Nanomaterial-Based Antivascular Therapy in the Multimodal Treatment of Cancer
Abstract
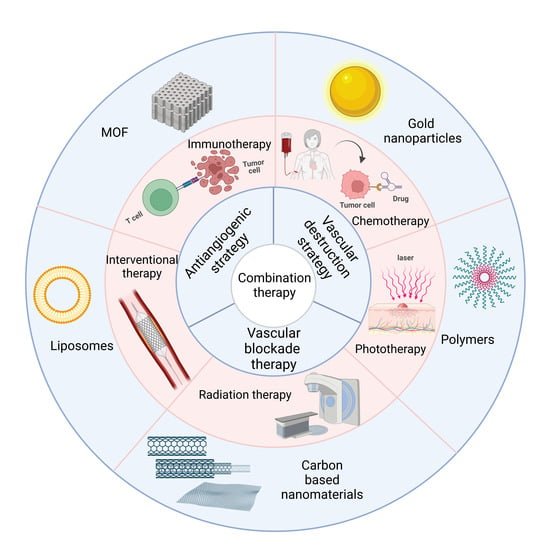
:1. Introduction
2. Immunotherapy
3. Chemotherapy
4. Phototherapy
5. Radiation Therapy
6. Interventional Therapy
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cheng, Q.; Huang, J.; Ma, M.; Zhang, D.; Lei, X.; Xiao, Z.; Zhang, D.; Shi, C.; Luo, L. Monitoring tumour microenvironment changes during anti-angiogenesis therapy using functional MRI. Angiogenesis 2019, 22, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat. Med. 2001, 7, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Fang, W.; Huang, C.; Chen, Z.; Nie, T.; Wang, J.; Luo, L.; Xiao, Z. MR imaging guided iron-based nanoenzyme for synergistic Ferroptosis−Starvation therapy in triple negative breast cancer. Smart Mater. Med. 2021, 3, 159–167. [Google Scholar] [CrossRef]
- Shi, C.; Liu, D.; Xiao, Z.; Zhang, D.; Liu, G.; Liu, G.; Chen, H.; Luo, L. Monitoring Tumor Response to Antivascular Therapy Using Non-Contrast Intravoxel Incoherent Motion Diffusion-Weighted MRI. Cancer Res. 2017, 77, 3491–3501. [Google Scholar] [CrossRef] [Green Version]
- Smolarczyk, R.; Czapla, J.; Jarosz-Biej, M.; Czerwinski, K.; Cichoń, T. Vascular disrupting agents in cancer therapy. Eur. J. Pharmacol. 2021, 891, 173692. [Google Scholar] [CrossRef]
- Siemann, D.W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Di, C.; Li, S.; Yang, X.; Nie, G. Smart Nanotherapeutic Targeting of Tumor Vasculature. Acc. Chem. Res. 2019, 52, 2703–2712. [Google Scholar] [CrossRef]
- Tong, S.; Zhao, W.; Zhao, D.; Zhang, W.; Zhang, Z. Biomaterials-Mediated Tumor Infarction Therapy. Front. Bioeng. Biotechnol. 2022, 10, 916926. [Google Scholar] [CrossRef]
- Plate, K.H.; Scholz, A.; Dumont, D.J. Tumor angiogenesis and anti-angiogenic therapy in malignant gliomas revisited. Acta Neuropathol. 2012, 124, 763–775. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, challenges, and future of nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Lungare, S.; Hallam, K.; Badhan, R.K. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, J.; Tian, Y.; Zhang, L.; Han, X.; Wang, Q.; Cheng, W. Targeted delivery of reduced graphene oxide nanosheets using multifunctional ultrasound nanobubbles for visual and enhanced photothermal therapy [Corrigendum]. Int. J. Nanomed. 2019, 14, 2449–2450. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Li, S.; Nie, G. Nanotechnological strategies for therapeutic targeting of tumor vasculature. Nanomedicine 2013, 8, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Sun, J.; Li, S.; Shi, J.; Gao, H.; Leong, W.S.; Wu, Y.; Li, M.; Liu, C.; Li, P.; et al. Blood-triggered generation of platinum nanoparticle functions as an anti-cancer agent. Nat. Commun. 2020, 11, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Ji, T.; Wang, H.; Li, S.; Zhao, Y.; Nie, G. Self-assembled peptide nanoparticles as tumor microenvironment activatable probes for tumor targeting and imaging. J. Control. Release 2014, 177, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, J.; Zhao, X.; Qi, Y.; Zhang, Y.; Han, X.; Ding, Y.; Guan, J.; Ji, T.; Zhao, Y.; Nie, G. Reshaping Prostate Tumor Microenvironment to Suppress Metastasis via Cancer-Associated Fibroblast Inactivation with Peptide-Assembly-Based Nanosystem. ACS Nano 2019, 13, 12357–12371. [Google Scholar] [CrossRef]
- Du, C.; Qi, Y.; Zhang, Y.; Wang, Y.; Zhao, X.; Min, H.; Han, X.; Lang, J.; Qin, H.; Shi, Q.; et al. Epidermal Growth Factor Receptor-Targeting Peptide Nanoparticles Simultaneously Deliver Gemcitabine and Olaparib to Treat Pancreatic Cancer with Breast Cancer 2 (BRCA2) Mutation. ACS Nano 2018, 12, 10785–10796. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Z.; Xu, W.; Sun, T.; Zhuang, X.; Ding, J.; Chen, X. Spatiotemporally Targeted Nanomedicine Overcomes Hypoxia-Induced Drug Resistance of Tumor Cells after Disrupting Neovasculature. Nano Lett. 2020, 20, 6191–6198. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, J.; Shen, N.; Tang, Z.; Chen, X. A self-activating nanoized vascular disrupting agent for selective anti-tumor therapy. Biomaterials 2022, 288, 121736. [Google Scholar] [CrossRef] [PubMed]
- Darge, H.F.; Hanurry, E.Y.; Birhan, Y.S.; Mekonnen, T.W.; Andrgie, A.T.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C. Multifunctional drug-loaded micelles encapsulated in thermo-sensitive hydrogel for in vivo local cancer treatment: Synergistic effects of anti-vascular and immuno-chemotherapy. Chem. Eng. J. 2021, 406, 126879. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Taleb, M.; Ding, Y.; Wang, B.; Yang, N.; Han, X.; Du, C.; Qi, Y.; Zhang, Y.; Sabet, Z.F.; Alanagh, H.R.; et al. Dopamine Delivery via pH-Sensitive Nanoparticles for Tumor Blood Vessel Normalization and an Improved Effect of Cancer Chemotherapeutic Drugs. Adv. Healthc. Mater. 2019, 8, 1900283. [Google Scholar] [CrossRef]
