Ginkgo Biloba and Long COVID: In Vivo and In Vitro Models for the Evaluation of Nanotherapeutic Efficacy
Abstract
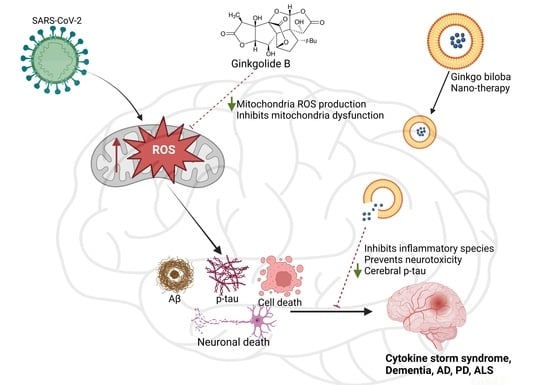
:1. Introduction
2. Long-Term Neurological Damage and the Role of Oxidative Stress
2.1. Neuroinvasive and Neurotoxic Potential of Coronavirus Linked to Neurodegeneration
2.2. Oxidative Stress and Redox Signaling, Players in SARS-CoV-2 Neurological Damage
2.3. In Vivo and In Vitro Models of Oxidative Stress
3. Ginkgo Biloba Extract (EGb) for Neuroprotection and Potential Regeneration from Long COVID Syndrome
3.1. Ginkgo Biloba Antioxidative and Anti-Inflammatory Effects
3.2. Neuroprotective, Anti-Apoptotic, and Anxiolytic Drug Effects of EGb
3.3. Bioavailability and Safety of EGb
4. Perspectives for Use of Ginkgo Biloba in Nanotherapies of Neurological Disorders
4.1. Intranasal Administration and Biodistribution of Nanoparticulate Carriers
4.2. Ginkgo-Biloba-Based Nanotherapy for Neuroprotection and Regeneration from SARS-CoV-2 Neurological Damage
4.3. Green Synthesis of Ginkgo Biloba nanoEGb
4.4. Characterization Techniques for Nanotherapeutics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An Overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Wieczfinska, J.; Kleniewska, P.; Pawliczak, R. Oxidative Stress-Related Mechanisms in SARS-CoV-2 Infections. Oxidative Med. Cell. Longev. 2022, 2022, 5589089. [Google Scholar] [CrossRef] [PubMed]
- Kase, Y.; Okano, H. Neurological Pathogenesis of SARS-CoV-2 (COVID-19): From Virological Features to Clinical Symptoms. Inflamm. Regen. 2021, 41, 15. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, D.; Picone, P. Potential Neurological Effects of Severe COVID-19 Infection. Neurosci. Res. 2020, 158, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Chappidi, M.; Alpaugh, E.S.; Turbeville, B.C.; Falgoust, E.P.; Cornett, E.M.; Murnane, K.S.; Kaye, A.M.; Kaye, A.D. Neurological and Psychiatric Symptoms of COVID-19: A Narrative Review. Psychiatry Int. 2022, 3, 158–168. [Google Scholar] [CrossRef]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What Is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Ahamed, J.; Laurence, J. Long COVID Endotheliopathy: Hypothesized Mechanisms and Potential Therapeutic Approaches. J. Clin. Investig. 2022, 132, e161167. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603. [Google Scholar] [CrossRef]
- Pilotto, A.; Cristillo, V.; Cotti Piccinelli, S.; Zoppi, N.; Bonzi, G.; Sattin, D.; Schiavolin, S.; Raggi, A.; Canale, A.; Gipponi, S.; et al. Long-Term Neurological Manifestations of COVID-19: Prevalence and Predictive Factors. Neurol. Sci. 2021, 42, 4903–4907. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Rass, V.; Beer, R.; Schiefecker, A.J.; Lindner, A.; Kofler, M.; Ianosi, B.A.; Mahlknecht, P.; Heim, B.; Peball, M.; Carbone, F.; et al. Neurological Outcomes 1 Year after COVID-19 Diagnosis: A Prospective Longitudinal Cohort Study. Euro. J. Neurol. 2022, 29, 1685–1696. [Google Scholar] [CrossRef]
- Vollbracht, C.; Kraft, K. Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C. Front. Pharmacol. 2022, 13, 899198. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Villa, C.; Rivellini, E.; Lavitrano, M.; Combi, R. Can SARS-CoV-2 Infection Exacerbate Alzheimer’s Disease? An Overview of Shared Risk Factors and Pathogenetic Mechanisms. JPM 2022, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.B.; Mendes-Correa, M.C.; de Moura Leite, F.B.V.; Sabino, E.C.; Salarini, D.Z.; Claro, I.; Santos, D.W.; de Jesus, J.G.; Ferreira, N.E.; Romano, C.M.; et al. First Case of SARS-COV-2 Sequencing in Cerebrospinal Fluid of a Patient with Suspected Demyelinating Disease. J. Neurol. 2020, 267, 3154–3156. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial Dysfunction and Immunothrombosis as Key Pathogenic Mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Lage, S.L.; Amaral, E.P.; Hilligan, K.L.; Laidlaw, E.; Rupert, A.; Namasivayan, S.; Rocco, J.; Galindo, F.; Kellogg, A.; Kumar, P.; et al. Persistent Oxidative Stress and Inflammasome Activation in CD14highCD16− Monocytes From COVID-19 Patients. Front. Immunol. 2022, 12, 799558. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative Stress and Parkinson’s Disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, G.-H.; Wang, H.-N.; Hu, Q.; Chen, L.-L.; Guan, X.-Q.; Li, H.-L.; Chen, H.-Z.; Tang, H.; Ge, G.-B. Discovery of Naturally Occurring Inhibitors against SARS-CoV-2 3CLpro from Ginkgo Biloba Leaves via Large-Scale Screening. Fitoterapia 2021, 152, 104909. [Google Scholar] [CrossRef]
- Chen, Z.; Cui, Q.; Cooper, L.; Zhang, P.; Lee, H.; Chen, Z.; Wang, Y.; Liu, X.; Rong, L.; Du, R. Ginkgolic Acid and Anacardic Acid Are Specific Covalent Inhibitors of SARS-CoV-2 Cysteine Proteases. Cell Biosci. 2021, 11, 45. [Google Scholar] [CrossRef]
- Noor-E-Tabassum; Das, R.; Lami, M.S.; Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Idroes, R.; Mohamed, A.A.-R.; Hossain, M.J.; Dhama, K.; et al. Ginkgo Biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid. Based Complement. Altern. Med. 2022, 2022, 8288818. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Ochnik, M.; Sobczyński, M.; Gębura, K.; Zambrowicz, A.; Naporowski, P.; Leszek, J. Ginkgo Biloba Leaf Extract Improves an Innate Immune Response of Peripheral Blood Leukocytes of Alzheimer’s Disease Patients. Nutrients 2022, 14, 2022. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.D.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo Biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Tomino, C.; Ilari, S.; Solfrizzi, V.; Malafoglia, V.; Zilio, G.; Russo, P.; Proietti, S.; Marcolongo, F.; Scapagnini, G.; Muscoli, C.; et al. Mild Cognitive Impairment and Mild Dementia: The Role of Ginkgo Biloba (EGb 761®). Pharmaceuticals 2021, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo Biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Kaushik, A.; Kujawska, M.; Batiha, G.E. Ginkgo Biloba in the Management of the COVID-19 Severity. Arch. Pharm. 2022, 355, 2200188. [Google Scholar] [CrossRef]
- Baig, A.M. Deleterious Outcomes in Long-Hauler COVID-19: The Effects of SARS-CoV-2 on the CNS in Chronic COVID Syndrome. ACS Chem. Neurosci. 2020, 11, 4017–4020. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, J.; Gao, J. Sars-Cov-2: Underestimated Damage to Nervous System. Travel Med. Infect. Dis. 2020, 36, 101642. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Zhang, Y.; Scheuermann, R.H.; Peters, B.; Sette, A. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell Host Microbe 2020, 27, 671–680.e2. [Google Scholar] [CrossRef]
- Arbour, N.; Côté, G.; Lachance, C.; Tardieu, M.; Cashman, N.R.; Talbot, P.J. Acute and Persistent Infection of Human Neural Cell Lines by Human Coronavirus OC43. J. Virol. 1999, 73, 3338–3350. [Google Scholar] [CrossRef]
