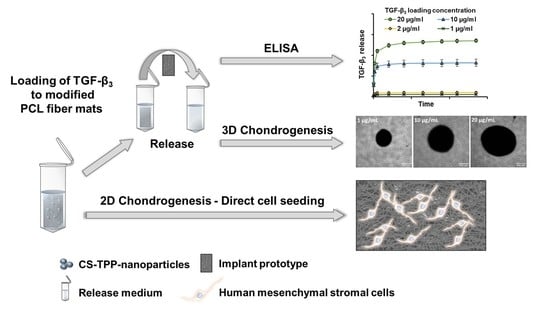
Release of TGF-β3 from Surface-Modified PCL Fiber Mats Triggers a Dose-Dependent Chondrogenic Differentiation of Human Mesenchymal Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implant Prototypes
2.2. Nanoparticle Preparation and Protein Loading
2.3. Particle Size and Zeta Potential Measurements
2.4. Release Experiments
2.5. ELISA and Data Processing
2.6. Cultivation of Bone Marrow-Derived Mesenchymal Stromal Cells
2.7. Chondrogenic Differentiation
2.8. GAG/DNA
2.9. RNA Isolation and cDNA Synthesis
2.10. qRT-PCR
3. Results
3.1. Particle Size and Zeta Potential of CS/TPP Nanoparticles
3.2. TGF-β3 Release from Implant Samples
3.3. Correlation between TGF-β3 Loading Concentration and Total Release
3.4. Evolution of TGF-β3 Release Rate over Time
3.5. Calculation of Expected In Vivo Dose
3.6. Chondrogenic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michaud, C.M.; McKenna, M.T.; Begg, S.; Tomijima, N.; Majmudar, M.; Bulzacchelli, M.T.; Ebrahim, S.; Ezzati, M.; Salomon, J.A.; Kreiser, J.G.; et al. The burden of disease and injury in the United States 1996. Popul. Health Metr. 2006, 4, 11. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Martin, J.A. Osteoarthritis. Adv. Drug Deliv. Rev. 2006, 58, 150–167. [Google Scholar] [CrossRef]
- Milgrom, C.; Schaffler, M.; Gilbert, S.; van Holsbeeck, M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J. Bone Jt. Surg. Br. 1995, 77-B, 296–298. [Google Scholar] [CrossRef]
- Minagawa, H.; Yamamoto, N.; Abe, H.; Fukuda, M.; Seki, N.; Kikuchi, K.; Kijima, H.; Itoi, E. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. J. Orthop. 2013, 10, 8–12. [Google Scholar] [CrossRef]
- Yamamoto, A.; Takagishi, K.; Osawa, T.; Yanagawa, T.; Nakajima, D.; Shitara, H.; Kobayashi, T. Prevalence and risk factors of a rotator cuff tear in the general population. J. Shoulder Elb. Surg. 2010, 19, 116–120. [Google Scholar] [CrossRef]
- Lee, C.H.; Cook, J.L.; Mendelson, A.; Moioli, E.K.; Yao, H.; Mao, J.J. Regeneration of the articular surface of the rabbit synovial joint by cell homing: A proof of concept study. Lancet 2010, 376, 440–448. [Google Scholar] [CrossRef]
- Martino, M.M.; Briquez, P.S.; Ranga, A.; Lutolf, M.P.; Hubbell, J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. USA 2013, 110, 4563–4568. [Google Scholar] [CrossRef]
- Zumstein, M.-A.; Lädermann, A.; Raniga, S.; Schär, M.-O. The biology of rotator cuff healing. Orthop. Traumatol. Surg. Res. OTSR 2017, 103, S1–S10. [Google Scholar] [CrossRef]
- Hays, P.L.; Kawamura, S.; Deng, X.-H.; Dagher, E.; Mithoefer, K.; Ying, L.; Rodeo, S.A. The role of macrophages in early healing of a tendon graft in a bone tunnel. J. Bone Jt. Surg. Am. Vol. 2008, 90, 565–579. [Google Scholar] [CrossRef]
- Kawamura, S.; Ying, L.; Kim, H.-J.; Dynybil, C.; Rodeo, S.A. Macrophages accumulate in the early phase of tendon-bone healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2005, 23, 1425–1432. [Google Scholar] [CrossRef]