- Zhang, N.; Xin, X.; Feng, N.; Wu, D.; Zhang, J.; Yu, T.; Jiang, Q.; Gao, M.; Yang, H.; Zhao, S.; et al. Combining Fruquintinib and Doxorubicin in Size-Converted Nano-Drug Carriers for Tumor Therapy. ACS Biomater. Sci. Eng. 2022, 8, 1907–1920. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.-Y.; et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef]
- Li, B.; Chu, T.; Wei, J.; Zhang, Y.; Qi, F.; Lu, Z.; Gao, C.; Zhang, T.; Jiang, E.; Xu, J.; et al. Platelet-Membrane-Coated Nanoparticles Enable Vascular Disrupting Agent Combining Anti-Angiogenic Drug for Improved Tumor Vessel Impairment. Nano Lett. 2021, 21, 2588–2595. [Google Scholar] [CrossRef]
- Alamzadeh, Z.; Beik, J.; Mirrahimi, M.; Shakeri-Zadeh, A.; Ebrahimi, F.; Komeili, A.; Ghalandari, B.; Ghaznavi, H.; Kamrava, S.K.; Moustakis, C. Gold nanoparticles promote a multimodal synergistic cancer therapy strategy by co-delivery of thermo-chemo-radio therapy. Eur. J. Pharm. Sci. 2020, 145, 105235. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, L.; Zheng, L.; Liu, K.; Mao, Y.; Song, S. Sonodynamic therapy-derived multimodal synergistic cancer therapy. Cancer Lett. 2020, 497, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, M.; Alamzadeh, Z.; Beik, J.; Sarikhani, A.; Mousavi, M.; Irajirad, R.; Khani, T.; Davani, E.S.; Farashahi, A.; Ardakani, T.S.; et al. A 2D nanotheranostic platform based on graphene oxide and phase-change materials for bimodal CT/MR imaging, NIR-activated drug release, and synergistic thermo-chemotherapy. Nanotheranostics 2022, 6, 350–364. [Google Scholar] [CrossRef]
- You, Q.; Zhang, K.; Liu, J.; Liu, C.; Wang, H.; Wang, M.; Ye, S.; Gao, H.; Lv, L.; Wang, C.; et al. Persistent Regulation of Tumor Hypoxia Microenvironment via a Bioinspired Pt-Based Oxygen Nanogenerator for Multimodal Imaging-Guided Synergistic Phototherapy. Adv. Sci. 2020, 7, 1903341. [Google Scholar] [CrossRef]
- Lu, D.; Chen, M.; Yu, L.; Chen, Z.; Guo, H.; Zhang, Y.; Han, Z.; Xu, T.; Wang, H.; Zhou, X.; et al. Smart-Polypeptide-Coated Mesoporous Fe3O4 Nanoparticles: Non-Interventional Target-Embolization/Thermal Ablation and Multimodal Imaging Combination Theranostics for Solid Tumors. Nano Lett. 2021, 21, 10267–10278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, X.; Hou, Z.; Wang, N.; Jiang, Y.; Luan, Y. Engineering a photosensitizer nanoplatform for amplified photodynamic immunotherapy via tumor microenvironment modulation. Nanoscale Horiz. 2021, 6, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.; Sakurai, Y.; Jones, H.S.; Akita, H.; Hisaka, A.; Hatakeyama, H. Silencing of VEGFR2 by RGD-Modified Lipid Nanoparticles Enhanced the Efficacy of Anti-PD-1 Antibody by Accelerating Vascular Normalization and Infiltration of T Cells in Tumors. Cancers 2020, 12, 3630. [Google Scholar] [CrossRef]
- Wang, D.; Feng, C.; Xiao, Z.; Huang, C.; Chen, Z.; Fang, W.; Ma, X.; Wang, X.; Luo, L.; Hu, K.; et al. Therapeutic hydrogel for enhanced immunotherapy: A powerful combination of MnO2 nanosheets and vascular disruption. Nano Today 2022, 47, 101673. [Google Scholar] [CrossRef]
- Zhou, P.; Qin, J.; Zhou, C.; Wan, G.; Liu, Y.; Zhang, M.; Yang, X.; Zhang, N.; Wang, Y. Multifunctional nanoparticles based on a polymeric copper chelator for combination treatment of metastatic breast cancer. Biomaterials 2019, 195, 86–99. [Google Scholar] [CrossRef]
- Bao, X.; Shen, N.; Lou, Y.; Yu, H.; Wang, Y.; Liu, L.; Tang, Z.; Chen, X. Enhanced anti-PD-1 therapy in hepatocellular carcinoma by tumor vascular disruption and normalization dependent on combretastatin A4 nanoparticles and DC101. Theranostics 2021, 11, 5955–5969. [Google Scholar] [CrossRef] [PubMed]
- Taleb, M.; Atabakhshi-Kashi, M.; Wang, Y.; Alanagh, H.R.; Sabet, Z.F.; Li, F.; Cheng, K.; Li, C.; Qi, Y.; Nie, G.; et al. Bifunctional Therapeutic Peptide Assembled Nanoparticles Exerting Improved Activities of Tumor Vessel Normalization and Immune Checkpoint Inhibition. Adv. Healthc. Mater. 2021, 10, 2100051. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Liu, Y.; Fang, Y.; Zheng, S.; Wu, J.; Wang, M.; Zhong, W.; Shi, M.; Xing, M.; Liao, W. Gold Nanoparticles Induce Tumor Vessel Normalization and Impair Metastasis by Inhibiting Endothelial Smad2/3 Signaling. ACS Nano 2020, 14, 7940–7958. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wu, J.; Chen, J.; Lin, L.; Zhang, S.; Yang, Z.; Sun, P.; Li, Y.; Tian, H.; Chen, X. Targeting dual gene delivery nanoparticles overcomes immune checkpoint blockade induced adaptive resistance and regulates tumor microenvironment for improved tumor immunotherapy. Nano Today 2021, 38, 101194. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Wu, Z.; Chu, T.; Yang, Z.; Xu, S.; Wu, S.; Qie, Y.; Lu, Z.; Qi, F.; et al. Reducing Postoperative Recurrence of Early-Stage Hepatocellular Carcinoma by a Wound-Targeted Nanodrug. Adv. Sci. 2022, 9, 2200477. [Google Scholar] [CrossRef]