- Collins, A.R.; Sorensen, O. Regulation of Viral Persistence in Human Glioblastoma and Rhabdomyosarcoma Cells Infected with Coronavirus OC43. Microb. Pathog. 1986, 1, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Chaná-Cuevas, P.; Salles-Gándara, P.; Rojas-Fernandez, A.; Salinas-Rebolledo, C.; Milán-Solé, A. The Potential Role of SARS-COV-2 in the Pathogenesis of Parkinson’s Disease. Front. Neurol. 2020, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Boroujeni, M.E.; Simani, L.; Bluyssen, H.A.R.; Samadikhah, H.R.; Zamanlui Benisi, S.; Hassani, S.; Akbari Dilmaghani, N.; Fathi, M.; Vakili, K.; Mahmoudiasl, G.-R.; et al. Inflammatory Response Leads to Neuronal Death in Human Post-Mortem Cerebral Cortex in Patients with COVID-19. ACS Chem. Neurosci. 2021, 12, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The Role of Microglia and Astrocytes in Huntington’s Disease. Front. Mol. Neurosci. 2019, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, X.; Chen, Z.; Duan, J.; Hashimoto, K.; Yang, L.; Liu, C.; Yang, C. Nervous System Involvement after Infection with COVID-19 and Other Coronaviruses. Brain Behav. Immun. 2020, 87, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, W.; Hashikawa, T. Response to Commentary on “The Neuroinvasive Potential of SARS-CoV-2 May Play a Role in the Respiratory Failure of COVID-19 Patients”. J. Med. Virol. 2020, 92, 707–709. [Google Scholar] [CrossRef]
- Devaux, C.A.; Lagier, J.-C.; Raoult, D. New Insights Into the Physiopathology of COVID-19: SARS-CoV-2-Associated Gastrointestinal Illness. Front. Med. 2021, 8, 640073. [Google Scholar] [CrossRef]
- Qian, Q.; Fan, L.; Liu, W.; Li, J.; Yue, J.; Wang, M.; Ke, X.; Yin, Y.; Chen, Q.; Jiang, C. Direct Evidence of Active SARS-CoV-2 Replication in the Intestine. Clin. Infect. Dis. 2021, 73, 361–366. [Google Scholar] [CrossRef]
- Lehmann, M.; Allers, K.; Heldt, C.; Meinhardt, J.; Schmidt, F.; Rodriguez-Sillke, Y.; Kunkel, D.; Schumann, M.; Böttcher, C.; Stahl-Hennig, C.; et al. Human Small Intestinal Infection by SARS-CoV-2 Is Characterized by a Mucosal Infiltration with Activated CD8+ T Cells. Mucosal Immunol. 2021, 14, 1381–1392. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The Gut-Brain Axis: Is Intestinal Inflammation a Silent Driver of Parkinson’s Disease Pathogenesis? NPJ Park. Dis. 2017, 3, 3. [Google Scholar] [CrossRef]
- Politi, L.S.; Salsano, E.; Grimaldi, M. Magnetic Resonance Imaging Alteration of the Brain in a Patient With Coronavirus Disease 2019 (COVID-19) and Anosmia. JAMA Neurol. 2020, 77, 1028. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Palaiodimou, L.; Katsanos, A.H.; Caso, V.; Köhrmann, M.; Molina, C.; Cordonnier, C.; Fischer, U.; Kelly, P.; Sharma, V.K.; et al. Neurological Manifestations and Implications of COVID-19 Pandemic. Ther. Adv. Neurol. Disord. 2020, 13, 175628642093203. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Fragkou, P.C.; Lachanis, S.; Palaiodimou, L.; Lambadiari, V.; Papathanasiou, M.; Sfikakis, P.P.; Voumvourakis, K.I.; Tsiodras, S. Olfactory Bulb and Mucosa Abnormalities in Persistent COVID-19-induced Anosmia: A Magnetic Resonance Imaging Study. Eur. J. Neurol. 2021, 28, e6–e8. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Fragkou, P.C.; Karofylakis, E.; Paneta, M.; Papathanasiou, K.; Palaiodimou, L.; Psarros, C.; Papathanasiou, M.; Lachanis, S.; Sfikakis, P.P.; et al. Hypothyroidism Is Associated with Prolonged COVID-19-Induced Anosmia: A Case–Control Study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 911–912. [Google Scholar] [CrossRef]
- Stefanou, M.-I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C.; et al. Neurological Manifestations of Long-COVID Syndrome: A Narrative Review. Ther. Adv. Chronic Dis. 2022, 13, 204062232210768. [Google Scholar] [CrossRef]
- Welcome, M.O.; Mastorakis, N.E. Neuropathophysiology of Coronavirus Disease 2019: Neuroinflammation and Blood Brain Barrier Disruption Are Critical Pathophysiological Processes That Contribute to the Clinical Symptoms of SARS-CoV-2 Infection. Inflammopharmacology 2021, 29, 939–963. [Google Scholar] [CrossRef]
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in Human and Mouse Brain. BioRxiv 2020. [Google Scholar] [CrossRef]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 Spike Protein Alters Barrier Function in 2D Static and 3D Microfluidic in-Vitro Models of the Human Blood–Brain Barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction, Oxidative Stress, and Neuroinflammation: Intertwined Roads to Neurodegeneration. Antioxidants 2020, 9, 647. [Google Scholar] [CrossRef]
- Sulzer, D.; Antonini, A.; Leta, V.; Nordvig, A.; Smeyne, R.J.; Goldman, J.E.; Al-Dalahmah, O.; Zecca, L.; Sette, A.; Bubacco, L.; et al. COVID-19 and Possible Links with Parkinson’s Disease and Parkinsonism: From Bench to Bedside. NPJ Park. Dis. 2020, 6, 18. [Google Scholar] [CrossRef]
- Chaudhry, Z.; Klenja, D.; Janjua, N.; Cami-Kobeci, G.; Ahmed, B. COVID-19 and Parkinson’s Disease: Shared Inflammatory Pathways Under Oxidative Stress. Brain Sci. 2020, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Castro, P.J.; Estivill-Torrús, G.; Cabezudo-García, P.; Reyes-Bueno, J.A.; Ciano Petersen, N.; Aguilar-Castillo, M.J.; Suárez-Pérez, J.; Jiménez-Hernández, M.D.; Moya-Molina, M.Á.; Oliver-Martos, B.; et al. Impact of SARS-CoV-2 Infection on Neurodegenerative and Neuropsychiatric Diseases: A Delayed Pandemic? Neurología 2020, 35, 245–251. [Google Scholar] [CrossRef]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, Iron, and Hypoxia beyond Inflammation. A Narrative Review. Clin. Pract. 2020, 10, 1271. [Google Scholar] [CrossRef]
- Idrees, D.; Kumar, V. SARS-CoV-2 Spike Protein Interactions with Amyloidogenic Proteins: Potential Clues to Neurodegeneration. Biochem. Biophys. Res. Commun. 2021, 554, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Pedraza-Chaverri, J.; Scholze, A. Nrf2 Activation in Chronic Kidney Disease: Promises and Pitfalls. Antioxidants 2022, 11, 1112. [Google Scholar] [CrossRef]
- Horie, Y.; Suzuki, T.; Inoue, J.; Iso, T.; Wells, G.; Moore, T.W.; Mizushima, T.; Dinkova-Kostova, A.T.; Kasai, T.; Kamei, T.; et al. Molecular Basis for the Disruption of Keap1–Nrf2 Interaction via Hinge & Latch Mechanism. Commun. Biol. 2021, 4, 576. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Olagnier, D.; Farahani, E.; Thyrsted, J.; Blay-Cadanet, J.; Herengt, A.; Idorn, M.; Hait, A.; Hernaez, B.; Knudsen, A.; Iversen, M.B.; et al. SARS-CoV2-Mediated Suppression of NRF2-Signaling Reveals Potent Antiviral and Anti-Inflammatory Activity of 4-Octyl-Itaconate and Dimethyl Fumarate. Nat. Commun. 2020, 11, 4938. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox Regulation of FoxO Transcription Factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Borniquel, S.; García-Quintáns, N.; Valle, I.; Olmos, Y.; Wild, B.; Martínez-Granero, F.; Soria, E.; Lamas, S.; Monsalve, M. Inactivation of Foxo3a and Subsequent Downregulation of PGC-1α Mediate Nitric Oxide-Induced Endothelial Cell Migration. Mol. Cell. Biol. 2010, 30, 4035–4044. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Monks, B.; Ge, Q.; Birnbaum, M.J. Akt/PKB Regulates Hepatic Metabolism by Directly Inhibiting PGC-1α Transcription Coactivator. Nature 2007, 447, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Bian, Q.; Liu, Y.; Fernandes, A.; Taylor, A.; Pereira, P.; Shang, F. Sustained Oxidative Stress Inhibits NF-ΚB Activation Partially via Inactivating the Proteasome. Free Radic. Biol. Med. 2009, 46, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, K.; Jampolska, M.; Zaremba, M.; Joniec-Maciejak, I.; Boguszewski, P.M.; Kaczyńska, K. Respiratory Pattern and Phrenic and Hypoglossal Nerve Activity during Normoxia and Hypoxia in 6-OHDA-Induced Bilateral Model of Parkinson’s Disease. J. Physiol. Sci. 2020, 70, 16. [Google Scholar] [CrossRef] [PubMed]
