Nova Science Publisher: Hauppauge, NY, USA, 2011; pp. 296–301. [Google Scholar]
- Raghavan, S.L.; Trividic, A.; Davis, A.F.; Hadgraft, J. Crystallization of hydrocortisone acetate: Influence of polymers. Int. J. Pharm. 2001, 212, 213–221. [Google Scholar] [CrossRef]
- Fornells, E.; Fuguet, E.; Mañé, M.; Ruiz, R.; Box, K.; Bosch, E.; Ràfols, C. Effect of vinylpyrrolidone polymers on the solubility and supersaturation of drugs; a study using the Cheqsol method. Eur. J. Pharm. Sci. 2018, 117, 227–235. [Google Scholar] [CrossRef]
- Hong, S.; Nowak, S.A.; Wah, C.L. Impact of Physicochemical Properties of Cellulosic Polymers on Supersaturation Maintenance in Aqueous Drug Solutions. AAPS PharmSciTech. 2018, 19, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Tros de Ilarduya, M.C.; Martín, C.; Goñi, M.M.; Martínez-Ohárriz, M.C. Solubilization and interaction of sulindac with polyvinylpyrrolidone K30 in the solid state and in aqueous solution. Drug Dev. Ind. Pharm. 1998, 24, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.D.; Anderson, B.D. Maintenance of supersaturation II: Indomethacin crystal growth kinetics versus degree of supersaturation. J. Pharm. Sci. 2013, 102, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Knopp, M.M.; Nguyen, J.H.; Becker, C.; Francke, N.M.; Jørgensen, E.B.; Holm, P.; Holm, R.; Mu, H.; Rades, T.; Langguth, P. Influence of polymer molecular weight on in vitro dissolution behavior and in vivo performance of celecoxib:PVP amorphous solid dispersions. Eur. J. Pharm. Biopharm. 2016, 101, 145–151. [Google Scholar] [CrossRef]
- Plum, J.; Bavnhøj, C.G.; Eliasen, J.N.; Rades, T.; Müllertz, A. Comparison of induction methods for supersaturation: Amorphous dissolution versus solvent shift. Eur. J. Pharm Biopharm. 2020, 152, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Mallepogu, P.; Kaur, H.; Singh, R.; Sodhi, I.; Samal, S.K.; Jena, K.C.; Sangamwar, A.T. Explicating the molecular level drug-polymer interactions at the interface of supersaturated solution of the model drug: Albendazole. Eur. J. Pharm. Sci. 2021, 167, 106014. [Google Scholar] [CrossRef]
- Kumara, P.; Mohanb, C.; Uma Shankara, M.K.S.; Gulatia, M. Physiochemical characterization and release rate studies of solid dispersions of Ketoconazole with Pluronic F127 and PVP K-30. Iran J. Pharm. Res. 2011, 10, 685–694. [Google Scholar]
- Mistry, P.; Mohapatra, S.; Gopinath, T.; Vogt, F.G.; Suryanarayanan, R. Role of the Strength of Drug-Polymer Interactions on the Molecular Mobility and Crystallization Inhibition in Ketoconazole Solid Dispersions. Mol. Pharm. 2015, 12, 3339–3350. [Google Scholar] [CrossRef]
- Lindfors, L.; Forssén, S.; Westergren, J.; Olsson, U. Nucleation and crystal growth in supersaturated solutions of a model drug. J. Colloid Interface Sci. 2008, 325, 404–413. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Guidance for Monomers and Polymers; European Chemicals Agency: Helsinki, Finland, 2012.
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Evaluation of the inhibitory potential of HPMC, PVP and HPC polymers on nucleation and crystal growth. RSC Adv. 2016, 6, 77569–77576. [Google Scholar] [CrossRef]
- Patel, D.D.; Anderson, B.D. Effect of precipitation inhibitors on indomethacin supersaturation maintenance: Mechanisms and modeling. Mol. Pharm. 2014, 11, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Poornachary, S.K.; Chow, P.S.; Tan, R.B.H. Direct precipitation of micron-size salbutamol sulfate: New insights into the action of surfactants and polymeric additives. Cryst. Growth Des. 2010, 10, 3363–3371. [Google Scholar] [CrossRef]
- Sekikawa, H.; Nakano, M.; Arita, T. Inhibitory Effect of Polyvinylpyrrolidone on the Crystallization of Drugs. Chem. Pharm. Bull. 1978, 26, 118–126. [Google Scholar] [CrossRef]
- Dai, W.G.; Dong, L.C.; Li, S.; Deng, Z. Combination of Pluronic/Vitamin E TPGS as a potential inhibitor of drug precipitation. Int. J. Pharm. 2008, 355, 31–37. [Google Scholar] [CrossRef] [PubMed]








| K15 | K30 | K60 | K120 | |
|---|---|---|---|---|
| Concentration (% (w/v)) | 3.13 | 2.81 | 0.33 | 0.25 |
| Viscosity (mPa.s) mean ± SD | 0.98 ± 0.03 | 1.05 ± 0.12 | 1.05 ± 0.12 | 1.04 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odeh, A.B.; El-Sayed, B.; Knopp, M.M.; Rades, T.; Blaabjerg, L.I. Influence of Polyvinylpyrrolidone Molecular Weight and Concentration on the Precipitation Inhibition of Supersaturated Solutions of Poorly Soluble Drugs. Pharmaceutics 2023, 15, 1601. https://doi.org/10.3390/pharmaceutics15061601
Odeh AB, El-Sayed B, Knopp MM, Rades T, Blaabjerg LI. Influence of Polyvinylpyrrolidone Molecular Weight and Concentration on the Precipitation Inhibition of Supersaturated Solutions of Poorly Soluble Drugs. Pharmaceutics. 2023; 15(6):1601. https://doi.org/10.3390/pharmaceutics15061601
Chicago/Turabian StyleOdeh, Afnan Bany, Boushra El-Sayed, Matthias Manne Knopp, Thomas Rades, and Lasse Ingerslev Blaabjerg. 2023. "Influence of Polyvinylpyrrolidone Molecular Weight and Concentration on the Precipitation Inhibition of Supersaturated Solutions of Poorly Soluble Drugs" Pharmaceutics 15, no. 6: 1601. https://doi.org/10.3390/pharmaceutics15061601