- Juneja, V.R.; McGuire, K.A.; Manguso, R.T.; LaFleur, M.W.; Collins, N.; Haining, W.N.; Freeman, G.J.; Sharpe, A.H. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 2017, 214, 895–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadzadeh, M.; Johnson, L.A.; Heemskerk, B.; Wunderlich, J.R.; Dudley, M.E.; White, D.E.; Rosenberg, S.A. Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009, 114, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, F.; Zhong, M.; Yarden, Y.; Fu, L. The biomarkers of hyperprogressive disease in PD-1/PD-L1 blockage therapy. Mol. Cancer 2020, 19, 81. [Google Scholar] [CrossRef]
- Grimm, M.-O.; Leucht, K.; Grünwald, V.; Foller, S. New First Line Treatment Options of Clear Cell Renal Cell Cancer Patients with PD-1 or PD-L1 Immune-Checkpoint Inhibitor-Based Combination Therapies. J. Clin. Med. 2020, 9, 565. [Google Scholar] [CrossRef] [Green Version]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Jiao, L.; Dong, Q.; Zhai, W.; Zhao, W.; Shi, P.; Wu, Y.; Zhou, X.; Gao, Y. A PD-L1 and VEGFR2 dual targeted peptide and its combination with irradiation for cancer immunotherapy. Pharmacol. Res. 2022, 182, 106343. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Boucher, Y.; Kumar, A.S.; Posada, J.M.; Gjini, E.; Pfaff, K.; Lipschitz, M.; Lako, A.; Duda, D.G.; Rodig, S.J.; Hodi, F.S.; et al. Bevacizumab improves tumor infiltration of mature dendritic cells and effector T-cells in triple-negative breast cancer patients. npj Precis. Oncol. 2021, 5, 62. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, T.; Zhang, L.; Zhao, C.; Zhang, Z.; Wang, Z.; Gu, M.; Li, W.; Li, B. Combined application of bevacizumab and PD-1 blockade displays durable treatment effects by increasing the infiltration and cytotoxic function of CD8+T cells in lung cancer. Immunotherapy 2022, 14, 695–708. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, J.; Di, W.; Wu, X. Anti-angiogenesis therapy overcomes the innate resistance to PD-1/PD-L1 blockade in VEGFA-overexpressed mouse tumor models. Cancer Immunol. Immunother. 2020, 69, 1781–1799. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Gao, G.; Na Wang, Y.; Zhao, J.; Chen, G.; Liu, Z.; Gu, K.; Huang, M.; He, J.; Chen, J.; et al. Efficacy of PD-1 monoclonal antibody SHR-1210 plus apatinib in patients with advanced nonsquamous NSCLC with wild-type EGFR and ALK. J. Clin. Oncol. 2019, 37. [Google Scholar] [CrossRef]
- Tang, H.; Qiao, J.; Fu, Y.-X. Immunotherapy and tumor microenvironment. Cancer Lett. 2015, 370, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rad, H.S.; Monkman, J.; Warkiani, M.E.; Ladwa, R.; O’Byrne, K.; Rezaei, N.; Kulasinghe, A. Understanding the tumor microenvironment for effective immunotherapy. Med. Res. Rev. 2021, 41, 1474–1498. [Google Scholar] [CrossRef]
- DeBerardinis, R.J. Tumor microenvironment, metabolism, and immunotherapy. N. Engl. J. Med. 2020, 382, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xiong, H.; Yang, S.; Lu, Y.; Deng, Y.; Yao, J.; Yao, J. Jet-Lagged Nanoparticles Enhanced Immunotherapy Efficiency through Synergistic Reconstruction of Tumor Microenvironment and Normalized Tumor Vasculature. Adv. Healthc. Mater. 2020, 9, e2000075. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, Z.; Tang, J.; Zhang, M.; Yang, Y.; Song, H.; Yu, C. MnO2 Nanoflowers Induce Immunogenic Cell Death under Nutrient Deprivation: Enabling an Orchestrated Cancer Starvation-Immunotherapy. Adv. Sci. 2021, 8, 2002667. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Jiang, X.; Li, Y.; Han, W.; Rodriguez, M.; Xu, Z.; Lin, W. Sequential Treatment of Bioresponsive Nanoparticles Elicits Antiangiogenesis and Apoptosis and Synergizes with a CD40 Agonist for Antitumor Immunity. ACS Nano 2020, 15, 765–780. [Google Scholar] [CrossRef]
- Chang, C.-C.; Dinh, T.K.; Lee, Y.-A.; Wang, F.-N.; Sung, Y.-C.; Yu, P.-L.; Chiu, S.-C.; Shih, Y.-C.; Wu, C.-Y.; Huang, Y.-D.; et al. Nanoparticle Delivery of MnO2 and Antiangiogenic Therapy to Overcome Hypoxia-Driven Tumor Escape and Suppress Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2020, 12, 44407–44419. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Liu, H.; Zhang, K.; Zeng, Q.; Yang, S.; Jiang, Z.; Zhang, X.; Chen, T.; Li, D.; et al. Bi/Se-Based Nanotherapeutics Sensitize CT Image-Guided Stereotactic Body Radiotherapy through Reprogramming the Microenvironment of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2021, 13, 42473–42485. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Li, S.; Savvides, P.; Ohr, J.P.; Gilbert, J.; Levine, M.A.; Chakravarti, A.; Haigentz, M., Jr.; Saba, N.F.; Ikpeazu, C.V.; et al. Phase III Randomized Trial of Chemotherapy with or without Bevacizumab in Patients with Recurrent or Metastatic Head and Neck Cancer. J. Clin. Oncol. 2019, 37, 3266–3274. [Google Scholar] [CrossRef]