- Deumens, R.; Blokland, A.; Prickaerts, J. Modeling Parkinson’s Disease in Rats: An Evaluation of 6-OHDA Lesions of the Nigrostriatal Pathway. Exp. Neurol. 2002, 175, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Glinka, Y.Y.; Youdim, M.B.H. Inhibition of Mitochondrial Complexes I and IV by 6-Hydroxydopamine. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1995, 292, 329–332. [Google Scholar] [CrossRef]
- Tanaka, K.; Ogawa, N.; Asanuma, M. Molecular Basis of 6-Hydroxydopamine-Induced Caspase Activations Due to Increases in Oxidative Stress in the Mouse Striatum. Neurosci. Lett. 2006, 410, 85–89. [Google Scholar] [CrossRef]
- Tirmenstein, M.A.; Hu, C.X.; Scicchitano, M.S.; Narayanan, P.K.; McFarland, D.C.; Thomas, H.C.; Schwartz, L.W. Effects of 6-Hydroxydopamine on Mitochondrial Function and Glutathione Status in SH-SY5Y Human Neuroblastoma Cells. Toxicol. Vitr. 2005, 19, 471–479. [Google Scholar] [CrossRef]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.-F.; Benabid, A.-L.; Sadoul, R.; Verna, J.-M. Molecular Pathways Involved in the Neurotoxicity of 6-OHDA, Dopamine and MPTP: Contribution to the Apoptotic Theory in Parkinson’s Disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Gong, P.; Deng, F.; Zhang, W.; Ji, J.; Liu, J.; Sun, Y.; Hu, J. Tectorigenin Attenuates the MPP+-induced SH-SY5Y Cell Damage, Indicating a Potential Beneficial Role in Parkinson’s Disease by Oxidative Stress Inhibition. Exp. Ther. Med. 2017, 14, 4431–4437. [Google Scholar] [CrossRef]
- Vestuto, V.; Amodio, G.; Pepe, G.; Basilicata, M.G.; Belvedere, R.; Napolitano, E.; Guarnieri, D.; Pagliara, V.; Paladino, S.; Rodriquez, M.; et al. Cocoa Extract Provides Protection against 6-OHDA Toxicity in SH-SY5Y Dopaminergic Neurons by Targeting PERK. Biomedicines 2022, 10, 2009. [Google Scholar] [CrossRef]
- Chansiw, N.; Kulprachakarn, K.; Paradee, N.; Prommaban, A.; Srichairatanakool, S. Protection of Iron-Induced Oxidative Damage in Neuroblastoma (SH-SY5Y) Cells by Combination of 1-(N-Acetyl-6-Aminohexyl)-3-Hydroxy-2-Methylpyridin-4-One and Green Tea Extract. Bioinorg. Chem. Appl. 2021, 2021, 5539666. [Google Scholar] [CrossRef]
- Chen, D.; Kanthasamy, A.G.; Reddy, M.B. EGCG Protects against 6-OHDA-Induced Neurotoxicity in a Cell Culture Model. Park. Dis. 2015, 2015, 843906. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Mediator of Physiological and Pathophysiological Responses to Hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480. [Google Scholar] [CrossRef]
- Chazelas, P.; Steichen, C.; Favreau, F.; Trouillas, P.; Hannaert, P.; Thuillier, R.; Giraud, S.; Hauet, T.; Guillard, J. Oxidative Stress Evaluation in Ischemia Reperfusion Models: Characteristics, Limits and Perspectives. IJMS 2021, 22, 2366. [Google Scholar] [CrossRef]
- Irani, K. Oxidant Signaling in Vascular Cell Growth, Death, and Survival: A Review of the Roles of Reactive Oxygen Species in Smooth Muscle and Endothelial Cell Mitogenic and Apoptotic Signaling. Circ. Res. 2000, 87, 179–183. [Google Scholar] [CrossRef]
- Saikumar, P.; Dong, Z.; Weinberg, J.M.; Venkatachalam, M.A. Mechanisms of Cell Death in Hypoxia/Reoxygenation Injury. Oncogene 1998, 17, 3341–3349. [Google Scholar] [CrossRef]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef]
- Martin, I.; Dawson, V.L.; Dawson, T.M. Recent Advances in the Genetics of Parkinson’s Disease. Annu. Rev. Genom. Hum. Genet. 2011, 12, 301–325. [Google Scholar] [CrossRef]
- Scott, L.; Dawson, V.L.; Dawson, T.M. Trumping Neurodegeneration: Targeting Common Pathways Regulated by Autosomal Recessive Parkinson’s Disease Genes. Exp. Neurol. 2017, 298, 191–201. [Google Scholar] [CrossRef]
- Ittner, L.M.; Halliday, G.M.; Kril, J.J.; Götz, J.; Hodges, J.R.; Kiernan, M.C. FTD and ALS—Translating Mouse Studies into Clinical Trials. Nat. Rev. Neurol. 2015, 11, 360–366. [Google Scholar] [CrossRef]
- Haass, C.; Strooper, B.D. The Presenilins in Alzheimer’s Disease--Proteolysis Holds the Key. Science 1999, 286, 916–919. [Google Scholar] [CrossRef]
- Dawson, T.M.; Golde, T.E.; Lagier-Tourenne, C. Animal Models of Neurodegenerative Diseases. Nat. Neurosci. 2018, 21, 1370–1379. [Google Scholar] [CrossRef]
- Johnson, T.E.; de Castro, E.; Hegi de Castro, S.; Cypser, J.; Henderson, S.; Tedesco, P. Relationship between Increased Longevity and Stress Resistance as Assessed through Gerontogene Mutations in Caenorhabditis Elegans. Exp. Gerontol. 2001, 36, 1609–1617. [Google Scholar] [CrossRef]
- Melov, S. Animal Models of Oxidative Stress, Aging, and Therapeutic Antioxidant Interventions. Int. J. Biochem. Cell Biol. 2002, 34, 1395–1400. [Google Scholar] [CrossRef]
- Larsen, P.L. Aging and Resistance to Oxidative Damage in Caenorhabditis Elegans. Proc. Natl. Acad. Sci. USA 1993, 90, 8905–8909. [Google Scholar] [CrossRef]
- Johnson, T.E. Increased Life-Span of Age-1 Mutants in Caenorhabditis Elegans and Lower Gompertz Rate of Aging. Science 1990, 249, 908–912. [Google Scholar] [CrossRef]
- Chang, H.-C.; Liu, K.-F.; Teng, C.-J.; Lai, S.-C.; Yang, S.-E.; Ching, H.; Wu, C.-R. Sophora Tomentosa Extract Prevents MPTP-Induced Parkinsonism in C57BL/6 Mice Via the Inhibition of GSK-3β Phosphorylation and Oxidative Stress. Nutrients 2019, 11, 252. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic Systemic Pesticide Exposure Reproduces Features of Parkinson’s Disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Yang, W.; Tiffany-Castiglioni, E.; Lee, M.-Y.; Son, I.-H. Paraquat Induces Cyclooxygenase-2 (COX-2) Implicated Toxicity in Human Neuroblastoma SH-SY5Y Cells. Toxicol. Lett. 2010, 199, 239–246. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to Cause Oxidative Stress, the Catalase Issue. IJMS 2020, 21, 9149. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Lee, S.R.; Park, Y.J.; Ra, M.; Lee, Y.; Pang, C.; Kim, K.H. Suppression of 6-Hydroxydopamine-Induced Oxidative Stress by Hyperoside Via Activation of Nrf2/HO-1 Signaling in Dopaminergic Neurons. IJMS 2019, 20, 5832. [Google Scholar] [CrossRef]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate Toxicity in a Neuronal Cell Line Involves Inhibition of Cystine Transport Leading to Oxidative Stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef]
- Salińska, E.; Danysz, W.; Łazarewicz, J.W. The Role of Excitotoxicity in Neurodegeneration. Folia Neuropathol. 2005, 43, 322–339. [Google Scholar]
- Kumar, P.; Kalonia, H.; Kumar, A. Novel Protective Mechanisms of Antidepressants against 3-Nitropropionic Acid Induced Huntington’s-like Symptoms: A Comparative Study. J. Psychopharmacol. 2011, 25, 1399–1411. [Google Scholar] [CrossRef]
- Salvi, A.; Patki, G.; Khan, E.; Asghar, M.; Salim, S. Protective Effect of Tempol on Buthionine Sulfoximine-Induced Mitochondrial Impairment in Hippocampal Derived HT22 Cells. Oxidative Med. Cell. Longev. 2016, 2016, 5059043. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelov, B.; Drechsler, M.; Nicolas, V.; Bizien, T.; Gorshkova, Y.E.; Deng, Y.; Angelova, A. Liquid Crystalline Lipid Nanoparticles for Combined Delivery of Curcumin, Fish Oil and BDNF: In Vitro Neuroprotective Potential in a Cellular Model of Tunicamycin-Induced Endoplasmic Reticulum Stress. Smart Mater. Med. 2022, 3, 274–288. [Google Scholar] [CrossRef]
