Anisotropic, Hydrogel Microparticles as pH-Responsive Drug Carriers for Oral Administration of 5-FU
Abstract
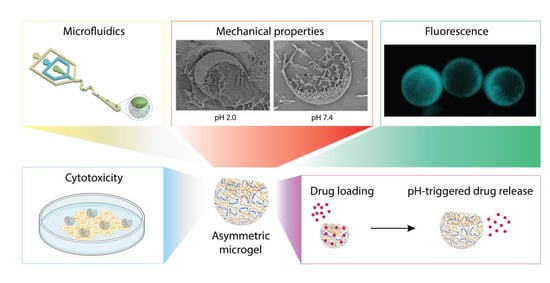
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PDMS Microfluidic Device
2.3. PDMS Generation of Hydrogel Microparticles
2.3.1. PEGDA–ALMA Spherical Microbeads
2.3.2. PEGDA–ALMA:Dextran Asymmetric Microparticles
2.4. Experimental Buffers and Solutions
2.5. Characterization of Microgels
2.5.1. FT-IT Spectroscopy
2.5.2. Cryo-SEM Analysis
2.5.3. Rheology Studies
2.5.4. Microgels Shrinking and Swelling Measurements
2.6. Fluorescence of PEGDA–ALMA Asymmetric Microgels
2.6.1. Fluorescence Labelling of ALMA
2.6.2. Fluorescently Labelled PEGDA–ALMA Asymmetric Microgels
2.6.3. Fluorescent Images of PEGDA–ALMA Asymmetric Microgels
2.7. Loading and Release of 5-FU
2.7.1. Cumulative Loading of 5-FU
2.7.2. pH-Responsive Drug Release
2.8. Cell Viability of Empty Microgels
2.8.1. Cell Culture and Cytotoxicity Assay
2.8.2. Cytotoxicity Measurements
2.9. Cell Viability of 5-FU-Loaded Microgels
2.9.1. Cell Culture and Cytotoxicity Assay
2.9.2. Cytotoxicity Measurements
2.10. Live–Dead Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. PEGDA–ALMA Cross-Linked Polymeric Microgels
3.2. Microfluidics for the Generation of PEGDA–ALMA Microparticles
3.3. Morphological Characterization of Microgels
3.3.1. Differences between Spherical and Asymmetric Microparticles
3.3.2. Influence of pH on the Microgels Structure
3.4. Mechanical Properties
3.5. Swelling Measurements
3.6. Fluorescence Images
3.7. Cumulative Drug Loading
3.8. In Vitro pH-Triggered 5-FU Release
3.9. Cytotoxicity and Live/Dead Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Horkay, F.; Basser, P.J. Hydrogel composite mimics biological tissues. Soft Matter. 2022, 18, 4414–4426. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon-Ganorkar, C.; Liu, F.; Baudyš, M.; Kim, S.W. Modulating insulin-release profile from pH/thermosensitive polymeric beads through polymer molecular weight. J. Control. Release 1999, 59, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef]
- Pillai, O.; Panchagnula, R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Dong, C.; Jiang, H.; Bin, X.; Gao, X.; Qin, M.; Wang, W.; Bin, C.; Cao, Y. Rationally designed synthetic protein hydrogels with predictable mechanical properties. Nat. Commun. 2018, 9, 620. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S. Mechanical Properties of Cellularly Responsive Hydrogels and Their Experimental Determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef]
- Kędzierska, M.; Jamroży, M.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bańkosz, M.; Gruca, M.; Potemski, P.; Tyliszczak, B. Analysis of the Influence of Both the Average Molecular Weight and the Content of Crosslinking Agent on Physicochemical Properties of PVP-Based Hydrogels Developed as Innovative Dressings. Int. J. Mol. Sci. 2022, 23, 11618. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Galaev, I.Y.; Mattiasson, B. ‘Smart’ polymers and what they could do in biotechnology and medicine. Trends Biotechnol. 1999, 17, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Jagur-grodzinski, J. Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym. Adv. Technol. 2010, 21, 27–47. [Google Scholar] [CrossRef]
- Talei Franzesi, G.; Ni, B.; Ling, Y.; Khademhosseini, A. A Controlled-Release Strategy for the Generation of Cross-Linked Hydrogel Microstructures. J. Am. Chem. Soc. 2006, 128, 15064–15065. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Landfester, K. Stimuli-responsive microgels for the loading and release of functional compounds: Fundamental concepts and applications. Polymer 2012, 53, 5209–5231. [Google Scholar] [CrossRef]