- Cheung-Ong, K.; Giaever, G.; Nislow, C. DNA-Damaging Agents in Cancer Chemotherapy: Serendipity and Chemical Biology. Chem. Biol. 2013, 20, 648–659. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kim, Y.J.; Siriwon, N.; Rohrs, J.A.; Yu, Z.; Wanga, P.; Wang, P. Combination drug delivery via multilamellar vesicles enables targeting of tumor cells and tumor vasculature. Biotechnol. Bioeng. 2018, 115, 1403–1415. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, N.; Ci, T.; Tang, Z.; Gu, Z.; Li, G.; Chen, X. Combretastatin A4 Nanodrug-Induced MMP9 Amplification Boosts Tumor-Selective Release of Doxorubicin Prodrug. Adv. Mater. 2019, 31, e1904278. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, J.; Li, X.; Xu, L.; Li, C.; Ma, L.; Liu, J.; Ma, R.; An, Y.; Huang, F.; et al. Rational design of drug delivery systems for potential programmable drug release and improved therapeutic effect. Mater. Chem. Front. 2019, 3, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Zhong, X.; Peng, Y.; Hao, C.; Liang, X.; Yang, Y.; Shi, X.; Chen, X.; Yi, X.; Li, X.; et al. Self-anti-angiogenesis nanoparticles enhance anti-metastatic-tumor efficacy of chemotherapeutics. Bioact. Mater. 2022, 13, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Jiang, H.; Li, C.; Li, Z.; Qi, C.; Li, Z.; Gao, Z.; Zhang, B.; Wu, J. Dual-Ligand-Modified Liposomes Co-Loaded with Anti-Angiogenic and Chemotherapeutic Drugs for Inhibiting Tumor Angiogenesis and Metastasis. Int. J. Nanomed. 2021, 16, 4001–4016. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, B.; Cheng, G. Reshaping Tumor Blood Vessels to Enhance Drug Penetration with a Multistrategy Synergistic Nanosystem. Mol. Pharm. 2020, 17, 3151–3164. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Dahmani, F.Z.; Qiao, J.; Ni, J.; Xiong, H.; Liu, T.; Zhou, J.; Yao, J. A targeted nanoplatform co-delivering chemotherapeutic and antiangiogenic drugs as a tool to reverse multidrug resistance in breast cancer. Acta Biomater. 2018, 75, 398–412. [Google Scholar] [CrossRef]
- Du, S.; Xiong, H.; Xu, C.; Lu, Y.; Yao, J. Attempts to strengthen and simplify the tumor vascular normalization strategy using tumor vessel normalization promoting nanomedicines. Biomater. Sci. 2019, 7, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Wang, H.; Zhang, Y.; Cai, H.; Zhang, P.; Li, L.; Zhou, J.; Yin, T. Co-delivery of silybin and paclitaxel by dextran-based nanoparticles for effective anti-tumor treatment through chemotherapy sensitization and microenvironment modulation. J. Control. Release 2020, 321, 198–210. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ho, S.-H.; Li, B.; Wang, M.; Deng, X.; Na Yang, N.; Liu, G.; Lu, Z.; Xu, J.; et al. Combination of tumour-infarction therapy and chemotherapy via the co-delivery of doxorubicin and thrombin encapsulated in tumour-targeted nanoparticles. Nat. Biomed. Eng. 2020, 4, 732–742. [Google Scholar] [CrossRef]
- Lai, X.; Liu, X.; Pan, H.; Zhu, M.; Long, M.; Yuan, Y.; Zhang, Z.; Dong, X.; Lu, Q.; Sun, P.; et al. Light-Triggered Efficient Sequential Drug Delivery of Biomimetic Nanosystem for Multimodal Chemo-, Antiangiogenic, and Anti-MDSC Therapy in Melanoma. Adv. Mater. 2022, 34, 2106682. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Chen, X.-J.; Li, B.-Z.; Xu, S.; Wu, Z.-L.; Hu, M.-G.; Zhao, Z.-M.; Zhao, G.-D.; Wang, C.-R.; Hong, W.; et al. Active targeted Janus nanoparticles enable anti-angiogenic drug combining chemotherapy agent to prevent postoperative hepatocellular carcinoma recurrence. Biomaterials 2022, 281, 121362. [Google Scholar] [CrossRef]
- Wu, H.; Huang, C.; Wang, L.; Li, Q.; Li, Y.; Zhang, L.; Zhu, D. Folate-targeted co-delivery polymersomes for efficient photo-chemo-antiangiogenic therapy against breast cancer and in vivo evaluation via OCTA/NIRF dual-modal imaging. Chin. Chem. Lett. 2022, 33, 5035–5041. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Shi, Z.; Yang, Y.; Xie, X.; Lee, S.M.; Wang, Y.; Leong, K.W.; Chen, M. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017, 58, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Assali, M.; Kittana, N.; Qasem, S.A.; Adas, R.; Saleh, D.; Arar, A.; Zohud, O. Combretastatin A4-camptothecin micelles as combination therapy for effective anticancer activity. RSC Adv. 2019, 9, 1055–1061. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Shen, J.; Liu, C.; Du, A.; Chen, M.; Meng, X.; Wang, H.; Zhang, S.; Zhang, L.; Li, Z.; et al. Modularly designed peptide-based nanomedicine inhibits angiogenesis to enhance chemotherapy for post-surgical recurrence of esophageal squamous cell carcinomas. Nano Res. 2023, 1–8. [Google Scholar] [CrossRef]
