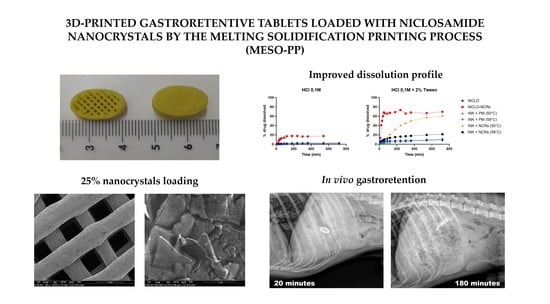
3D-Printed Gastroretentive Tablets Loaded with Niclosamide Nanocrystals by the Melting Solidification Printing Process (MESO-PP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Niclosamide Nanocrystals Manufacturing
2.2.1. Nanomilling
2.2.2. Freeze Drying and Moisture Content
2.3. Particle Size and Polydispersity Index
2.4. Nanoparticles Tracking Analysis (NTA)
2.5. Scanning Electron Microscopy (SEM) and Scanning Transmission Electron Microscopy (STEM)
2.6. Cell Culture
Cell Viability MTS Assays
2.7. Ink Formulation
2.8. Infrared Spectroscopy
2.9. X-ray Diffraction
2.10. Thermogravimetric Analyses and Differential Scanning Calorimetry
2.11. Hot-Stage Microscopy
2.12. Rheological Analysis
2.13. Printlet Set Up
2.14. Printing Process
2.15. Weight/Volume Ratio and Weight Variation
2.16. Buoyancy and In Vitro Drug Release Evaluation
2.17. In Vivo Gastroretention
2.18. Statistical Analysis
3. Result and Discussion
3.1. Formulation and Characterization of Niclosamide Nanocrystals
3.2. Ink Formulation and Physicochemical Characterization
3.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2.2. Thermal Analysis: TGA and DSC
3.2.3. Hot Stage Microscopy
3.2.4. Rheological Behavior
3.3. Printing Process and 3D Printed Tablets (Printlets) Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Low, Z.Y.; Farouk, I.A.; Lal, S.K. Drug Repositioning: New Approaches and Future Prospects for Life-Debilitating Diseases and the COVID-19 Pandemic Outbreak. Viruses 2020, 12, 1058. [Google Scholar] [CrossRef]
- Brown, D. Antibiotic resistance breakers: Can repurposed drugs fill the antibiotic discovery void? Nat. Rev. Drug Discov. 2015, 14, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, L.H.; Zagari, R.M.; Bazzoli, F. Epidemiology of Helicobacter pylori infection. Helicobacter 2014, 19 (Suppl. S1), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Peek, R.M.; Blaser, M.J. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2002, 2, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef] [PubMed]
- Hagymási, K.; Tulassay, Z. Helicobacter pylori infection: New pathogenetic and clinical aspects. World J. Gastroenterol. 2014, 20, 6386–6399. Available online: http://www.wjgnet.com (accessed on 30 December 2022).
- La OMS Publica La Lista De Las Bacterias Para Las Que Se Necesitan Urgentemente Nuevos Antibióticos. Available online: https://www.who.int/es/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 30 December 2022).
- The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2007: (Including the 15th Model List of Essential Medicines). Available online: https://apps.who.int/iris/handle/10665/43745 (accessed on 30 December 2022).