- Jiang, Z.; Tan, M.L.; Taheri, M.; Yan, Q.; Tsuzuki, T.; Gardiner, M.G.; Diggle, B.; Connal, L.A. Strong, Self-Healable, and Recyclable Visible-Light-Responsive Hydrogel Actuators. Angew. Chem. Int. Ed. 2020, 59, 7049–7056. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Huang, J.; Fang, R.; Liu, M. Imparting Functionality to the Hydrogel by Magnetic-Field-Induced Nano-assembly and Macro-response. ACS Appl. Mater. Interfaces 2020, 12, 5177–5194. [Google Scholar] [CrossRef] [PubMed]
- Murdan, S. Electro-responsive drug delivery from hydrogels. J. Control. Release 2003, 92, 1–17. [Google Scholar] [CrossRef]
- Huang, X.; Lowe, T.L. Biodegradable Thermoresponsive Hydrogels for Aqueous Encapsulation and Controlled Release of Hydrophilic Model Drugs. Biomacromolecules 2005, 6, 2131–2139. [Google Scholar] [CrossRef]
- Gui, H.; Yang, T.; Li, L.L.; Liang, F.; Yang, Z. Temperature-Sensitive Anti-Inflammatory Organohydrogels Containing Janus Particle Stabilized Phase-Change Microinclusions. ACS Nano 2022, 16, 9859–9870. [Google Scholar] [CrossRef]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Zhong, D.; Yiming, B.; Hu, X.; Jia, Z.; Nian, G.; Qu, S.; et al. Dual pH-Responsive Hydrogel Actuator for Lipophilic Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.H.; Choi, J.S.; Choi, Y.C.; Kim, J.D.; Cho, Y.W. Fabrication of drug-loaded polymer microparticles with arbitrary geometries using a piezoelectric inkjet printing system. Int. J. Pharm. 2012, 427, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Berkland, C.; Kim, K.; Pack, D.W. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J. Control. Release 2001, 73, 59–74. [Google Scholar] [CrossRef]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef]
- Moore, T.L.; Cook, A.B.; Bellotti, E.; Palomba, R.; Manghnani, P.; Spanò, R.; Brahmachari, S.; Di Francesco, M.; Palange, A.L.; Di Mascolo, D.; et al. Shape-specific microfabricated particles for biomedical applications: A review. Drug Deliv. Transl. Res. 2022, 12, 2019–2037. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Z.; Li, G.; Cai, Z.; Wu, J.; Wang, L.; Deng, L.; Cai, M.; Cui, W. Injectable Microfluidic Hydrogel Microspheres for Cell and Drug Delivery. Adv. Funct. Mater. 2021, 31, 2103339. [Google Scholar] [CrossRef]
- Dirksen, M.; Dargel, C.; Meier, L.; Brändel, T.; Hellweg, T. Smart microgels as drug delivery vehicles for the natural drug aescin: Uptake, release and interactions. Colloid Polym. Sci. 2020, 298, 505–518. [Google Scholar] [CrossRef]
- Hadidi, N.; Pazuki, G. Preparation, characterization and in-vivo efficacy study of glatiramer acetate (GA)-hydrogel-microparticles as novel drug delivery system for GA in RRMS. Sci. Rep. 2022, 12, 22042. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Liu, J.; Zhang, W. Particle morphology: An important factor affecting drug delivery by nanocarriers into solid tumors. Expert Opin. Drug Deliv. 2018, 15, 379–395. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Noordhuis, P.; Holwerda, U.; Van der Wilt, C.L.; Van Groeningen, C.J.; Smid, K.; Meijer, S.; Pinedo, H.M.; Peters, G.J. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004, 15, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kinouchi, M.; Ishida, K.; Fujibuchi, W.; Naitoh, T.; Ogawa, H.; Ando, T.; Yazaki, N.; Watanabe, K.; Haneda, S.; et al. 5-fu metabolism in cancer and orally-administrable 5-fu drugs. Cancers 2010, 2, 1717–1730. [Google Scholar] [CrossRef]
- Skipper, H.; Schabel Jr, F.; Wilcox, W. On the criteria and kinetics associated with curability of experimental leukaemia. Cancer Chemother. Rep. 1964, 35, 1307–1317. [Google Scholar]