- Itoi, E.; Minagawa, H.; Yamamoto, N.; Seki, N.; Abe, H. Are pain location and physical examinations useful in locating a tear site of the rotator cuff? Am. J. Sport. Med. 2006, 34, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Carbone, E.J.; Lo, K.W.-H.; Laurencin, C.T. Electrospinning of polymer nanofibers for tissue regeneration. Prog. Polym. Sci. 2015, 46, 1–24. [Google Scholar] [CrossRef]
- Li, W.-J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; Attawia, M.A.; Elgendy, H.E.; Herbert, K.M. Tissue engineered bone-regeneration using degradable polymers: The formation of mineralized matrices. Bone 1996, 19, 93S–99S. [Google Scholar] [CrossRef]
- Borden, M.; El-Amin, S.F.; Attawia, M.; Laurencin, C.T. Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair. Biomaterials 2003, 24, 597–609. [Google Scholar] [CrossRef]
- Kovacevic, D.; Fox, A.J.; Bedi, A.; Ying, L.; Deng, X.-H.; Warren, R.F.; Rodeo, S.A. Calcium-phosphate matrix with or without TGF-β3 improves tendon-bone healing after rotator cuff repair. Am. J. Sport. Med. 2011, 39, 811–819. [Google Scholar] [CrossRef]
- Wang, X.; Wenk, E.; Zhang, X.; Meinel, L.; Vunjak-Novakovic, G.; Kaplan, D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J. Control. Release 2009, 134, 81–90. [Google Scholar] [CrossRef]
- Park, J.Y.; Shim, J.-H.; Choi, S.-A.; Jang, J.; Kim, M.; Lee, S.H.; Cho, D.-W. 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J. Mater. Chem. B 2015, 3, 5415–5425. [Google Scholar] [CrossRef]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Prantl, L.; Kujat, R.; Nerlich, M.; Tuan, R.S.; Angele, P. Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs 2010, 192, 158–166. [Google Scholar] [CrossRef]
- Barry, F.; Boynton, R.E.; Liu, B.; Murphy, J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp. Cell Res. 2001, 268, 189–200. [Google Scholar] [CrossRef]
- Fricke, D.; Becker, A.; Jütte, L.; Bode, M.; de Cassan, D.; Wollweber, M.; Glasmacher, B.; Roth, B. Mueller matrix measurement of electrospun fiber scaffolds for tissue engineering. Polymers 2019, 11, 2062. [Google Scholar] [CrossRef] [PubMed]
- de Cassan, D.; Sydow, S.; Schmidt, N.; Behrens, P.; Roger, Y.; Hoffmann, A.; Hoheisel, A.L.; Glasmacher, B.; Hänsch, R.; Menzel, H. Attachment of nanoparticulate drug-release systems on poly(ε-caprolactone) nanofibers via a graftpolymer as interlayer. Colloids Surf. B Biointerfaces 2018, 163, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, J.; Sydow, S.; Burmeister, L.; Hoffmann, A.; Menzel, H.; Bunjes, H. Spatially and temporally controllable BMP-2 and TGF-β3 double release from polycaprolactone fiber scaffolds via chitosan-based polyelectrolyte coatings. ACS Biomater. Sci. Eng. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Sydow, S.; de Cassan, D.; Hänsch, R.; Gengenbach, T.R.; Easton, C.D.; Thissen, H.; Menzel, H. Layer-by-layer deposition of chitosan nanoparticles as drug-release coatings for PCL nanofibers. Biomater. Sci. 2018, 7, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Roger, Y.; Sydow, S.; Burmeister, L.; Menzel, H.; Hoffmann, A. Sustained release of TGF-β3 from polysaccharide nanoparticles induces chondrogenic differentiation of human mesenchymal stromal cells. Colloids Surfaces B Biointerfaces 2020, 189, 110843. [Google Scholar] [CrossRef]