- Vliet, S.M.F.; Dasgupta, S.; Sparks, N.R.L.; Kirkwood, J.S.; Vollaro, A.; Hur, M.; zur Nieden, N.I.; Volz, D.C. Maternal-to-zygotic transition as a potential target for niclosamide during early embryogenesis. Toxicol. Appl. Pharmacol. 2019, 380, 114699. [Google Scholar] [CrossRef]
- Barbosa, E.J.; Löbenberg, R.; de Araujo, G.L.B.; Bou-Chacra, N.A. Niclosamide repositioning for treating cancer: Challenges and nano-based drug delivery opportunities. Eur. J. Pharm. Biopharm. 2019, 141, 58–69. [Google Scholar] [CrossRef]
- Cairns, D.M.; Boorgu, D.S.S.K.; Levin, M.; Kaplan, D.L. Niclosamide rescues microcephaly in a humanized in vivo model of Zika infection using human induced neural stem cells. Biol. Open 2018, 7, bio031807. [Google Scholar] [CrossRef]
- Blake, S.; Shaabani, N.; Eubanks, L.M.; Maruyama, J.; Manning, J.T.; Beutler, N.; Paessler, S.; Ji, H.; Teijaro, J.R.; Janda, K.D. Salicylanilides Reduce SARS-CoV-2 Replication and Suppress Induction of Inflammatory Cytokines in a Rodent Model. ACS Infect. Dis. 2021, 7, 2229–2237. [Google Scholar] [CrossRef]
- Imperi, F.; Massai, F.; Pillai, C.R.; Longo, F.; Zennaro, E.; Rampioni, G.; Visc, P.; Leoni, L. New life for an old Drug: The anthelmintic drug niclosamide inhibits pseudomonas aeruginosa quorum sensing. Antimicrob. Agents Chemother. 2013, 57, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, N.; Port, J.; Castillo, D.; Mylonakis, E. Repurposing the anthelmintic drug niclosamide to combat Helicobacter pylori. Sci. Rep. 2018, 8, 3701. [Google Scholar] [CrossRef]
- Jara, M.O.; Warnken, Z.N.; Sahakijpijarn, S.; Thakkar, R.; Kulkarni, V.R.; Christensen, D.J.; Koleng, J.J.; Williams, R.O. Oral Delivery of Niclosamide as an Amorphous Solid Dispersion That Generates Amorphous Nanoparticles during Dissolution. Pharmaceutics 2022, 14, 2568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lv, Y.; Zhang, J.B.; Wang, B.; Lv, G.J.; Ma, X.J. Gastroretentive drug delivery systems for the treatment of Helicobacter pylori. World J. Gastroenterol. 2014, 20, 9321. [Google Scholar]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef]
- Real, D.; Leonardi, D.; Williams, R.O.; Repka, M.A.; Salomon, C.J. Solving the Delivery Problems of Triclabendazole Using Cyclodextrins. AAPS PharmSciTech 2018, 19, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- Real, D.A.; Orzan, L.; Leonardi, D.; Salomon, C. Improving the Dissolution of Triclabendazole from Stable Crystalline Solid Dispersions Designed for Oral Delivery (en revisión). AAPS PharmSciTech 2019, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Santos Souza, H.F.; Real, D.; Leonardi, D.; Silber, A.M.; Salomon, C.J. Development and in vitro/in vivo evaluation of a novel benznidazole liquid dosage form using a quality-by-design approach. Trop. Med. Int. Health 2017, 22, 1514–1522. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Maji, I.; Mahajan, S.; Sriram, A.; Medtiya, P.; Vasave, R.; Khatri, D.K.; Kumar, R.; Singh, S.B.; Madan, J.; Singh, P.K. Solid self emulsifying drug delivery system: Superior mode for oral delivery of hydrophobic cargos. J. Control. Release 2021, 337, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Real, D.; Formica, M.L.; Picchio, M.L.; Paredes, A.J. Manufacturing Techniques for Nanoparticles in Drug Delivery. In Drug Delivery Using Nanomaterials; CRC Press: Boca Raton, FL, USA, 2022; pp. 23–48. [Google Scholar] [CrossRef]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Paulino, É.T.; Ribeiro de Lima, M.; Viçosa, A.L.; da Silva, C.H.; Salomon, C.J.; Real, D.A.; Leonardi, D.; Mello Silva, C.C.; de Moraes Neto, A.H.A. The Effect of Different Formulations of Praziquantel in Reducing Worms in the Prepatent Period of Schistosomiasis in Murine Models. Front. Public Health 2022, 10, 848633. [Google Scholar] [CrossRef]