- Lembersky, B.C.; Wieand, H.S.; Petrelli, N.J.; O’Connell, M.J.; Colangelo, L.H.; Smith, R.E.; Seay, T.E.; Giguere, J.K.; Marshall, M.E.; Jacobs, A.D.; et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J. Clin. Oncol. 2006, 24, 2059–2064. [Google Scholar] [CrossRef]
- Boku, N.; Yamamoto, S.; Fukuda, H.; Shirao, K.; Doi, T.; Sawaki, A.; Koizumi, W.; Saito, H.; Yamaguchi, K.; Takiuchi, H.; et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: A randomised phase 3 study. Lancet Oncol. 2009, 10, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control Release 2007, 119, 5–24. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Sun, Y.; Yu, F.; Ma, J. Ionically cross-linked sodium alginate/ĸ-carrageenan double-network gel beads with low-swelling, enhanced mechanical properties, and excellent adsorption performance. Chem. Eng. J. 2019, 372, 1091–1103. [Google Scholar] [CrossRef]
- Karim, A.; Rehman, A.; Feng, J.; Noreen, A.; Assadpour, E.; Kharazmi, M.S.; Lianfu, Z.; Jafari, S.M. Alginate-based nanocarriers for the delivery and controlled-release of bioactive compounds. Adv. Colloid Interface Sci. 2022, 307, 102744. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Q.; Hui, Y.; Seth, A.; Petrovsky, N.; Zhao, C.-X. Microfluidic formation of core-shell alginate microparticles for protein encapsulation and controlled release. J. Colloid Interface Sci. 2019, 539, 497–503. [Google Scholar] [CrossRef]
- Lima, D.S.; Tenório-Neto, E.T.; Lima-Tenório, M.K.; Guilherme, M.R.; Scariot, D.B.; Nakamura, C.V.; Muniz, E.C.; Rubira, A.F. pH-responsive alginate-based hydrogels for protein delivery. J. Mol. Liq. 2018, 262, 29–36. [Google Scholar] [CrossRef]
- Kesarwani, A.; Sahu, P.; Jain, K.; Sinha, P.; Mohan, K.V.; Nagpal, P.S.; Singh, S.; Zaidi, R.; Nagarajan, P.; Upadhyay, P. The safety and efficacy of BCG encapsulated alginate particle (BEAP) against M.tb H37Rv infection in Macaca mulatta: A pilot study. Sci. Rep. 2021, 11, 3049. [Google Scholar] [CrossRef] [PubMed]
- Vaghasiya, K.; Eram, A.; Sharma, A.; Ray, E.; Adlakha, S.; Verma, R.K. Alginate Microspheres Elicit Innate M1-Inflammatory Response in Macrophages Leading to Bacillary Killing. AAPS PharmSciTech. 2019, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Chan, L.W.; Jin, Y.; Heng, P.W.S. Cross-linking mechanisms of calcium and zinc in production of alginate microspheres. Int. J. Pharm. 2002, 242, 255–258. [Google Scholar] [CrossRef]
- Agulhon, P.; Robitzer, M.; Habas, J.-P.; Quignard, F. Influence of both cation and alginate nature on the rheological behavior of transition metal alginate gels. Carbohydr. Polym. 2014, 112, 525–531. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef]
- Araiza-Verduzco, F.; Rodríguez-Velázquez, E.; Cruz, H.; Rivero, I.A.; Acosta-Martínez, D.R.; Pina-Luis, G.; Alatorre-Meda, M. Photocrosslinked Alginate-Methacrylate Hydrogels with Modulable Mechanical Properties: Effect of the Molecular Conformation and Electron Density of the Methacrylate Reactive Group. Materials 2020, 13, 534. [Google Scholar] [CrossRef]
- Hasany, M.; Talebian, S.; Sadat, S.; Ranjbar, N.; Mehrali, M.; Wallace, G.G.; Mehrali, M. Synthesis, properties, and biomedical applications of alginate methacrylate (ALMA)-based hydrogels: Current advances and challenges. Appl. Mater. Today 2021, 24, 101150. [Google Scholar] [CrossRef]