- Martin, I.; Jones, M.A.; Rhodenizer, D.; Zheng, J.; Warrick, J.M.; Seroude, L.; Grotewiel, M. Sod2 Knockdown in the Musculature Has Whole-Organism Consequences in Drosophila. Free Radic. Biol. Med. 2009, 47, 803–813. [Google Scholar] [CrossRef]
- Nowak, A.; Kojder, K.; Zielonka-Brzezicka, J.; Wróbel, J.; Bosiacki, M.; Fabiańska, M.; Wróbel, M.; Sołek-Pastuszka, J.; Klimowicz, A. The Use of Ginkgo Biloba L. as a Neuroprotective Agent in the Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 775034. [Google Scholar] [CrossRef]
- Bradley, P.R. (Ed.) British Herbal Compendium: A Handbook of Scientific Information on Widely Used Plant Drugs; British Herbal Medicine Association: Bournemouth, UK, 1992. [Google Scholar]
- Yang, Y.; Liu, P.; Chen, L.; Liu, Z.; Zhang, H.; Wang, J.; Sun, X.; Zhong, W.; Wang, N.; Tian, K.; et al. Therapeutic Effect of Ginkgo Biloba Polysaccharide in Rats with Focal Cerebral Ischemia/Reperfusion (I/R) Injury. Carbohydr. Polym. 2013, 98, 1383–1388. [Google Scholar] [CrossRef]
- Achete de Souza, G.; de Marqui, S.V.; Matias, J.N.; Guiguer, E.L.; Barbalho, S.M. Effects of Ginkgo Biloba on Diseases Related to Oxidative Stress. Planta Med. 2020, 86, 376–386. [Google Scholar] [CrossRef]
- Trebatická, J.; Ďuračková, Z. Psychiatric Disorders and Polyphenols: Can They Be Helpful in Therapy? Oxidative Med. Cell. Longev. 2015, 2015, 248529. [Google Scholar] [CrossRef]
- Malik, J.; Choudhary, S.; Kumar, P. Plants and Phytochemicals for Huntington′s Disease. Pharmacogn. Rev. 2013, 7, 81. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent Progress Regarding Kaempferol for the Treatment of Various Diseases (Review). Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Zhou, M.; Ren, H.; Han, J.; Wang, W.; Zheng, Q.; Wang, D. Protective Effects of Kaempferol against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart via Antioxidant Activity and Inhibition of Glycogen Synthase Kinase-3 β. Oxidative Med. Cell. Longev. 2015, 2015, 481405. [Google Scholar] [CrossRef]
- Yang, C.-C.; Hsiao, L.-D.; Wang, C.-Y.; Lin, W.-N.; Shih, Y.-F.; Chen, Y.-W.; Cho, R.-L.; Tseng, H.-C.; Yang, C.-M. HO-1 Upregulation by Kaempferol via ROS-Dependent Nrf2-ARE Cascade Attenuates Lipopolysaccharide-Mediated Intercellular Cell Adhesion Molecule-1 Expression in Human Pulmonary Alveolar Epithelial Cells. Antioxidants 2022, 11, 782. [Google Scholar] [CrossRef]
- Zhou, L.J.; Zhu, X.Z. Reactive Oxygen Species-Induced Apoptosis in PC12 Cells and Protective Effect of Bilobalide. J. Pharmacol. Exp. Ther. 2000, 293, 982–988. [Google Scholar]
- Ma, T.; Lv, L.; Yu, Y.; Jia, L.; Song, X.; Xu, X.; Li, T.; Sheng, X.; Wang, H.; Zhang, J.; et al. Bilobalide Exerts Anti-Inflammatory Effects on Chondrocytes Through the AMPK/SIRT1/MTOR Pathway to Attenuate ACLT-Induced Post-Traumatic Osteoarthritis in Rats. Front. Pharmacol. 2022, 13, 783506. [Google Scholar] [CrossRef]
- Wang, F.; Huang, J.; Li, J.; Chen, K.; Zhang, X.; Zhang, Y.; Zhu, Y. Bilobalide, a Bioactive Compound on Sepsis-Induced Acute Lung Injury through Its Anti-Inflammatory and Antioxidative Activity. Pharmacogn. Mag. 2021, 17, 163. [Google Scholar] [CrossRef]
- Tchantchou, F.; Lacor, P.N.; Cao, Z.; Lao, L.; Hou, Y.; Cui, C.; Klein, W.L.; Luo, Y. Stimulation of Neurogenesis and Synaptogenesis by Bilobalide and Quercetin via Common Final Pathway in Hippocampal Neurons. JAD 2009, 18, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-M.; Gu, S.-S.; Mei, W.H.; Zhou, J.; Wang, Z.Z.; Xiao, W. Ginkgolides and Bilobalide Protect BV2 Microglia Cells against OGD/Reoxygenation Injury by Inhibiting TLR2/4 Signaling Pathways. Cell Stress Chaperones 2016, 21, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qu, P. Effects of Ginkgo Biloba Leaf Extract on Local Renin-Angiotensin System through TLR4/NF-ΚB Pathway in Cardiac Myocyte. Exp. Ther. Med. 2017, 14, 5857–5862. [Google Scholar] [CrossRef]
- He, G.; Yuan, C.; Hao, L.; Xu, Y.; Zhang, Z. GBE50 Attenuates Inflammatory Response by Inhibiting the P38 MAPK and NF-κB Pathways in LPS-Stimulated Microglial Cells. Evid. Based Complement. Altern. Med. 2014, 2014, 368598. [Google Scholar] [CrossRef]
- Liu, F.-Q.; Gao, Q.; Wang, D.-D.; Zhang, Z.-X. Effects of GBE50 on LPS/ATP induced NLRP3 inflammasome activation in primary rat microglia. China J. Chin. Mater. Med. 2018, 43, 3346–3352. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A Role for Quercetin in Coronavirus Disease 2019 (COVID-19). Phytother. Res. 2021, 35, 1230–1236. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant Effects of Ginkgolides and Bilobalide against Cerebral Ischemia Injury by Activating the Akt/Nrf2 Pathway in Vitro and in Vivo. Cell Stress Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef]
- Kaur, N.; Dhiman, M.; Perez-Polo, J.R.; Mantha, A.K. Ginkgolide B Revamps Neuroprotective Role of Apurinic/Apyrimidinic Endonuclease 1 and Mitochondrial Oxidative Phosphorylation against Aβ 25-35 -Induced Neurotoxicity in Human Neuroblastoma Cells: Neuroprotective Role of APE1. J. Neurosci. Res. 2015, 93, 938–947. [Google Scholar] [CrossRef]
- Lin, H.; Guo, X.; Zhang, S.; Dial, S.L.; Guo, L.; Manjanatha, M.G.; Moore, M.M.; Mei, N. Mechanistic Evaluation of Ginkgo Biloba Leaf Extract-Induced Genotoxicity in L5178Y Cells. Toxicol. Sci. 2014, 139, 338–349. [Google Scholar] [CrossRef]
- Parimoo, H.A.; Sharma, R.; Patil, R.D.; Sharma, O.P.; Kumar, P.; Kumar, N. Hepatoprotective Effect of Ginkgo Biloba Leaf Extract on Lantadenes-Induced Hepatotoxicity in Guinea Pigs. Toxicon 2014, 81, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wang, C.; Li, J.; Hou, Q.; Li, J.; Ma, J.; Wang, W.; Wang, Z. Ginkgolide B Protects Hippocampal Neurons from Apoptosis Induced by Beta-Amyloid 25–35 Partly via up-Regulation of Brain-Derived Neurotrophic Factor. Eur. J. Pharmacol. 2010, 647, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.-J.; Lai, X.-Y.; Chen, Y.-L.; Wang, C.-L.; Wei, C.-H.; Huang, W.-C. Ginkgolide C Suppresses Adipogenesis in 3T3-L1 Adipocytes via the AMPK Signaling Pathway. Evid. Based Complement. Altern. Med. 2015, 2015, 298635. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Chen, Y.-L.; Liu, H.-C.; Wu, S.-J.; Liou, C.-J. Ginkgolide C Reduced Oleic Acid-Induced Lipid Accumulation in HepG2 Cells. Saudi Pharm. J. 2018, 26, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, D.; Li, Z.; Shen, C.; Zhang, Y.; Li, J.; Yan, G.; Li, S.; Hu, B.; Li, J.; et al. Ginkgolide C Alleviates Myocardial Ischemia/Reperfusion-Induced Inflammatory Injury via Inhibition of CD40-NF-ΚB Pathway. Front. Pharmacol. 2018, 9, 109. [Google Scholar] [CrossRef]
- Zhao, M.; Qin, J.; Shen, W.; Wu, A. Bilobalide Enhances AMPK Activity to Improve Liver Injury and Metabolic Disorders in STZ-Induced Diabetes in Immature Rats via Regulating HMGB1/TLR4/NF-ΚB Signaling Pathway. BioMed Res. Int. 2021, 2021, 8835408. [Google Scholar] [CrossRef]
- Lu, L.; Wang, S.; Fu, L.; Liu, D.; Zhu, Y.; Xu, A. Bilobalide Protection of Normal Human Melanocytes from Hydrogen Peroxide-Induced Oxidative Damage via Promotion of Antioxidase Expression and Inhibition of Endoplasmic Reticulum Stress. Clin. Exp. Dermatol. 2016, 41, 64–73. [Google Scholar] [CrossRef]
