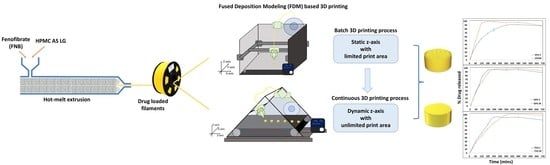
Veering to a Continuous Platform of Fused Deposition Modeling Based 3D Printing for Pharmaceutical Dosage Forms: Understanding the Effect of Layer Orientation on Formulation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solid-State Analysis (Pre-Printing Process)
2.2.1. Thermogravimetric Analysis (TGA)
2.2.2. Differential Scanning Calorimetry (DSC) Analysis
2.2.3. Powder X-Ray Diffraction (XRD) Analysis
2.2.4. Polarized Light Microscopy (PLM)
2.2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Hot-Melt Extrusion (HME)
2.4. Designing and FDM 3D Printing of Formulations
2.5. Assessment of Printed Tablets
2.6. Evaluation of Dosage Form Breaking Force
2.7. In Vitro Release Performance Testing
2.8. Analytical Method
2.9. Statistics
3. Results
3.1. Pre-Formulation Characterization and Thermal Assessment
3.2. Processing FNB-Loaded Drug Filaments Using the HME Process
3.3. Characterization of FNB-Loaded Extruded Filaments
3.4. Print Quality Comparison between the Two Processes
3.5. Effect of Layer Orientation on Printlet Strength
- Testing when the printlets are placed horizontally: At both a 25% and 50% infill density, the print differences proved to be statistically significant as compared to the 75% infill density prints, where the difference in the force required to break them was not significant. In addition, as the infill density increased, the force required to break the printed tablet increased, which correlated to an increased strength due to reduced void spaces in the prints (Figure 6c). The printlets formulated using the continuous method broke with the print splitting in two pieces and with the split occurring along the axis of printing (45°), across all infill densities tested for the study. The reason for this split along the axis can be attributed to the fact that, when a force is applied at a 45° angle, it becomes resolved into two components, one that acts in line and the other along the axis of the printed angle. This split in the applied force prevents the crushing of the tablet and in turn, ends up splitting it along its printed axis. Comparatively, when printed using the conventionally used pharmaceutical FDM printing process, the tablets when tested in the horizontal orientation were crushed into small fragments.
- Testing when the printlets are placed vertically: As compared to the horizontal orientation for testing, the force required to break the printlet was significantly different across all the infill densities tested in this study. The force required to break a printlet with layers printed at 45° axes is greater as compared to the force required to break a printlet with layers printed at a 0° axis. This was the case for both the 50% and 75% infill printlets (Figure 6c). The printlets printed using both printers, when tested after being placed vertically, collapsed on their structure without showing any breakage post removal and had their height reduced due to compression because of the applied force (Figure 6b). For both prints, breakages or crushing of the printlets was seen for 4 and 5 units for the continuous and batch processes, respectively. This can be attributed to the large void spaces and weak internal structural strength of the print at low infill densities.
3.6. Performance Comparison Using In Vitro Release Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Salvi, S.; Sood, P.; Karsiya, J.; Kumar, D. Recent Advancements in Additive Manufacturing Techniques Employed in the Pharmaceutical Industry: A Bird’s Eye View. Ann. 3d Print. Med. 2022, 8, 100081. [Google Scholar] [CrossRef]
- Samiei, N. Recent Trends on Applications of 3D Printing Technology on the Design and Manufacture of Pharmaceutical Oral Formulation: A Mini Review. Beni Suef Univ. J. Basic Appl. Sci. 2020, 9, 12. [Google Scholar] [CrossRef]
- Kulkarni, V.R.; Kazi, M.; Shahba, A.A.W.; Radhanpuri, A.; Maniruzzaman, M. Three-Dimensional Printing of a Container Tablet: A New Paradigm for Multi-Drug-Containing Bioactive Self-Nanoemulsifying Drug-Delivery Systems (Bio-SNEDDSs). Pharmaceutics 2022, 14, 1082. [Google Scholar] [CrossRef]
- Salazar-Gamarra, R.; Contreras-Pulache, H.; Cruz-Gonzales, G.; Binasco, S.; Cruz-Gonzales, W.; Moya-Salazar, J. Three-Dimensional Printing and Digital Flow in Human Medicine: A Review and State-of-the-Art. Appl. Syst. Innov. 2022, 5, 126. [Google Scholar] [CrossRef]
- Mohapatra, S.; Kar, R.K.; Biswal, P.K.; Bindhani, S. Approaches of 3D Printing in Current Drug Delivery. Sens. Int. 2022, 3, 100146. [Google Scholar] [CrossRef]