- Strand, B.L.; Mørch, Y.A.; Espevik, T.; Skjåk-Bræk, G. Visualization of alginate–poly-L-lysine–alginate microcapsules by confocal laser scanning microscopy. Biotechnol. Bioeng. 2003, 82, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Beamish, J.A.; Zhu, J.; Kottke-Marchant, K.; Marchant, R.E. The effects of monoacrylated poly(ethylene glycol) on the properties of poly(ethylene glycol) diacrylate hydrogels used for tissue engineering. J. Biomed. Mater. Res. A 2010, 92, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Teora, S.P.; van der Knaap, K.H.; Keller, S.; Rijpkema, S.J.; Wilson, D.A. Reversible speed control of one-stimulus-double-response, temperature-sensitive asymmetric hydrogel micromotors. Chem. Commun. 2022, 58, 10333–10336. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Teora, S.P.; Keskin, A.; Daris, L.J.C.; Samuels, N.A.P.E.; Boujemaa, M.; Wilson, D.A. Spatial Control over Catalyst Positioning for Increased Micromotor Efficiency. Gels 2023, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Teora, S.P.; Boujemaa, M.; Wilson, D.A. Exploring New Horizons in Liquid Compartmentalization via Microfluidics. Biomacromolecules 2021, 22, 1759–1769. [Google Scholar] [CrossRef]
- Mace, C.R.; Akbulut, O.; Kumar, A.A.; Shapiro, N.D.; Derda, R.; Patton, M.R.; Whitesides, G.M. Aqueous Multiphase Systems of Polymers and Surfactants Provide Self-Assembling Step-Gradients in Density. J. Am. Chem. Soc. 2012, 134, 9094–9097. [Google Scholar] [CrossRef]
- Keller, S.; Teora, S.P.; Hu, G.X.; Nijemeisland, M.; Wilson, D.A. High-Throughput Design of Biocompatible Enzyme-Based Hydrogel Microparticles with Autonomous Movement. Angew. Chem. Int. Ed. 2018, 57, 9814–9817. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Fenn, S.L.; Miao, T.; Scherrer, R.M.; Floreani, R.A. Dual-Cross-Linked Methacrylated Alginate Sub-Microspheres for Intracellular Chemotherapeutic Delivery. ACS Appl. Mater. Interfaces 2016, 8, 17775–17783. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Grasdalen, H.; Smidsrød, O. Inhomogeneous polysaccharide ionic gels. Carbohydr. Polym. 1989, 10, 31–54. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Yoshimoto, F.K. Formation and Cleavage of C-C Bonds by Enzymatic Oxidation-Reduction Reactions. Chem. Rev. 2018, 118, 6573–6655. [Google Scholar] [CrossRef] [PubMed]
- Golubeva, O.Y.; Alikina, Y.A.; Brazovskaya, E.Y.; Ugolkov, V.V. Peculiarities of the 5-fluorouracil adsorption on porous aluminosilicates with different morphologies. Appl. Clay Sci. 2020, 184, 105401. [Google Scholar] [CrossRef]
- Mioduszewska, K.; Dołżonek, J.; Wyrzykowski, D.; Kubik, Ł.; Wiczling, P.; Sikorska, C.; Toński, M.; Kaczyński, Z.; Stepnowski, P.; Białk-Bielińska, A. Overview of experimental and computational methods for the determination of the pKa values of 5-fluorouracil, cyclophosphamide, ifosfamide, imatinib and methotrexate. TrAC Trends Anal. Chem. 2017, 97, 283–296. [Google Scholar] [CrossRef]
- Egodawatte, S.; Dominguez, S.; Larsen, S.C. Solvent effects in the development of a drug delivery system for 5-fluorouracil using magnetic mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2017, 237, 108–116. [Google Scholar] [CrossRef]
- Han, M.; Fang, Q.-L.; Zhan, H.-W.; Luo, T.; Liang, W.-Q.; Gao, J.-Q. In vitro and in vivo evaluation of a novel capsule for colon-specific drug delivery. J. Pharm. Sci. 2009, 98, 2626–2635. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teora, S.P.; Panavaité, E.; Sun, M.; Kiffen, B.; Wilson, D.A. Anisotropic, Hydrogel Microparticles as pH-Responsive Drug Carriers for Oral Administration of 5-FU. Pharmaceutics 2023, 15, 1380. https://doi.org/10.3390/pharmaceutics15051380
Teora SP, Panavaité E, Sun M, Kiffen B, Wilson DA. Anisotropic, Hydrogel Microparticles as pH-Responsive Drug Carriers for Oral Administration of 5-FU. Pharmaceutics. 2023; 15(5):1380. https://doi.org/10.3390/pharmaceutics15051380
Chicago/Turabian StyleTeora, Serena P., Elada Panavaité, Mingchen Sun, Bas Kiffen, and Daniela A. Wilson. 2023. "Anisotropic, Hydrogel Microparticles as pH-Responsive Drug Carriers for Oral Administration of 5-FU" Pharmaceutics 15, no. 5: 1380. https://doi.org/10.3390/pharmaceutics15051380