- Alshaman, R.; Alattar, A.; El-Sayed, R.M.; Gardouh, A.R.; Elshaer, R.E.; Elkazaz, A.Y.; Eladl, M.A.; El-Sherbiny, M.; Farag, N.E.; Hamdan, A.M.; et al. Formulation and Characterization of Doxycycline-Loaded Polymeric Nanoparticles for Testing Antitumor/Antiangiogenic Action in Experimental Colon Cancer in Mice. Nanomaterials 2022, 12, 857. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.E.; Priolo, D.; Antonelli, G.; Libra, M.; Mccubrey, J.A.; Ferraù, F. Bevacizumab in the treatment of NSCLC: Patient selection and perspectives. Lung Cancer Targets Ther. 2017, 8, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Raguz, S.; Yagüe, E. Resistance to chemotherapy: New treatments and novel insights into an old problem. Br. J. Cancer 2008, 99, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Luqmani, Y.A. Mechanisms of Drug Resistance in Cancer Chemotherapy. Med Princ. Pract. 2005, 14, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Takara, K.; Sakaeda, T.; Okumura, K. An Update on Overcoming MDR1-Mediated Multidrug Resistance in Cancer Chemotherapy. Curr. Pharm. Des. 2006, 12, 273–286. [Google Scholar] [CrossRef]
- Ji, C.; Cheng, W.; Hu, Y.; Liu, Y.; Liu, F.; Yin, M. A nano vector with photothermally enhanced drug release and retention to overcome cancer multidrug resistance. Nano Today 2021, 36, 101020. [Google Scholar] [CrossRef]
- Kankala, R.K.; Liu, C.-G.; Yang, D.-Y.; Wang, S.-B.; Chen, A.-Z. Ultrasmall platinum nanoparticles enable deep tumor penetration and synergistic therapeutic abilities through free radical species-assisted catalysis to combat cancer multidrug resistance. Chem. Eng. J. 2020, 383, 123138. [Google Scholar] [CrossRef]
- Wang, X.; Xiong, T.; Cui, M.; Li, N.; Li, Q.; Zhu, L.; Duan, S.; Wang, Y.; Guo, Y. A novel targeted co-delivery nanosystem for enhanced ovarian cancer treatment via multidrug resistance reversion and mTOR-mediated signaling pathway. J. Nanobiotechnol. 2021, 19, 444. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, Y.; Min, H.; Ni, C.; Wang, F.; Wang, B.; Qin, H.; Zhang, Y.; Liu, G.; Qin, Y.; et al. Cooperatively responsive peptide nanotherapeutic that regulates angiopoietin receptor Tie2 activity in tumor microenvironment to prevent breast tumor relapse after chemotherapy. ACS Nano 2019, 13, 5091–5102. [Google Scholar] [CrossRef]
- Jing, L.; Qu, H.; Wu, D.; Zhu, C.; Yang, Y.; Jin, X.; Zheng, J.; Shi, X.; Yan, X.; Wang, Y. Platelet-camouflaged nanococktail: Simultaneous inhibition of drug-resistant tumor growth and metastasis via a cancer cells and tumor vasculature dual-targeting strategy. Theranostics 2018, 8, 2683–2695. [Google Scholar] [CrossRef]
- Hong, S.; Huang, Q.-X.; Ji, P.; Pang, X.; Sun, Y.; Cheng, S.-X.; Zhang, X.-Z.; Chen, X. Vascular disrupting agent-induced amplification of tumor targeting and prodrug activation boosts anti-tumor efficacy. Sci. China Chem. 2022, 65, 1994–2004. [Google Scholar] [CrossRef]
- Martin, J.D.; Seano, G.; Jain, R.K. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu. Rev. Physiol. 2019, 81, 505–534. [Google Scholar] [CrossRef]
- Cesca, M.; Bizzaro, F.; Zucchetti, M.; Giavazzi, R. Tumor Delivery of Chemotherapy Combined with Inhibitors of Angiogenesis and Vascular Targeting Agents. Front. Oncol. 2013, 3, 259. [Google Scholar] [CrossRef] [Green Version]
- Kosharskyy, B.; Solban, N.; Chang, S.K.; Rizvi, I.; Chang, Y.; Hasan, T. A Mechanism-Based Combination Therapy Reduces Local Tumor Growth and Metastasis in an Orthotopic Model of Prostate Cancer. Cancer Res. 2006, 66, 10953–10958. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Liang, P.; Xie, J.; Song, C.; Tang, C.; Wang, Y.; Yin, X.; Cai, Y.; Han, W.; Dong, X. Carrier-free nano-integrated strategy for synergetic cancer anti-angiogenic therapy and phototherapy. Chem. Sci. 2019, 10, 2778–2784. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Sang, R.; Tang, Y.; Xia, H.; Liu, J.; Huang, W.; Fan, Q.; Wang, Q. Fabrication of a phototheranostic nanoplatform for single laser-triggered NIR-II fluorescence imaging-guided photothermal/chemo/antiangiogenic combination therapy. Acta Biomater. 2022, 151, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Yu, H.; Yang, L.; Chen, L. Combretastatin A4-combined photodynamic therapy for enhanced tumor therapeutic efficacy. Mater. Today Commun. 2021, 28, 102616. [Google Scholar] [CrossRef]
- Li, J.; Qu, B.; Wang, Q.; Ning, X.; Ren, S.; Liu, C.; Zhang, R. Hollow Manganese-Doped Calcium Phosphate Nanoparticles Treated with Melanin Nanoparticles and Thalidomide for Photothermal and Anti-angiogenic Cancer Therapy. ACS Appl. Nano Mater. 2022, 5, 7733–7743. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Deng, X.; Wang, X.; Jia, F.; Zhong, S.; Cui, X.; Pan, Z.; Shao, L.; Wu, Y. Self-assembling combretastatin A4 incorporated protamine/nanodiamond hybrids for combined anti-angiogenesis and mild photothermal therapy in liver cancer. Nanotechnology 2021, 32, 465101. [Google Scholar] [CrossRef]