- Real, D.A.; Hoffmann, S.; Leonardi, D.; Goycoolea, F.M.; Salomon, C.J. A quality by design approach for optimization of Lecithin/Span® 80 based nanoemulsions loaded with hydrophobic drugs. J. Mol. Liq. 2020, 321, 114743. [Google Scholar] [CrossRef]
- McGuckin, M.B.; Wang, J.; Ghanma, R.; Qin, N.; Palma, S.D.; Donnelly, R.F.; Paredes, A.J. Nanocrystals as a master key to deliver hydrophobic drugs via multiple administration routes. J. Control. Release 2022, 345, 334–353. [Google Scholar] [CrossRef]
- Lopez-Vidal, L.; Real, D.A.; Paredes, A.J.; Real, J.P.; Palma, S.D. 3D-Printed Nanocrystals for Oral Administration of the Drugs. In Drug Delivery Using Nanomaterials; CRC Press: Boca Raton, FL, USA, 2022; pp. 109–133. [Google Scholar] [CrossRef]
- Mauludin, R.; Müller, R.H.; Keck, C.M. Development of an oral rutin nanocrystal formulation. Int. J. Pharm. 2009, 370, 202–209. [Google Scholar] [CrossRef]
- Müller, R. Junghanns Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295. [Google Scholar] [CrossRef]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef]
- Lopez-Vidal, L.; Real, J.P.; Real, D.A.; Camacho, N.; Kogan, M.J.; Paredes, A.J.; Palma, S.D. Nanocrystal-based 3D-printed tablets: Semi-solid extrusion using melting solidification printing process (MESO-PP) for oral administration of poorly soluble drugs. Int. J. Pharm. 2022, 611, 121311. [Google Scholar] [CrossRef] [PubMed]
- Real, J.P.; Barberis, M.E.; Camacho, N.M.; Sánchez Bruni, S.; Palma, S.D. Design of novel oral ricobendazole formulation applying melting solidification printing process (MESO-PP): An innovative solvent-free alternative method for 3D printing using a simplified concept and low temperature. Int. J. Pharm. 2020, 587, 119653. [Google Scholar] [CrossRef]
- Real, J.P.; Palma, S.D.; Aquino, R.P.; Gaudio, D.; Garofalo, P.; Russo, E.; Zavan, B.; Sivolella, S.; Ronca, A.; Falcone, G.; et al. Floating Ricobendazole Delivery Systems: A 3D Printing Method by Co-Extrusion of Sodium Alginate and Calcium Chloride. Int. J. Mol. Sci. 2022, 23, 1280. [Google Scholar] [CrossRef]
- Gallo, L.; Peña, J.F.; Palma, S.D.; Real, J.P.; Cotabarren, I. Design and production of 3D printed oral capsular devices for the modified release of urea in ruminants. Int. J. Pharm. 2022, 628, 122353. [Google Scholar] [CrossRef]
- Barberis, M.E.; Palma, S.D.; Gonzo, E.E.; Bermúdez, J.M.; Lorier, M.; Ibarra, M.; Real, J.P. Mathematical and Pharmacokinetic Approaches for the Design of New 3D Printing Inks Using Ricobendazole. Pharm. Res. 2022, 39, 2277–2290. [Google Scholar] [CrossRef] [PubMed]
- Pistone, M.; Racaniello, G.F.; Arduino, I.; Laquintana, V.; Lopalco, A.; Cutrignelli, A.; Rizzi, R.; Franco, M.; Lopedota, A.; Denora, N. Direct cyclodextrin-based powder extrusion 3D printing for one-step production of the BCS class II model drug niclosamide. Drug Deliv. Transl. Res. 2022, 12, 1895–1910. [Google Scholar] [CrossRef]