- Serrano-García, N.; Pedraza-Chaverri, J.; Mares-Sámano, J.J.; Orozco-Ibarra, M.; Cruz-Salgado, A.; Jiménez-Anguiano, A.; Sotelo, J.; Trejo-Solís, C. Antiapoptotic Effects of EGb 761. Evid. Based Complement. Altern. Med. 2013, 2013, 495703. [Google Scholar] [CrossRef]
- Ku, S.-K.; Kim, T.H.; Bae, J.-S. Anticoagulant Activities of Persicarin and Isorhamnetin. Vasc. Pharmacol. 2013, 58, 272–279. [Google Scholar] [CrossRef]
- Yang, J.H.; Shin, B.Y.; Han, J.Y.; Kim, M.G.; Wi, J.E.; Kim, Y.W.; Cho, I.J.; Kim, S.C.; Shin, S.M.; Ki, S.H. Isorhamnetin Protects against Oxidative Stress by Activating Nrf2 and Inducing the Expression of Its Target Genes. Toxicol. Appl. Pharmacol. 2014, 274, 293–301. [Google Scholar] [CrossRef]
- Mango, D.; Weisz, F.; Nisticò, R. Ginkgolic Acid Protects against Aβ-Induced Synaptic Dysfunction in the Hippocampus. Front. Pharmacol. 2016, 7, 401. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumaran, S.; Nakamura, Y.; Henley, J.M.; Pountney, D.L. Ginkgolic Acid Promotes Autophagy-Dependent Clearance of Intracellular Alpha-Synuclein Aggregates. Mol. Cell. Neurosci. 2019, 101, 103416. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Drent, M.; de Boer, V.C.J.; Bast, A.; Haenen, G.R.M.M. Quercetin Reduces Markers of Oxidative Stress and Inflammation in Sarcoidosis. Clin. Nutr. 2011, 30, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9343460. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Zhang, Y.; Luo, M.; Wu, Q.; Yu, L.; Chu, H. Transcriptional Upregulation Centra of HO-1 by EGB via the MAPKs/Nrf2 Pathway in Mouse C2C12 Myoblasts. Toxicol. Vitr. 2015, 29, 380–388. [Google Scholar] [CrossRef]
- Ali, F.; Rahul; Jyoti, S.; Naz, F.; Ashafaq, M.; Shahid, M.; Siddique, Y.H. Therapeutic Potential of Luteolin in Transgenic Drosophila Model of Alzheimer’s Disease. Neurosci. Lett. 2019, 692, 90–99. [Google Scholar] [CrossRef]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid That Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef]
- Smith, J.V.; Burdick, A.J.; Golik, P.; Khan, I.; Wallace, D.; Luo, Y. Anti-Apoptotic Properties of Ginkgo Biloba Extract EGb 761 in Differentiated PC12 Cells. Cell. Mol. Biol. 2002, 48, 699–707. [Google Scholar]
- Perianayagam, M.C.; Madias, N.E.; Pereira, B.J.G.; Jaber, B.L. CREB Transcription Factor Modulates Bcl2 Transcription in Response to C5a in HL-60-Derived Neutrophils. Eur. J. Clin. Investig. 2006, 36, 353–361. [Google Scholar] [CrossRef]
- Tchantchou, F.; Xu, Y.; Wu, Y.; Christen, Y.; Luo, Y. EGb 761 Enhances Adult Hippocampal Neurogenesis and Phosphorylation of CREB in Transgenic Mouse Model of Alzheimer’s Disease. FASEB J. 2007, 21, 2400–2408. [Google Scholar] [CrossRef]
- Hao, F.; Li, A.; Yu, H.; Liu, M.; Wang, Y.; Liu, J.; Liang, Z. Enhanced Neuroprotective Effects of Combination Therapy with Bone Marrow-Derived Mesenchymal Stem Cells and Ginkgo Biloba Extract (EGb761) in a Rat Model of Experimental Autoimmune Encephalomyelitis. Neuroimmunomodulation 2016, 23, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s Disease Hypothesis and Related Therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef]
- Andersson, U. The Cholinergic Anti-Inflammatory Pathway Alleviates Acute Lung Injury. Mol. Med. 2020, 26, 64. [Google Scholar] [CrossRef] [PubMed]
- Ivic, L.; Sands, T.T.J.; Fishkin, N.; Nakanishi, K.; Kriegstein, A.R.; Strømgaard, K. Terpene Trilactones from Ginkgo Biloba Are Antagonists of Cortical Glycine and GABAA Receptors. J. Biol. Chem. 2003, 278, 49279–49285. [Google Scholar] [CrossRef] [PubMed]
- Alexandris, N.; Lagoumintzis, G.; Chasapis, C.T.; Leonidas, D.D.; Papadopoulos, G.E.; Tzartos, S.J.; Tsatsakis, A.; Eliopoulos, E.; Poulas, K.; Farsalinos, K. Nicotinic Cholinergic System and COVID-19: In Silico Evaluation of Nicotinic Acetylcholine Receptor Agonists as Potential Therapeutic Interventions. Toxicol. Rep. 2021, 8, 73–83. [Google Scholar] [CrossRef]
- Spiegel, R.; Kalla, R.; Mantokoudis, G.; Maire, R.; Mueller, H.; Hoerr, R.; Ihl, R. Ginkgo Biloba Extract EGb 761® Alleviates Neurosensory Symptoms in Patients with Dementia: A Meta-Analysis of Treatment Effects on Tinnitus and Dizziness in Randomized, Placebo-Controlled Trials. CIA 2018, 13, 1121–1127. [Google Scholar] [CrossRef]
- Di Meo, F.; Cuciniello, R.; Margarucci, S.; Bergamo, P.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Ginkgo Biloba Prevents Oxidative Stress-Induced Apoptosis Blocking P53 Activation in Neuroblastoma Cells. Antioxidants 2020, 9, 279. [Google Scholar] [CrossRef]
- Wang, L.P.; Zhang, X.Y.; Liu, N.; Ma, Z.Z.; Fang, D.S. Comparison of Integrated Traditional Chinese and Western Medicine Therapy on Vascular Cognitive Impairment with No Dementia. Genet. Mol. Res. 2015, 14, 4896–4902. [Google Scholar] [CrossRef]
- Gschwind, Y.J.; Bridenbaugh, S.A.; Reinhard, S.; Granacher, U.; Monsch, A.U.; Kressig, R.W. Ginkgo Biloba Special Extract LI 1370 Improves Dual-Task Walking in Patients with MCI: A Randomised, Double-Blind, Placebo-Controlled Exploratory Study. Aging Clin. Exp. Res. 2017, 29, 609–619. [Google Scholar] [CrossRef]
- Kuo, L.-C.; Song, Y.-Q.; Yao, C.-A.; Cheng, I.H.; Chien, C.-T.; Lee, G.-C.; Yang, W.-C.; Lin, Y. Ginkgolide A Prevents the Amyloid-β-Induced Depolarization of Cortical Neurons. J. Agric. Food Chem. 2019, 67, 81–89. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, P.; Li, J.; Liu, T.; Zhang, Y.; Wang, Q.; Zhang, J.; Lu, X.; Fan, X. Neuroprotective Effects of Ginkgo Biloba Dropping Pills in Parkinson’s Disease. J. Pharm. Anal. 2021, 11, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Schindowski, K.; Leutner, S.; Kressmann, S.; Eckert, A.; Müller, W.E. Age-Related Increase of Oxidative Stress-Induced Apoptosis in MicePrevention by Ginkgo Biloba Extract (EGb761). J. Neural Transm. 2001, 108, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Xiao, S.; Liu, J.; Guo, K.; Wu, F.; Yew, D.T.; Xu, J. Ginkgo Biloba Extract EGb761 Protects against Aging-Associated Mitochondrial Dysfunction in Platelets and Hippocampi of SAMP8 Mice. Platelets 2010, 21, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Wang, M.-Y.; Fu, X.-R.; Peng-Yu; Gao, G.-F.; Fan, Y.-M.; Duan, X.-L.; Zhao, B.-L.; Chang, Y.-Z.; Shi, Z.-H. Neuroprotective Effects of Ginkgetin against Neuroinjury in Parkinson’s Disease Model Induced by MPTP via Chelating Iron. Free Radic. Res. 2015, 49, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- You, O.H.; Kim, S.-H.; Kim, B.; Sohn, E.J.; Lee, H.-J.; Shim, B.-S.; Yun, M.; Kwon, B.-M.; Kim, S.-H. Ginkgetin Induces Apoptosis via Activation of Caspase and Inhibition of Survival Genes in PC-3 Prostate Cancer Cells. Bioorganic Med. Chem. Lett. 2013, 23, 2692–2695. [Google Scholar] [CrossRef]
- Rhein, V.; Giese, M.; Baysang, G.; Meier, F.; Rao, S.; Schulz, K.L.; Hamburger, M.; Eckert, A. Ginkgo Biloba Extract Ameliorates Oxidative Phosphorylation Performance and Rescues Aβ-Induced Failure. PLoS ONE 2010, 5, e12359. [Google Scholar] [CrossRef]