- Tao, N.; Liu, Y.; Wu, Y.; Li, X.; Li, J.; Sun, X.; Chen, S.; Liu, Y.-N. Minimally Invasive Antitumor Therapy Using Biodegradable Nanocomposite Micellar Hydrogel with Functionalities of NIR-II Photothermal Ablation and Vascular Disruption. ACS Appl. Bio Mater. 2020, 3, 4531–4542. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, H.; Zou, H.; Song, C.; Zhao, S.; Cao, Z.; Zhang, X.; Zhang, G.; Cai, Y.; Han, W. A novel second near-infrared theranostic agent: A win–win strategy of tracing and blocking tumor-associated vessels for oral squamous cell carcinoma. Mater. Today Nano 2022, 17, 100172. [Google Scholar] [CrossRef]
- Paris, J.L.; Villaverde, G.; Gómez-Graña, S.; Vallet-Regí, M. Nanoparticles for multimodal antivascular therapeutics: Dual drug release, photothermal and photodynamic therapy. Acta Biomater. 2020, 101, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-L.; Lin, H.-C.; Chiang, W.-L.; Shih, Y.-H.; Chiang, P.-F.; Luo, T.-Y.; Cheng, C.-C.; Shieh, M.-J. Anti-angiogenic treatment (Bevacizumab) improves the responsiveness of photodynamic therapy in colorectal cancer. Photodiagn. Photodyn. Ther. 2018, 23, 111–118. [Google Scholar] [CrossRef]
- Min, H.; Wang, J.; Qi, Y.; Zhang, Y.; Han, X.; Xu, Y.; Xu, J.; Li, Y.; Chen, L.; Cheng, K.; et al. Biomimetic Metal–Organic Framework Nanoparticles for Cooperative Combination of Antiangiogenesis and Photodynamic Therapy for Enhanced Efficacy. Adv. Mater. 2019, 31, e1808200. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, F.; Zheng, R.; Chen, X.; Zhao, L.; Yu, B.; Chen, A.; Jiang, X.; Cheng, H.; Li, S. Self-delivery nanomedicine for vascular disruption-supplemented chemo-photodynamic tumor therapy. J. Colloid Interface Sci. 2022, 612, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Hao, Y.; Wu, Y.; Zhou, Z.; Li, J.; Sun, X.; Liu, Y.-N. Integrated Hydrogel Platform for Programmed Antitumor Therapy Based on Near Infrared-Triggered Hyperthermia and Vascular Disruption. ACS Appl. Mater. Interfaces 2019, 11, 21381–21390. [Google Scholar] [CrossRef]
- Li, B.; Jiang, Z.; Xie, D.; Wang, Y.; Lao, X. Cetuximab-modified CuS nanoparticles integrating near-infrared-II-responsive photothermal therapy and anti-vessel treatment. Int. J. Nanomed. 2018, 13, 7289–7302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhu, J.; Huang, G.; Zhu, J.; He, D. Potential applications of multifunctional mesoporous carbon nanoplatform for tumor microenvironment improving by combined chemo-/phototherapy. Carbon 2020, 163, 128–136. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, S.; Wen, L.; Zhu, Y.; Tan, Y.; Qiu, G.; Meng, T.; Yu, F.; Yuan, H.; Hu, F. Enhancing Drug Delivery for Overcoming Angiogenesis and Improving the Phototherapy Efficacy of Glioblastoma by ICG-Loaded Glycolipid-Like Micelles. Int. J. Nanomed. 2020, 15, 2717–2732. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Yu, Q.; Huang, X.; Dai, H.; Luo, T.; Shao, J.; Chen, J.; Huang, W.; Dong, X. A Highly-Efficient Type I Photosensitizer with Robust Vascular-Disruption Activity for Hypoxic-and-Metastatic Tumor Specific Photodynamic Therapy. Small 2020, 16, e2001059. [Google Scholar] [CrossRef]
- Liang, P.; Huang, X.; Wang, Y.; Chen, D.; Ou, C.; Zhang, Q.; Shao, J.; Huang, W.; Dong, X. Tumor-Microenvironment-Responsive Nanoconjugate for Synergistic Antivascular Activity and Phototherapy. ACS Nano 2018, 12, 11446–11457. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Zheng, D.-W.; Zhang, C.; Huang, Q.-X.; Cheng, S.-X.; Zhang, X.-Z. Vascular disrupting agent induced aggregation of gold nanoparticles for photothermally enhanced tumor vascular disruption. Sci. Adv. 2020, 6, eabb0020. [Google Scholar] [CrossRef]
- Jung, E.; Lee, J.; Lee, Y.; Seon, S.; Park, M.; Song, C.; Lee, D. Tumor-Targeting H2O2-Responsive Photosensitizing Nanoparticles with Antiangiogenic and Immunogenic Activities for Maximizing Anticancer Efficacy of Phototherapy. ACS Appl. Bio Mater. 2021, 4, 4450–4461. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, S.; Liu, Z.; Sun, Y.; Cao, W.; Tong, L.; Cui, G.; Tang, B. Targeting and destroying tumor vasculature with a near-infrared laser-activated “nanobomb” for efficient tumor ablation. Biomaterials 2017, 139, 1–11. [Google Scholar] [CrossRef]
- Zou, H.; Wei, Z.; Song, C.; Ran, J.; Cao, Z.; Tang, C.; Zhang, G.; Cai, Y.; Lu, M.; Han, W. Novel NIR-II semiconducting molecule incorporating sorafenib for imaging guided synergetic cancer phototherapy and anti-angiogenic therapy. J. Mater. Chem. B 2021, 9, 3235–3248. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Liu, P.; Yang, W.; Shi, L.; Zhang, H.; Xu, Y.; Wang, P.; Zhang, G.; Chen, W.R.; Zhang, B.; Wang, X. Concurrent photothermal therapy and photodynamic therapy for cutaneous squamous cell carcinoma by gold nanoclusters under a single NIR laser irradiation. J. Mater. Chem. B 2019, 7, 6924–6933. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z. Organic Nanomaterials for Photothermal Therapy of Cancer. Chemistry 2015, 78, 292–293. [Google Scholar]