- Chakka, L.R.J.; Chede, S. 3D Printing of Pharmaceuticals for Disease Treatment. Front. Med. Technol. 2022, 4, 1040052. [Google Scholar] [CrossRef]
- Wallis, M.; Al-Dulimi, Z.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D Printing for Enhanced Drug Delivery: Current State-of-the-Art and Challenges. Drug Dev. Ind. Pharm. 2020, 46, 1385–1401. [Google Scholar] [CrossRef]
- Krueger, L.; Miles, J.A.; Popat, A. 3D Printing Hybrid Materials Using Fused Deposition Modelling for Solid Oral Dosage Forms. J. Control. Release 2022, 351, 444–455. [Google Scholar] [CrossRef]
- Lu, A.; Zhang, J.; Jiang, J.; Zhang, Y.; Giri, B.R.; Kulkarni, V.R.; Aghda, N.H.; Wang, J.; Maniruzzaman, M. Novel 3D Printed Modular Tablets Containing Multiple Anti-Viral Drugs: A Case of High Precision Drop-on-Demand Drug Deposition. Pharm. Res. 2022, 39, 2905–2918. [Google Scholar] [CrossRef]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive Manufacturing Technologies with Emphasis on Stereolithography 3D Printing in Pharmaceutical and Medical Applications: A Review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef] [PubMed]
- Crișan, A.G.; Iurian, S.; Porfire, A.; Rus, L.M.; Bogdan, C.; Casian, T.; Lucacel, R.C.; Turza, A.; Porav, S.; Tomuță, I. QbD Guided Development of Immediate Release FDM-3D Printed Tablets with Customizable API Doses. Int. J. Pharm. 2022, 613, 121411. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Aghda, N.H.; Pillai, A.R.; Thakkar, R.; Nokhodchi, A.; Maniruzzaman, M. Emerging 3D Printing Technologies for Drug Delivery Devices: Current Status and Future Perspective. Adv. Drug Deliv. Rev. 2021, 174, 294–316. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.; Thakkar, R.; Pillai, A.; Ashour, E.A.; Repka, M.A. Systematic Screening of Pharmaceutical Polymers for Hot Melt Extrusion Processing: A Comprehensive Review. Int. J. Pharm. 2020, 576, 118989. [Google Scholar] [CrossRef]
- Ong, J.J.; Castro, B.M.; Gaisford, S.; Cabalar, P.; Basit, A.W.; Pérez, G.; Goyanes, A. Accelerating 3D Printing of Pharmaceutical Products Using Machine Learning. Int. J. Pharm. X 2022, 4, 100120. [Google Scholar] [CrossRef]
- Tracy, T.; Wu, L.; Liu, X.; Cheng, S.; Li, X. 3D Printing: Innovative Solutions for Patients and Pharmaceutical Industry. Int. J. Pharm. 2023, 631, 122480. [Google Scholar] [CrossRef] [PubMed]
- Dhoppalapudi, S.; Illa, N. A Review of Hot Melt Extrusion Paired Fused Deposition Modeling Three-Dimensional Printing for Developing Patient Centric Dosage Forms. GSC Biol. Pharm. Sci. 2022, 21, 065–079. [Google Scholar] [CrossRef]
- Velez, N.L.; Drennen, J.K.; Anderson, C.A. Challenges, Opportunities and Recent Advances in near Infrared Spectroscopy Applications for Monitoring Blend Uniformity in the Continuous Manufacturing of Solid Oral Dosage Forms. Int. J. Pharm. 2022, 615, 121462. [Google Scholar] [CrossRef]
- Hausner, D.B.; Moore, C.M.V. Regulatory Considerations for Continuous Solid Oral Dose Pharmaceutical Manufacturing. In How to Design and Implement Powder-to-Tablet Continuous Manufacturing Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 301–318. ISBN 9780128134795. [Google Scholar]
- Wu, C.; Dai, C.; Fang, G.; Liu, Y.J.; Wang, C.C.L. RoboFDM: A Robotic System for Support-Free Fabrication Using FDM. In Proceedings of the IEEE International Conference on Robotics and Automation, Singapore, 29 May–3 June 2017; pp. 1175–1180. [Google Scholar]
- Sammaiah, P.; Chaitanya Krishna, D.; Sai Mounika, S.; Rajasri Reddy, I.; Karthik, T. Effect of the Support Structure on Flexural Properties of Fabricated Part at Different Parameters in the Fdm Process. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 981. [Google Scholar]
- Wójcik-Pastuszka, D.; Krzak, J.; Prządka, P.; Twarda, M.; Osiński, B.; Musiał, W. Release of Bupivacaine from Artificial Ligament Implants Modified with the Silica Coating. Ceram. Int. 2023, 49, 2852–2859. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, P.; Chung, S.; Bandari, S.; Repka, M.A. Fabrication of Bilayer Tablets Using Hot Melt Extrusion-Based Dual-Nozzle Fused Deposition Modeling 3D Printing. Int. J. Pharm. 2022, 624, 121972. [Google Scholar] [CrossRef]