- Adebayo, O.G.; Asiwe, J.N.; Ben-Azu, B.; Aduema, W.; Onyeleonu, I.; Akpotu, A.E.; Wopara, I.; Kolawole, T.A.; Umoren, E.B.; Igbokwe, V.; et al. Ginkgo Biloba Protects Striatal Neurodegeneration and Gut Phagoinflammatory Damage in Rotenone-induced Mice Model of Parkinson’s Disease: Role of Executioner Caspase-3/Nrf2/ ARE Signaling. J. Food Biochem. 2022, 46, e14253. [Google Scholar] [CrossRef]
- Dong, Z.-H.; Zhang, C.-Y.; Pu, B.-H. Effects of ginkgo biloba tablet in treating mild cognitive impairment. Chin. J. Integr. Tradit. West. Med. 2012, 32, 1208–1211. [Google Scholar]
- Mahdy, H.M.; Tadros, M.G.; Mohamed, M.R.; Karim, A.M.; Khalifa, A.E. The Effect of Ginkgo Biloba Extract on 3-Nitropropionic Acid-Induced Neurotoxicity in Rats. Neurochem. Int. 2011, 59, 770–778. [Google Scholar] [CrossRef]
- Beg, T.; Jyoti, S.; Naz, F.; Rahul; Ali, F.; Ali, S.K.; Reyad, A.M.; Siddique, Y.H. Protective Effect of Kaempferol on the Transgenic Drosophila Model of Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2018, 17, 421–429. [Google Scholar] [CrossRef]
- Jiang, M.; Li, J.; Peng, Q.; Liu, Y.; Liu, W.; Luo, C.; Peng, J.; Li, J.; Yung, K.K.L.; Mo, Z. Neuroprotective Effects of Bilobalide on Cerebral Ischemia and Reperfusion Injury Are Associated with Inhibition of Pro-Inflammatory Mediator Production and down-Regulation of JNK1/2 and P38 MAPK Activation. J. Neuroinflamm. 2014, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Z.; Yan, M.; He, P.; Chen, Z.; Dai, H. The Protective Role of Isorhamnetin on Human Brain Microvascular Endothelial Cells from Cytotoxicity Induced by Methylglyoxal and Oxygen-Glucose Deprivation. J. Neurochem. 2016, 136, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.-H.; Shah, F.-A.; Cho, E.-H.; Gim, S.-A.; Jeon, S.-J.; Kim, K.-M.; Kim, Y.-M.; Kim, M.-O.; Koh, P.-O. Ginkgo Biloba Extract (EGb 761) Prevents the Ischemic Brain Injury-Induced Decrease in Parvalbumin Expression. Lab. Anim. Res. 2012, 28, 77. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shi, Y.; Zhao, L. Ginkgolide B Alleviates Learning and Memory Impairment in Rats With Vascular Dementia by Reducing Neuroinflammation via Regulating NF-ΚB Pathway. Front. Pharmacol. 2021, 12, 676392. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qinghai, S.; Kai, L.; Xue, M.; Lili, N.; Jihua, R.; Zhengxiang, L.; Xiaoling, L.; Di, G.; Qi, Y.; et al. Oral Administration of Ginkgolide B Alleviates Hypoxia-Induced Neuronal Damage in Rat Hippocampus by Inhibiting Oxidative Stress and Apoptosis. Iran. J. Basic Med. Sci. 2019, 22, 140–145. [Google Scholar] [CrossRef]
- Wang, L.; Lei, Q.; Zhao, S.; Xu, W.; Dong, W.; Ran, J.; Shi, Q.; Fu, J. Ginkgolide B Maintains Calcium Homeostasis in Hypoxic Hippocampal Neurons by Inhibiting Calcium Influx and Intracellular Calcium Release. Front. Cell. Neurosci. 2021, 14, 627846. [Google Scholar] [CrossRef]
- Chan, P.-C.; Xia, Q.; Fu, P.P. Ginkgo Biloba Leave Extract: Biological, Medicinal, and Toxicological Effects. J. Environ. Sci. Health Part C 2007, 25, 211–244. [Google Scholar] [CrossRef]
- Al-Yahya, A.A.; Al-Majed, A.A.; Al-Bekairi, A.M.; Al-Shabanah, O.A.; Qureshi, S. Studies on the Reproductive, Cytological and Biochemical Toxicity of Ginkgo Biloba in Swiss Albino Mice. J. Ethnopharmacol. 2006, 107, 222–228. [Google Scholar] [CrossRef]
- Bent, S.; Goldberg, H.; Padula, A.; Avins, A.L. Spontaneous Bleeding Associated with Ginkgo Biloba: A Case Report and Systematic Review of the Literature: A Case Report and Systematic Review of the Literature. J. Gen. Intern. Med. 2005, 20, 657–661. [Google Scholar] [CrossRef]
- Pedroso, J.L.; Henriques Aquino, C.C.; Escórcio Bezerra, M.L.; Baiense, R.F.; Suarez, M.M.; Dutra, L.A.; Braga-Neto, P.; Povoas Barsottini, O.G. Ginkgo Biloba and Cerebral Bleeding: A Case Report and Critical Review. Neurologist 2011, 17, 89–90. [Google Scholar] [CrossRef]
- Rosenblatt, M.; Mindel, J. Spontaneous Hyphema Associated with Ingestion of Ginkgo Biloba Extract. N. Engl. J. Med. 1997, 336, 1108. [Google Scholar] [CrossRef] [PubMed]
- Oken, B.S.; Storzbach, D.M.; Kaye, J.A. The Efficacy of Ginkgo Biloba on Cognitive Function in Alzheimer Disease. Arch. Neurol. 1998, 55, 1409. [Google Scholar] [CrossRef]
- Jiang, L.; Su, L.; Cui, H.; Ren, J.; Li, C. Ginkgo Biloba Extract for Dementia: A Systematic Review. Shanghai Arch Psychiatry 2013, 25, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Bal Dit Sollier, C.; Caplain, H.; Drouet, L. No Alteration in Platelet Function or Coagulation Induced by EGb761 in a Controlled Study: No Effect of Egb761 on Platelet and Coagulation. Clin. Lab. Haematol. 2003, 25, 251–253. [Google Scholar] [CrossRef]
- Kudolo, G.B.; Wang, W.; Barrientos, J.; Elrod, R.; Blodgett, J. The Ingestion of Ginkgo Biloba Extract (EGb 761) Inhibits Arachidonic Acid-Mediated Platelet Aggregation and Thromboxane B2 Production in Healthy Volunteers. J. Herb. Pharmacother. 2004, 4, 13–26. [Google Scholar] [CrossRef]
- Snitz, B.E. Ginkgo Biloba for Preventing Cognitive Decline in Older AdultsA Randomized Trial. JAMA 2009, 302, 2663. [Google Scholar] [CrossRef] [PubMed]
- Solomon, P.R.; Adams, F.; Silver, A.; Zimmer, J.; DeVeaux, R. Ginkgo for Memory Enhancement: A Randomized Controlled Trial. JAMA 2002, 288, 835. [Google Scholar] [CrossRef] [PubMed]
- Dodge, H.H.; Zitzelberger, T.; Oken, B.S.; Howieson, D.; Kaye, J. A Randomized Placebo-Controlled Trial of Ginkgo Biloba for the Prevention of Cognitive Decline. Neurology 2008, 70, 1809–1817. [Google Scholar] [CrossRef]
- Akanchise, T.; Angelova, A. Potential of Nano-Antioxidants and Nanomedicine for Recovery from Neurological Disorders Linked to Long COVID Syndrome. Antioxidants 2023, 12, 393. [Google Scholar] [CrossRef]
- Li, M.; Al-Jamal, K.T.; Kostarelos, K.; Reineke, J. Physiologically Based Pharmacokinetic Modeling of Nanoparticles. ACS Nano 2010, 4, 6303–6317. [Google Scholar] [CrossRef]
- De Matteis, V.; Rinaldi, R. Toxicity Assessment in the Nanoparticle Era. In Cellular and Molecular Toxicology of Nanoparticles; Saquib, Q., Faisal, M., Al-Khedhairy, A.A., Alatar, A.A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1048, pp. 1–19. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Archunan, G.; Sivakumar, M.; Tamil Selvan, S.; Fred, A.L.; Kumar, S.; Gulyás, B.; Padmanabhan, P. Theranostic Applications of Nanoparticles in Neurodegenerative Disorders. IJN 2018, 13, 5561–5576. [Google Scholar] [CrossRef] [PubMed]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef] [PubMed]
- Cady, R. A Novel Intranasal Breath-Powered Delivery System for Sumatriptan: A Review of Technology and Clinical Application of the Investigational Product AVP-825 in the Treatment of Migraine. Expert Opin. Drug Deliv. 2015, 12, 1565–1577. [Google Scholar] [CrossRef]
- Yamada, K.; Hasegawa, M.; Kametani, S.; Ito, S. Nose-to-Brain Delivery of TS-002, Prostaglandin D 2 Analogue. J. Drug Target. 2007, 15, 59–66. [Google Scholar] [CrossRef]
- Sood, S.; Jain, K.; Gowthamarajan, K. Intranasal Therapeutic Strategies for Management of Alzheimer’s Disease. J. Drug Target. 2014, 22, 279–294. [Google Scholar] [CrossRef]
- Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Stanisçuaski Guterres, S.; Raffin Pohlmann, A.; Nicoli, S. Surface-Modified Nanocarriers for Nose-to-Brain Delivery: From Bioadhesion to Targeting. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef]