- Yang, Y.; Zhu, W.; Dong, Z.; Chao, Y.; Xu, L.; Chen, M.; Liu, Z. 1D Coordination Polymer Nanofibers for Low-Temperature Photothermal Therapy. Adv. Mater. 2017, 29, 1703588. [Google Scholar] [CrossRef]
- Fan, W.; Bu, W.; Zhang, Z.; Shen, B.; Zhang, H.; He, Q.; Ni, D.; Cui, Z.; Zhao, K.; Bu, J.; et al. X-ray Radiation-Controlled NO-Release for On-Demand Depth-Independent Hypoxic Radiosensitization. Angew. Chem. Int. Ed. 2015, 54, 14026–14030. [Google Scholar] [CrossRef] [PubMed]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef]
- Wang, X.; Niu, X.; Sha, W.; Feng, X.; Yu, L.; Zhang, Z.; Wang, W.; Yuan, Z. An oxidation responsive nano-radiosensitizer increases radiotherapy efficacy by remolding tumor vasculature. Biomater. Sci. 2021, 9, 6308–6324. [Google Scholar] [CrossRef]
- Tekin, V.; Aweda, T.; Guldu, O.K.; Muftuler, F.Z.B.; Bartels, J.; Lapi, S.E.; Unak, P. A novel anti-angiogenic radio/photo sensitizer for prostate cancer imaging and therapy: 89Zr-Pt@TiO2-SPHINX, synthesis and in vitro evaluation. Nucl. Med. Biol. 2021, 94–95, 20–31. [Google Scholar] [CrossRef]
- Lip, H.; Amini, M.A.; Zetrini, A.; Cai, P.; Abbasi, A.Z.; Bristow, R.G.; Rauth, A.M.; Wu, X.Y. Redox-responsive nanoparticles enhance radiation therapy by altering multifaceted radio-resistance mechanisms in human castration-resistant prostate cancer cells and xenografts. Radiother. Oncol. 2022, 170, 213–223. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, C.; Wang, X.; Shao, J.; Chen, C.; Li, H.; Ju, C.; He, J.; Gu, H.; Xia, D. Overcoming Hypoxia-Restrained Radiotherapy Using an Erythrocyte-Inspired and Glucose-Activatable Platform. Nano Lett. 2020, 20, 4211–4219. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Griffith, J.; Panneerselvam, J.; Babu, A.; Mani, J.; Herman, T.; Ramesh, R.; Munshi, A. Regorafenib sensitizes human breast cancer cells to radiation by inhibiting multiple kinases and inducing DNA damage. Int. J. Radiat. Biol. 2021, 97, 1109–1120. [Google Scholar] [CrossRef]
- Yoon, S.M.; Ryoo, B.-Y.; Lee, S.J.; Kim, J.H.; Shin, J.H.; An, J.; Lee, H.C.; Lim, Y.-S. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A randomized clinical trial. JAMA Oncol. 2018, 4, 661–669. [Google Scholar] [CrossRef]
- Zheng, L.; Li, C.; Huang, X.; Lin, X.; Lin, W.; Yang, F.; Chen, T. Thermosensitive hydrogels for sustained-release of sorafenib and selenium nanoparticles for localized synergistic chemoradiotherapy. Biomaterials 2019, 216, 119220. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, Q.; Tang, J.; Jiang, Y.; Yang, L.; Shi, X.; Chen, Y.; Zhang, Y.; Fu, S.; Lin, S. Anti-tumor effect of local injectable hydrogel-loaded endostatin alone and in combination with radiotherapy for lung cancer. Drug Deliv. 2021, 28, 183–194. [Google Scholar] [CrossRef]
- Zhang, W.; Duan, R.; Zhang, J.; Cheung, W.K.C.; Gao, X.; Zhang, R.; Zhang, Q.; Wei, M.; Wang, G.; Mei, P.-J.; et al. H1/pHGFK1 nanoparticles exert anti-tumoural and radiosensitising effects by inhibition of MET in glioblastoma. Br. J. Cancer 2018, 118, 522–533. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Yi, X.; Dong, Z.; Xu, J.; Liang, C.; Chao, Y.; Wang, Y.; Yang, K.; Liu, Z. Calcium Bisphosphonate Nanoparticles with Chelator-Free Radiolabeling to Deplete Tumor-Associated Macrophages for Enhanced Cancer Radioisotope Therapy. ACS Nano 2018, 12, 11541–11551. [Google Scholar] [CrossRef]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G.; et al. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 11134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penninckx, S.; Heuskin, A.-C.; Michiels, C.; Lucas, S. Gold Nanoparticles as a Potent Radiosensitizer: A Transdisciplinary Approach from Physics to Patient. Cancers 2020, 12, 2021. [Google Scholar] [CrossRef] [PubMed]
- Igaz, N.; Szőke, K.; Kovács, D.; Buhala, A.; Varga, Z.; Bélteky, P.; Rázga, Z.; Tiszlavicz, L.; Vizler, C.; Hideghéty, K.; et al. Synergistic Radiosensitization by Gold Nanoparticles and the Histone Deacetylase Inhibitor SAHA in 2D and 3D Cancer Cell Cultures. Nanomaterials 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, J.; Castle, K.D.; Qi, Y.; Kirsch, D.G.; West, J.L.; Badea, C.T. Dual-Energy CT Imaging of Tumor Liposome Delivery After Gold Nanoparticle-Augmented Radiation Therapy. Theranostics 2018, 8, 1782–1797. [Google Scholar] [CrossRef]
- Yang, C.; Gao, Y.; Fan, Y.; Cao, L.; Li, J.; Ge, Y.; Tu, W.; Liu, Y.; Cao, X.; Shi, X. Dual-mode endogenous and exogenous sensitization of tumor radiotherapy through antifouling dendrimer-entrapped gold nanoparticles. Theranostics 2021, 11, 1721–1731. [Google Scholar] [CrossRef]