- Klar, F.; Urbanetz, N.A. Solubility Parameters of Hypromellose Acetate Succinate and Plasticization in Dry Coating Procedures. Drug Dev. Ind. Pharm. 2016, 42, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Niederquell, A.; Dujovny, G.; Probst, S.E.; Kuentz, M. A Relative Permittivity Approach for Fast Drug Solubility Screening of Solvents and Excipients in Lipid-Based Delivery. J. Pharm. Sci. 2019, 108, 3457–3460. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A. Hydroxypropyl Methylcellulose Acetate Succinate as an Exceptional Polymer for Amorphous Solid Dispersion Formulations: A Review from Bench to Clinic. Eur. J. Pharm. Biopharm. 2022, 177, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Thakkar, R.; Kulkarni, V.R.; Zhang, Y.; Lu, A.; Maniruzzaman, M. Investigation of the Fused Deposition Modeling Additive Manufacturing I: Influence of Process Temperature on the Quality and Crystallinity of the Dosage Forms. AAPS PharmSciTech 2021, 22, 258. [Google Scholar] [CrossRef] [PubMed]
- Dedroog, S.; Pas, T.; Vergauwen, B.; Huygens, C.; van den Mooter, G. Solid-State Analysis of Amorphous Solid Dispersions: Why DSC and XRPD May Not Be Regarded as Stand-Alone Techniques. J. Pharm. Biomed. Anal. 2020, 178, 112937. [Google Scholar] [CrossRef] [PubMed]
- Karmarkar, A.; Karmarkar, A.B. Effect of Ceolus KG-802 on the Dissolution Rate of Fenofibrate Liquisolid Tablets: Preformulation and Formulation Development Studies. Drug Discov. Ther. 2010, 4, 493–498. [Google Scholar]
- Raman Kallakunta, V.; Sarabu, S.; Bandari, S.; Batra, A.; Bi, V.; Durig, T.; Repka, M.A.; Repka, M.A. Stable Amorphous Solid Dispersions of Fenofibrate Using Hot Melt Extrusion Technology: Effect of Formulation and Process Parameters for a Low Glass Transition Temperature Drug HHS Public Access. J. Drug Deliv. Sci. Technol. 2020, 58, 101395. [Google Scholar] [CrossRef]
- Prasanthi, D.; Jagadish, G.; Aishwarya, K.L. Solid Dispersions of Fenofibrate: Comparision of Natural and Synthetic Carriers. Pharma Innov. J. 2019, 8, 42–50. [Google Scholar]
- Tambe, S.; Jain, D.; Meruva, S.K.; Rongala, G.; Juluri, A.; Nihalani, G.; Mamidi, H.K.; Nukala, P.K.; Bolla, P.K. Recent Advances in Amorphous Solid Dispersions: Preformulation, Formulation Strategies, Technological Advancements and Characterization. Pharmaceutics 2022, 14, 2203. [Google Scholar] [CrossRef]
- Xu, W.; Sun, Y.; Dong, X.; Li, S.; Wang, H.; Xue, J.; Zheng, X. Local Order and Vibrational Coupling of the C=O Stretching Mode of γ-Caprolactone in Liquid Binary Mixtures. Sci. Rep. 2017, 7, 12182. [Google Scholar] [CrossRef]
- Davis, D.A.; Miller, D.A.; Su, Y.; Williams, R.O. Thermally Conductive Excipient Expands KinetiSol® Processing Capabilities. AAPS PharmSciTech 2020, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Duceac, I.A.; Coseri, S. Biopolymers and Their Derivatives: Key Components of Advanced Biomedical Technologies. Biotechnol. Adv. 2022, 61, 108056. [Google Scholar] [CrossRef]
- Ashland. AquaSolveTM Hydroxypropylmethylcellulose Acetate Succinate: Physical and Chemical Properties Handbook; Ashland: Wilmington, DE, USA, 2016. [Google Scholar]
- Ashland. AquaSolveTM Hypromellose Acetate Succinate (HPMCAS): Versatile Pharmaceutical Polymers for Improved Solubility; Ashland Inc.: Wilmington, DE, USA, 2014. [Google Scholar]
- Thakkar, R.; Pillai, A.R.; Zhang, J.; Zhang, Y.; Kulkarni, V.; Maniruzzaman, M. Novel On-Demand 3-Dimensional (3-d) Printed Tablets Using Fill Density as an Effective Release-Controlling Tool. Polymers 2020, 12, 1872. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug Release Kinetics and Transport Mechanisms of Non-Degradable and Degradable Polymeric Delivery Systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. [Google Scholar]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D Printing of Tablets Containing Multiple Drugs with Defined Release Profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.; Ervasti, T.; Laitinen, R. Production and Characterization of Glibenclamide Incorporated PLA Filaments for 3D Printing by Fused Deposition Modeling. J. Drug Deliv. Sci. Technol. 2022, 77, 103843. [Google Scholar] [CrossRef]
- Pawar, R.; Pawar, A. 3D Printing of Pharmaceuticals: Approach from Bench Scale to Commercial Development. Future J. Pharm. Sci. 2022, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.W.; Gaisford, S. 3D Printing of Pharmaceuticals; Springer International Publishing: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D Printing in Personalized Drug Delivery: An Overview of Hot-Melt Extrusion-Based Fused Deposition Modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef] [PubMed]
- Kamio, T.; Onda, T. Fused Deposition Modeling 3D Printing in Oral and Maxillofacial Surgery: Problems and Solutions. Cureus 2022, 14, e28906. [Google Scholar] [CrossRef] [PubMed]
- Conradie, D. 3D Printer Automated Bed Swapping System Loads from a Magazine. Available online: https://schleuss.online/@itnewsbot/106694149154551774 (accessed on 9 March 2023).