- Paredes, A.J.; Camacho, N.M.; Schofs, L.; Dib, A.; Zarazaga, M.d.P.; Litterio, N.; Allemandi, D.A.; Sánchez Bruni, S.; Lanusse, C.; Palma, S.D. Ricobendazole nanocrystals obtained by media milling and spray drying: Pharmacokinetic comparison with the micronized form of the drug. Int. J. Pharm. 2020, 585, 119501. [Google Scholar] [CrossRef]
- Ford, R.R.; Gilbert, P.H.; Gillilan, R.; Huang, Q.; Donnelly, R.; Qian, K.K.; Allen, D.P.; Wagner, N.J.; Liu, Y. Micelle Formation and Phase Separation of Poloxamer 188 and Preservative Molecules in Aqueous Solutions Studied by Small Angle X-ray Scattering. J. Pharm. Sci. 2023, 112, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Finholt, P.; Solvang, S. Dissolution Kinetics of Drugs in Human Gastric Juice—The Role of Surface Tension. J. Pharm. Sci. 1968, 57, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.B.; Berthelsen, R.; Rades, T.; Jørgensen, S.A.; Vilmann, P.; Bar-Shalom, D.; Baldursdottir, S.; Müllertz, A. Physico-chemical characterization of aspirated and simulated human gastric fluids to study their influence on the intrinsic dissolution rate of cinnarizine. Int. J. Pharm. 2022, 622, 121856. [Google Scholar] [CrossRef]
- Real, D.A.; Gagliano, A.; Sonsini, N.; Wicky, G.; Orzan, L.; Leonardi, D.; Salomon, C. Design and optimization of pH-sensitive Eudragit nanoparticles for improved oral delivery of triclabendazole. Int. J. Pharm. 2022, 617, 121594. [Google Scholar] [CrossRef]
















| Time (h) | Z-Average (nm) | PDI |
|---|---|---|
| 2 h | 233 | 0.226 |
| 4 h | 198 | 0.192 |
| 6 h | 188 | 0.186 |
| Time (Days) | Refrigerator (4 °C) Z-Average-PDI | Room Temperature (25 °C) Z-Average-PDI |
|---|---|---|
| 0 | 188 nm–0.186 | 188 nm–0.186 |
| 7 | 208 nm–0.186 | 205 nm–0.170 |
| 14 | 204 nm–0.183 | 219 nm–0.170 |
| 21 | 224 nm–0.174 | 215 nm–0.179 |
| 28 | 204 nm–0.164 | 232 nm–0.147 |
| 35 | 208 nm–0.176 | 230 nm–0.189 |
| Time (Days) | Z-Average | PDI |
|---|---|---|
| 0 | 357 | 0.304 |
| 7 | 356 | 0.249 |
| 14 | 361 | 0.289 |
| 21 | 350 | 0.262 |
| Name | % Matrix Agent (Gelucire 50/13) | % Niclo:P188 (Ratio 1:1) | Niclo:P188 State | Formulation Temperature |
|---|---|---|---|---|
| INK + PM (50 °C) | 75% | 25% | Raw powders | 50 °C |
| INK + PM (58 °C) | Raw powders | 58 °C | ||
| INK + NCR (50 °C) | Nanomilled | 50 °C | ||
| INK + NCR (58 °C) | Nanomilled | 58 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Real, J.P.; Real, D.A.; Lopez-Vidal, L.; Barrientos, B.A.; Bolaños, K.; Tinti, M.G.; Litterio, N.J.; Kogan, M.J.; Palma, S.D. 3D-Printed Gastroretentive Tablets Loaded with Niclosamide Nanocrystals by the Melting Solidification Printing Process (MESO-PP). Pharmaceutics 2023, 15, 1387. https://doi.org/10.3390/pharmaceutics15051387
Real JP, Real DA, Lopez-Vidal L, Barrientos BA, Bolaños K, Tinti MG, Litterio NJ, Kogan MJ, Palma SD. 3D-Printed Gastroretentive Tablets Loaded with Niclosamide Nanocrystals by the Melting Solidification Printing Process (MESO-PP). Pharmaceutics. 2023; 15(5):1387. https://doi.org/10.3390/pharmaceutics15051387
Chicago/Turabian StyleReal, Juan Pablo, Daniel Andrés Real, Lucía Lopez-Vidal, Bruno Andrés Barrientos, Karen Bolaños, Mariano Guillermo Tinti, Nicolás Javier Litterio, Marcelo Javier Kogan, and Santiago Daniel Palma. 2023. "3D-Printed Gastroretentive Tablets Loaded with Niclosamide Nanocrystals by the Melting Solidification Printing Process (MESO-PP)" Pharmaceutics 15, no. 5: 1387. https://doi.org/10.3390/pharmaceutics15051387