- Wen, Z.; Yan, Z.; Hu, K.; Pang, Z.; Cheng, X.; Guo, L.; Zhang, Q.; Jiang, X.; Fang, L.; Lai, R. Odorranalectin-Conjugated Nanoparticles: Preparation, Brain Delivery and Pharmacodynamic Study on Parkinson’s Disease Following Intranasal Administration. J. Control. Release 2011, 151, 131–138. [Google Scholar] [CrossRef]
- Karavelioglu, Z.; Cakir-Koc, R. Preparation of Chitosan Nanoparticles as Ginkgo Biloba Extract Carrier: In Vitro Neuroprotective Effect on Oxidative Stress-Induced Human Neuroblastoma Cells (SH-SY5Y). Int. J. Biol. Macromol. 2021, 192, 675–683. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Yang, F.; Wu, W.; Liu, Y.; Wang, L.; Wang, L.; Wang, Z. Enhanced Bioaccessibility in Vitro and Bioavailability of Ginkgo Biloba Extract Nanoparticles Prepared by Liquid Anti-solvent Precipitation. Int. J. Food Sci. Technol. 2019, 54, 2266–2276. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, S.; Liu, P.; Liu, W.; Wang, Q.; Liu, Y.; Tan, H.; Chen, X.; Shi, X.; Wang, Q.; et al. Polymeric Nanoparticles-Based Brain Delivery with Improved Therapeutic Efficacy of Ginkgolide B in Parkinson’s Disease. IJN 2020, 15, 10453–10467. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.; Kapil, R.; Singh, B. Formulation Development and Systematic Optimization of Solid Lipid Nanoparticles of Quercetin for Improved Brain Delivery. J. Pharm. Pharmacol. 2011, 63, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, P.; Ghaffari, S.; Arbabi Bidgoli, S.; Qomi, M.; Haghighat, S. Preparation, Characterization and Evaluation of Ginkgo Biloba Solid Lipid Nanoparticles. Nanomed. Res. J. 2018, 3, 71–78. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, Q.; Wang, M.; Zhao, H.; Lin, Y.; Zhou, S. Green Biosynthesized Silver Nanoparticles With Aqueous Extracts of Ginkgo Biloba Induce Apoptosis via Mitochondrial Pathway in Cervical Cancer Cells. Front. Oncol. 2020, 10, 575415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, R.; Liu, P.; Cheng, G.; Yang, Q.; Chen, X.; Wu, Z.; Yuan, D.; Chen, T. Efficient Sustained-Release Nanoparticle Delivery System Protects Nigral Neurons in a Toxin Model of Parkinson’s Disease. Pharmaceutics 2022, 14, 1731. [Google Scholar] [CrossRef]
- Wu, X.; Huo, Q.; Quan, Q.; Yang, X.; Yu, N.; Wang, Y. Optimizing the Formulation for Ginkgolide B Solid Dispersion. Open Life Sci. 2018, 13, 253–262. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, H.; Wang, T.; Wang, C. Green Synthesis and Antimicrobial Activity of Monodisperse Silver Nanoparticles Synthesized Using Ginkgo Biloba Leaf Extract. Phys. Lett. A 2016, 380, 3773–3777. [Google Scholar] [CrossRef]
- Osman, A.S.; Abu-Risha, S.E.; Bakr, S.M.; Altyar, A.E.; Fayad, E.; EL-Sawi, M.R.; EL-Kholy, W.M. Comparative Study between Effects of Ginkgo Biloba Extract and Extract Loaded on Gold Nanoparticles on Hepatotoxicity Induced by Potassium Bromate. Environ. Sci. Pollut. Res. 2023, 30, 5027–5036. [Google Scholar] [CrossRef]
- Elshazly, E.H.; Nasr, A.; Elnosary, M.E.; Gouda, G.A.; Mohamed, H.; Song, Y. Identifying the Anti-MERS-CoV and Anti-HcoV-229E Potential Drugs from the Ginkgo Biloba Leaves Extract and Its Eco-Friendly Synthesis of Silver Nanoparticles. Molecules 2023, 28, 1375. [Google Scholar] [CrossRef]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; El-Aleem, S.A.A.; El-Tahawy, N.F.G. Neuroprotective Effect of Quercetin Nanoparticles: A Possible Prophylactic and Therapeutic Role in Alzheimer’s Disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh Jajin, E.; Esmaeili, A.; Rahgozar, S.; Noorbakhshnia, M. Quercetin-Conjugated Superparamagnetic Iron Oxide Nanoparticles Protect AlCl3-Induced Neurotoxicity in a Rat Model of Alzheimer’s Disease via Antioxidant Genes, APP Gene, and MiRNA-101. Front. Neurosci. 2021, 14, 598617. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wen, J.; Garg, S.; Zhang, W.; Teng, L.R.; Liu, D.; Zhou, Y. Development of a Novel Niosomal System for Oral Delivery of Ginkgo Biloba Extract. IJN 2013, 8, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, I.; Al-Abbasi, F.A.; Afzal, M.; Altayb, H.N.; Nadeem, M.S.; Gupta, G. Formulation and Evaluation of Kaempferol Loaded Nanoparticles against Experimentally Induced Hepatocellular Carcinoma: In Vitro and In Vivo Studies. Pharmaceutics 2021, 13, 2086. [Google Scholar] [CrossRef]
- Abbas, H.; Sayed, N.S.E.; Youssef, N.A.H.A.; Gaafar, P.M.E.; Mousa, M.R.; Fayez, A.M.; Elsheikh, M.A. Novel Luteolin-Loaded Chitosan Decorated Nanoparticles for Brain-Targeting Delivery in a Sporadic Alzheimer’s Disease Mouse Model: Focus on Antioxidant, Anti-Inflammatory, and Amyloidogenic Pathways. Pharmaceutics 2022, 14, 1003. [Google Scholar] [CrossRef]
- Settu, K.; Vaiyapuri, M. FORMULATION AND EVALUATION OF ISORHAMNETIN LOADED POLY LACTIC-CO-GLYCOLIC ACID NANOPARTICLES. Asian J. Pharm. Clin. Res. 2017, 10, 177. [Google Scholar] [CrossRef]
- Tang, J.; Sun, J.; Cui, F.; Zhang, T.; Liu, X.; He, Z. Self-Emulsifying Drug Delivery Systems for Improving Oral Absorption of Ginkgo Biloba Extracts. Drug Deliv. 2008, 15, 477–484. [Google Scholar] [CrossRef]
- Wang, T.; Wu, C.; Fan, G.; Li, T.; Gong, H.; Cao, F. Ginkgo Biloba Extracts-Loaded Starch Nano-Spheres: Preparation, Characterization, and in Vitro Release Kinetics. Int. J. Biol. Macromol. 2018, 106, 148–157. [Google Scholar] [CrossRef]
- Bremer-Hoffmann, S.; Halamoda-Kenzaoui, B.; Borgos, S.E. Identification of Regulatory Needs for Nanomedicines: Regulatory Needs for Nanomedicines. J. Interdiscip. Nanomed. 2018, 3, 4–15. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Faria, M.; Björnmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum Information Reporting in Bio–Nano Experimental Literature. Nature Nanotech. 2018, 13, 777–785. [Google Scholar] [CrossRef] [PubMed]









| Stimuli | Model/Species | Disease Model | Administration/Protocol | Mechanism of Oxidative Stress | Ref. |
|---|---|---|---|---|---|
| N-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) | Male C57BL/6 mice (20–25 g) | PD, neurodegeneration | Intraperitoneal injection of MPTP (20 mg/kg), two times at 4 h intervals daily for 5 days, followed by oral administration of Sophora tomentosa (25 mg/kg, 50 mg/kg, and 100 mg/kg) for 15 consecutive days until behavioral tests |
| [88] |
| Rotenone | Rotenone-induced Sprague–Dawley and Lewis rats | PD | Infusion of a 2–3 mg/kg dose of rotenone per day via a jugular vein cannula attached to a subcutaneous osmotic minipump |
| [89] |
| Paraquat (N, N′-dimethyl-4-4′-bipiridinium) | Human neuroblastoma SH-SY5Y cells | PD | Treated with paraquat (0.5 mM PQ) for 48 h |
| [90] |
| Hydrogen peroxide (H2O2) | SH-SY5Y cells | PD, AD, Huntington’s disease | Incubation with varying concentrations of H2O2 (0 to 250 µM) for 30 min, followed by evaluation of cell viability |
| [91,92] |
| 6-hydroxydopamine (6-OHDA) | SH-SY5Y cells | PD, AD, and dementia | Cells incubated with 200 µM of 6-OHDA for 24 h with or without hyperoxide or NAC pretreatment |
| [93] |
| Glutamate analog; homocysteate quisqualate ibotenate | Neuronal hybridoma cell line, N18-RE-105 mouse neuroblastoma cells | ALS, AD, dementia, PD, multiple sclerosis (MS) | Continuous exposure of cells to ʟ-glutamate (1–10 mM) or quisqualate (0.1–1.0 mM) for 5 min |
| [94,95] |