- Sharma, B.; Williams, C.G.; Khan, M.; Manson, P.; Elisseeff, J.H. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast. Reconstr. Surg. 2007, 119, 112–120. [Google Scholar] [CrossRef]
- Bian, L.; Zhai, D.Y.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32, 6425–6434. [Google Scholar] [CrossRef]
- Sydow, S.; Aniol, A.; Hadler, C.; Menzel, H. Chitosan-azide nanoparticle coating as a degradation barrier in multilayered polyelectrolyte drug delivery systems. Biomolecules 2019, 9, 573. [Google Scholar] [CrossRef]
- Poth, N.; Seiffart, V.; Gross, G.; Menzel, H.; Dempwolf, W. Biodegradable chitosan nanoparticle coatings on titanium for the delivery of BMP-2. Biomolecules 2015, 5, 3–19. [Google Scholar] [CrossRef]
- de Cassan, D.; Hoheisel, A.L.; Glasmacher, B.; Menzel, H. Impact of sterilization by electron beam, gamma radiation and X-rays on electrospun poly-(ε-caprolactone) fiber mats. J. Mater. Sci. Mater. Med. 2019, 30, 42. [Google Scholar] [CrossRef]
- Sundermann, J.; Oehmichen, S.; Sydow, S.; Burmeister, L.; Quaas, B.; Hänsch, R.; Rinas, U.; Hoffmann, A.; Menzel, H.; Bunjes, H. Varying the sustained release of BMP-2 from chitosan nanogel-functionalized polycaprolactone fiber mats by different polycaprolactone surface modifications. J. Biomed. Mater. Res. Part A 2020, 109, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Scheper, V.; Schwieger, J.; Hamm, A.; Lenarz, T.; Hoffmann, A. BDNF-overexpressing human mesenchymal stem cells mediate increased neuronal protection in vitro. J. Neurosci. Res. 2019, 97, 1414–1429. [Google Scholar] [CrossRef] [PubMed]
- Uludag, H.; D’Augusta, D.; Golden, J.; Li, J.; Timony, G.; Riedel, R.; Wozney, J.M. Implantation of recombinant human bone morphogenetic proteins with biomaterial carriers: A correlation between protein pharmacokinetics and osteoinduction in the rat ectopic model. J. Biomed. Mater. Res. 2000, 50, 227–238. [Google Scholar] [CrossRef]
- Pugliese, E.; Sallent, I.; Ribeiro, S.; Trotier, A.; Korntner, S.H.; Bayon, Y.; Zeugolis, D.I. Development of three-layer collagen scaffolds to spatially direct tissue-specific cell differentiation for enthesis repair. Mater. Today Bio 2023, 19, 100584. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, P.; Ci, Z.; Hao, X.; Bai, B.; Zhang, W.; Jiang, H.; Zhou, G. Acellular cartilage matrix biomimetic scaffold with immediate enrichment of autologous bone marrow mononuclear cells to repair articular cartilage defects. Mater. Today Bio 2022, 15, 100310. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, H.; Tian, Y.; Fu, L.; Gao, C.; Zhao, T.; Cao, F.; Liao, Z.; Yuan, Z.; Liu, S.; et al. Biofunctionalized structure and ingredient mimicking scaffolds achieving recruitment and chondrogenesis for staged cartilage regeneration. Front. Cell Dev. Biol. 2021, 9, 655440. [Google Scholar] [CrossRef]