- Abeykoon, C. Sensing Technologies for Process Monitoring in Polymer Extrusion: A Comprehensive Review on Past, Present and Future Aspects. Meas. Sens. 2022, 22, 100381. [Google Scholar] [CrossRef]
- Vo, A.Q.; He, H.; Zhang, J.; Martin, S.; Chen, R.; Repka, M.A. Application of FT-NIR Analysis for In-Line and Real-Time Monitoring of Pharmaceutical Hot Melt Extrusion: A Technical Note. AAPS PharmSciTech 2018, 19, 3425–3429. [Google Scholar] [CrossRef] [PubMed]
- P112_Process Monitoring of Extrusion Based 3D Printingvia Laser Scanning. Available online: https://www.academia.edu/26979547/Process_Monitoring_of_Extrusion_Based_3D_Printing_via_Laser_Scanning (accessed on 9 March 2023).
- Que, C.; Lou, X.; Zemlyanov, D.Y.; Mo, H.; Indulkar, A.S.; Gao, Y.; Zhang, G.G.Z.; Taylor, L.S. Insights into the Dissolution Behavior of Ledipasvir-Copovidone Amorphous Solid Dispersions: Role of Drug Loading and Intermolecular Interactions. Mol. Pharm. 2019, 16, 5054–5067. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Trivino, A.; Prasad, D.; Chauhan, H. Investigation and Correlation of Drug Polymer Miscibility and Molecular Interactions by Various Approaches for the Preparation of Amorphous Solid Dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. [Google Scholar] [CrossRef]
- Ma, X.; Williams, R.O. Characterization of Amorphous Solid Dispersions: An Update. J. Drug Deliv. Sci. Technol. 2019, 50, 113–124. [Google Scholar] [CrossRef]







| Process | Diameter (mm) | Height (mm) |
|---|---|---|
| Batch printing | 7.843 ± 0.294 | 4.967 ± 0.199 |
| Continuous printing | 8.007 ± 0.055 | 5.004 ± 0.018 |
| Reference | Test | Difference Factor (f1) | Similarity Factor (f2) |
|---|---|---|---|
| 25B | 25C | 20.862 | 36.982 |
| 50B | 50C | 5.815 | 65.517 |
| 75B | 75C | 13.437 | 54.846 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, V.R.; Chakka, J.; Alkadi, F.; Maniruzzaman, M. Veering to a Continuous Platform of Fused Deposition Modeling Based 3D Printing for Pharmaceutical Dosage Forms: Understanding the Effect of Layer Orientation on Formulation Performance. Pharmaceutics 2023, 15, 1324. https://doi.org/10.3390/pharmaceutics15051324
Kulkarni VR, Chakka J, Alkadi F, Maniruzzaman M. Veering to a Continuous Platform of Fused Deposition Modeling Based 3D Printing for Pharmaceutical Dosage Forms: Understanding the Effect of Layer Orientation on Formulation Performance. Pharmaceutics. 2023; 15(5):1324. https://doi.org/10.3390/pharmaceutics15051324
Chicago/Turabian StyleKulkarni, Vineet R., Jaidev Chakka, Faez Alkadi, and Mohammed Maniruzzaman. 2023. "Veering to a Continuous Platform of Fused Deposition Modeling Based 3D Printing for Pharmaceutical Dosage Forms: Understanding the Effect of Layer Orientation on Formulation Performance" Pharmaceutics 15, no. 5: 1324. https://doi.org/10.3390/pharmaceutics15051324