| Mycotoxin 3-nitropropionic acid (3-NP) | Male Wistar rats (300–350 g) | Huntington disease | Intraperitoneal administration of 3-NP (10 mg/kg) |
| [96] |
| Buthionine sulfoximine (BSO) | Hippocampus-derived immortalized cell line (HT22) | Chronic psychological stress | Treatment with 1 mM BSO for 14 h |
| [97] |
| Tunicamycin | SH-SY5Y cells | Endoplasmic reticulum stress | Incubation with 1 μM tunicamycin |
| [98] |
| RNAi | Drosophila | Oxidative damage | Knockdown of SOD2 using the Gal4/UAS system to express SOD2 inverted repeat (Sod2-IR) transgenes |
| [99] |
| Compound | Sources | Activity/Mechanism | Ref. |
|---|---|---|---|
 | Leaves, root, and bark |
| [120,121,122,123,124,125,126] |
Bilobalide | Leaves and bark |
| [119,127,128] |
Isorhamnetin | Leaves |
| [117,129,130,131] |
Ginkgolic acid | Leaves |
| [132,133] |
Quercetin | Leaves |
| [129,134,135,136] |
Luteolin | Leaves |
| [137,138] |
Kaempferol | Leaves |
| [104,105,107] |
| Ginkgo biloba Constituents | Neurological Condition | Model | Outcome | Ref. |
|---|---|---|---|---|
| EGb 761 | Age-associated mitochondrial dysfunction | SAMP8 mice, oral administration |
| [153,154] |
| Ginkgetin and bilobalide | PD | MPTP-induced mice, oral administration |
| [155,156] |
| Ginkgo biloba extract (EGb LI 1370) | AD | SH-SY5Y cells expressing amyloid precursor protein (APP) |
| [157] |
| Ginkgo biloba supplements (GBS) | PD | Rotenone-induced Swiss mice, oral administration |
| [158] |
| Ginkgo biloba dropping pill (GBDP) | PD | In vivo: 6-OHDA-induced zebrafish MPTP-induced male C57BL/6 mice, oral administration In vitro: MPP+-induced SH-SY5Y cells |
| [152] |
| Ginkgo biloba tablets | Vascular cognitive impairment of non-dementia (VCIND) | Randomized clinical study of 80 patients with VCIND |
| [159] |
| EGb (Symfona® forte 120 mg) | Mild cognitive impairment (MCI) | Randomized, double-blind, placebo exploratory study in 50–85-year-old patients with MCI and associated dual-task-related gait impairment |
| [150] |
| EGb 761 | Huntington’s disease | 3-NP-induced rats, I.P. injection |
| [105,160] |
| Kaempferol and luteolin | AD | Transgenic drosophila expressing wild-type human Aβ42 |
| [137,161] |
| Bilobalide | Cerebral ischemia and reperfusion (I/R) injury | MCAO male Sprague–Dawley rats |
| [162] |
| Isorhamnetin | Ischemia-induced cerebral vascular degeneration | Human brain microvascular endothelial cells (HBMECs) |
| [163] |
| EGb 761 | Ischemic brain injury | MCAO male Sprague–Dawley rats |
| [164] |
| Ginkgolide B (GB) | Vascular dementia (VD), hypoxic injury | In vivo: BCCAO rats, intraperitoneal injection In vitro: Oxygen-glucose deprivation (OGD) in SH-SY5Y cells, primary hippocampal neurons subjected to chemical hypoxia (0.7 mM CoCl2) |
| [165,166,167] |
| Molecule | Nanocarrier | Technique | ζ-Potential, mV | Size, nm | PDI | DL% | EE% | Morphology | Pathology | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Ginkgolide B | GB–PPNPs | Antisolvent precipitation | −10.37 ± 0.56 | 77.58 ± 0.77 | 0.124 ± 0.018 | 19.43% | 92.08 | Spherical | PD | [198] |
| Quercetin | QNPs | Antisolvent precipitation | — | <1000 | 0.734 | — | — | — | AD | [203] |
| Quercetin | QT–SPION conjugates | Co-precipitation technique | — | 30–50 | — | — | — | Spherical | AD | [204] |
| Ginkgo biloba extract | EGb niosomes | Freeze-drying and spray-drying methods | Noisome suspension, −0.1 ± 1.7 Freeze-drying, −11.6 ± 4.3 Spray-drying, −33.6 ± 1.6 | 141.3 ± 11.9 661.3 ± 78.6 680.2 ± 90.0 | — | — | 50.0 ± 1.9 50.1 ± 1.0 77.5 ± 1.0 | Spherical and smooth surface | Improving oral bioavailability | [205] |
| Kaempferol | Kaempferol-loaded nanoparticles (KFP–NPs) | Quasi-emulsion methods | −28.5 to −7.5 | 201 ± 0.45 | 0.12 to 0.95 | 11.34 to 15.06 | 30.14 to 46.72 | Solid sphere with a smooth surface | Hepatoprotective and antioxidant effects | [206] |
| Luteolin | Luteolin-loaded chitosomes (LUT–CHS) | Ethanol injection | 37.4 ± 2.13 | 412.8 ± 3.28 | 0.378 ± 0.07 | — | 86.6 ± 2.05 | Spherical vesicular system with a phospholipid bilayer membrane | Cognitive dysfunction in Alzheimer’s disease (AD) | [207] |
| Ginkgo biloba | EGb-loaded solid lipid nanoparticles (SLNs) | High-pressure homogenization | −12.6 to −28 | 104 to 621 | < 0.5 | — | 79 to 89 | Spherical, smooth, and rounded surface | Cytotoxicity and antibacterial activities | [196] |
| Isorhamnetin | Isorhamnetin-PLGA NPs | Double-emulsion solvent evaporation | — | 255 to 342 | — | — | — | — | — | [208] |
| Ginkgo biloba extract | Gb-extract -loaded chitosan nanoparticles (Gb–CsNPs) | Ionic gelation | 29.3 | 104.4 | 0.09 | 40 | 97.4 | Smooth and spherical morphology | Oxidative stress | [192] |
| Silver nanoparticles (AgNPs) | Biogenic synthesis | −74.2 ± 2.45 | 5.46 to 19.40 | — | — | — | Agglomerated spherical shapes | Antiviral activities against MERS-CoV and HCoV-229E | [202] | |
| Self-emulsifying drug delivery systems (SEEDS) | Self-emulsification | — | ~100 | — | — | — | — | Improving oral absorption | [209] | |
| EGb-loaded nanospheres | Nanoprecipitation | — | 100 to 200 | 0.428 to 0.478 | — | — | Oval or spherical shape with a smooth surface | In vitro release kinetics | [210] |
| Nanospecific Characteristics | Test Method |
|---|---|
| Size/size distribution | DLS |
| Physical form/shape/morphology | TEM, cryo-TEM |
| Surface charge | Zeta potential, electrophoretic mobility (EPM) |
| Aggregation behaviour | DLS |
| Stability and uniformity | DLS, UV–VIS spectroscopy |
| Density/weight/volume fraction of nanomaterial dispersed in the medium | Ultracentrifugation, densitometry |
| Drug encapsulation | UV–VIS spectrometry, high-performance liquid chromatography (HPLC) |
| Presence of targeting moieties | Kinetic turbidity assays, Spectroscopic assays (UV–VIS, circular dichroism), surface plasmon resonance (SPR) binding assays |
| Toxicity | Cytotoxicity assessment using MTT and LDH assays |
| Biocompatibility | Immunological response, hemolytic properties |
| Structural and functional properties | TEM, SEM, small-angle X-ray scattering (SAXS), NTA, high-resolution transmission electron microscopy (HRTEM), atomic force microscopy (AFM), extended X-ray absorption fine structure (EXAFS), ferromagnetic resonance (FMR), DSC, differential centrifugal sedimentation (DCS), inductively coupled plasma atomic emission spectroscopy (ICP-MS), UV–VIS, matrix-assisted laser desorption/ionization (MALDI), nuclear magnetic resonance (NMR), superparamagnetic relaxometry, tunable resistive pulse sensing (TRPS) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akanchise, T.; Angelova, A. Ginkgo Biloba and Long COVID: In Vivo and In Vitro Models for the Evaluation of Nanotherapeutic Efficacy. Pharmaceutics 2023, 15, 1562. https://doi.org/10.3390/pharmaceutics15051562
Akanchise T, Angelova A. Ginkgo Biloba and Long COVID: In Vivo and In Vitro Models for the Evaluation of Nanotherapeutic Efficacy. Pharmaceutics. 2023; 15(5):1562. https://doi.org/10.3390/pharmaceutics15051562
Chicago/Turabian StyleAkanchise, Thelma, and Angelina Angelova. 2023. "Ginkgo Biloba and Long COVID: In Vivo and In Vitro Models for the Evaluation of Nanotherapeutic Efficacy" Pharmaceutics 15, no. 5: 1562. https://doi.org/10.3390/pharmaceutics15051562