- Li, H.; Zhao, T.; Cao, F.; Deng, H.; He, S.; Li, J.; Liu, S.; Yang, Z.; Yuan, Z.; Guo, Q. Integrated bioactive scaffold with aptamer-targeted stem cell recruitment and growth factor-induced pro-differentiation effects for anisotropic meniscal regeneration. Bioeng. Transl. Med. 2022, 7, e10302. [Google Scholar] [CrossRef]
- Hua, Y.; Huo, Y.; Bai, B.; Hao, J.; Hu, G.; Ci, Z.; Wu, X.; Yu, M.; Wang, X.; Chen, H.; et al. Fabrication of biphasic cartilage-bone integrated scaffolds based on tissue-specific photo-crosslinkable acellular matrix hydrogels. Mater. Today Bio 2022, 17, 100489. [Google Scholar] [CrossRef]
- Branton, M.H.; Kopp, J.B. TGF-beta and fibrosis. Microbes Infect. 1999, 1, 1349–1365. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Bornstein, Q.L.; Leon, D.A.; Orozco, E.H.; Madrigal, V.K.; Cordero, E.M. Inflammatory cytokine production by immunological and foreign body multinucleated giant cells. Immunology 2000, 100, 352–358. [Google Scholar] [CrossRef]
- Ihn, H. Scleroderma, fibroblasts, signaling, and excessive extracellular matrix. Curr. Rheumatol. Rep. 2005, 7, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Yata, Y.; Gotwals, P.; Koteliansky, V.; Rockey, D.C. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: Implications for antifibrotic therapy. Hepatology 2002, 35, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ma, W.; Xiao, Y.; Wu, B.; Li, X.; Liu, F.; Qiu, J.; Zhang, G. High doses of baicalin induces kidney injury and fibrosis through regulating TGF-β/Smad signaling pathway. Toxicol. Appl. Pharmacol. 2017, 333, 1–9. [Google Scholar] [CrossRef] [PubMed]






| TGF-β3 Concentration | Z-average Diameter (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| 0 µg/mL | 267.8 ± 2.38 | 0.262 ± 0.003 | 16.2 ± 1.01 |
| 1 µg/mL | 259.9 ± 1.19 | 0.258 ± 0.002 | 16.6 ± 0.75 |
| 2 µg/mL | 278.3 ± 0.81 | 0.253 ± 0.006 | 17.6 ± 0.60 |
| 10 µg/mL | 264.1 ± 0.87 | 0.259 ± 0.003 | 16.9 ± 0.40 |
| 20 µg/mL | 249.4 ± 0.23 | 0.252 ± 0.002 | 16.6 ± 0.58 |
| Loading Concentration [µg/mL] | Calculated Total Release from 128 mm2 Fiber Mats (ng) | Calculated Total Release from 15 mm2 Fiber Mats (ng) | MEDin vivo Achieved |
|---|---|---|---|
| 20 | 3012.3 | 353.0 | yes |
| 10 | 1463.3 | 171.5 | yes |
| 2 | 224.1 | 26.3 | no |
| 1 | 69.2 | 8.1 | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berten-Schunk, L.; Roger, Y.; Bunjes, H.; Hoffmann, A. Release of TGF-β3 from Surface-Modified PCL Fiber Mats Triggers a Dose-Dependent Chondrogenic Differentiation of Human Mesenchymal Stromal Cells. Pharmaceutics 2023, 15, 1303. https://doi.org/10.3390/pharmaceutics15041303
Berten-Schunk L, Roger Y, Bunjes H, Hoffmann A. Release of TGF-β3 from Surface-Modified PCL Fiber Mats Triggers a Dose-Dependent Chondrogenic Differentiation of Human Mesenchymal Stromal Cells. Pharmaceutics. 2023; 15(4):1303. https://doi.org/10.3390/pharmaceutics15041303
Chicago/Turabian StyleBerten-Schunk, Leonie, Yvonne Roger, Heike Bunjes, and Andrea Hoffmann. 2023. "Release of TGF-β3 from Surface-Modified PCL Fiber Mats Triggers a Dose-Dependent Chondrogenic Differentiation of Human Mesenchymal Stromal Cells" Pharmaceutics 15, no. 4: 1303. https://doi.org/10.3390/pharmaceutics15041303