- Wu, W.; Klockow, J.L.; Mohanty, S.; Ku, K.S.; Aghighi, M.; Melemenidis, S.; Chen, Z.; Li, K.; Morais, G.R.; Zhao, N.; et al. Theranostic nanoparticles enhance the response of glioblastomas to radiation. Nanotheranostics 2019, 3, 299–310. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Xiao, Z.; Liu, Q.; Li, P.; Xu, F.; Liu, J.; Tao, H.; Feng, L.; Song, S.; Liu, Z.; et al. CaCO3 -Encapuslated Microspheres for Enhanced Transhepatic Arterial Embolization Treatment of Hepatocellular Carcinoma. Adv. Healthc. Mater. 2021, 10, e2100748. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liang, X.; Xu, X.; Zhang, X.; Wen, J.; Chen, K.; Su, X.; Teng, Z.; Lu, G.; Xu, J. Magnetic mesoporous embolic microspheres in transcatheter arterial chemoembolization for liver cancer. Acta Biomater. 2021, 130, 374–384. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, H.; Zhang, H.; Li, L.; Li, S.; Zhao, Y.; Zheng, C.; Nie, G.; Yang, X. The synergistic blood-vessel-embolization of coagulation fusion protein with temperature sensitive nanogels in interventional therapies on hepatocellular carcinoma. Chem. Eng. J. 2022, 433, 134357. [Google Scholar] [CrossRef]
- Ouyang, T.; Cao, Y.; Chen, L.; Zheng, C. Comparison of the Efficacy Among Transcatheter Arterial Chemoembolization (TACE)–Radiofrequency Ablation Plus Apatinib, TACE Plus Apatinib, and TACE Alone for Hepatocellular Carcinoma: A Retrospective Study. Cardiovasc. Interv. Radiol. 2022, 45, 780–790. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Q.; Liu, J.; Huang, S.; Yang, C.; Xiong, B. Effect of Inhibiting Tumor Angiogenesis After Embolization in the Treatment of HCC with Apatinib-Loaded p(N-Isopropyl-Acrylamide-co-Butyl Methyl Acrylate) Temperature-Sensitive Nanogel. J. Hepatocell. Carcinoma 2020, 7, 447–456. [Google Scholar] [CrossRef]
- Zhou, C.; Yao, Q.; Zhang, H.; Guo, X.; Liu, J.; Shi, Q.; Huang, S.; Xiong, B. Combining transcatheter arterial embolization with iodized oil containing Apatinib inhibits HCC growth and metastasis. Sci. Rep. 2020, 10, 2964. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Sun, J.; Yang, X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: Current status. Cancer Lett. 2016, 370, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Kong, J.; Pan, B.; Ke, S.; Dong, S.; Li, X.; Zhou, A.; Zheng, L.; Sun, W.B. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1α/VEGFA. PLoS ONE 2013, 7, e37266. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, H.; Zhao, H.; Shi, D.; Zheng, C.; Zhao, Y.; Yang, X. Radiofrequency-thermal effect of cisplatin-crosslinked nanogels for triple therapies of ablation-chemo-embolization. Chem. Eng. J. 2022, 450, 138421. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, H.; Zhou, L.; Chen, X.; Zheng, S. The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma. Anal. Cell. Pathol. 2019, 2019, 8619096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Guo, X.; Peng, X.; Zhang, H.; Liu, Y.; Li, H.; He, X.; Shi, D.; Xiong, B.; Zhao, Y.; et al. Radiofrequency-responsive dual-valent gold nanoclusters for enhancing synergistic therapy of tumor ablation and artery embolization. Nano Today 2020, 35, 100934. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Tang, J.; Zhou, M.; Liu, F. Local application of doxorubicin- loaded Iron oxid nanoparticles and the vascular disrupting agent via the hepatic artery: Chemoembolization–photothermal ablation treatment of hepatocellular carcinoma in rats. Cancer Imaging 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Y.; Li, Q.; Luo, Z.; Zhang, Z.; Huang, H.; Sun, J.; Zhang, L.; Sun, R.; Bain, D.J.; et al. Targeting Xkr8 via nanoparticle-mediated in situ co-delivery of siRNA and chemotherapy drugs for cancer immunochemotherapy. Nat. Nanotechnol. 2022, 18, 193–204. [Google Scholar] [CrossRef]
- He, T.; Hu, M.; Zhu, S.; Shen, M.; Kou, X.; Liang, X.; Li, L.; Li, X.; Zhang, M.; Wu, Q.; et al. A tactical nanomissile mobilizing antitumor immunity enables neoadjuvant chemo-immunotherapy to minimize postsurgical tumor metastasis and recurrence. Acta Pharm. Sin. B 2023, 13, 804–818. [Google Scholar] [CrossRef]
- Zhou, S.; Shang, Q.; Wang, N.; Li, Q.; Song, A.; Luan, Y. Rational design of a minimalist nanoplatform to maximize immunotherapeutic efficacy: Four birds with one stone. J. Control. Release 2020, 328, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Brawer, M.K. Hormonal therapy for prostate cancer. Rev. Urol. 2006, 8 (Suppl. 2), S35. [Google Scholar]
- Ma, C.-C.; Wang, Z.-L.; Xu, T.; He, Z.-Y.; Wei, Y.-Q. The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef]
- Yang, W.; Deng, C.; Shi, X.; Xu, Y.; Dai, C.; Wang, H.; Bian, K.; Cui, T.; Zhang, B. Structural and Molecular Fusion MRI Nanoprobe for Differential Diagnosis of Malignant Tumors and Follow-Up Chemodynamic Therapy. ACS Nano 2023, 17, 4009–4022. [Google Scholar] [CrossRef]
- Galle, P.R.; Finn, R.S.; Qin, S.; Ikeda, M.; Zhu, A.X.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.; et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 991–1001. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Krasner, C.N.; Campos, S.M.; Young, C.L.; Chadda, K.R.; Lee, H.; Birrer, M.J.; Horowitz, N.S.; Konstantinopoulos, P.A.; D’Ascanio, A.M.; Matulonis, U.A.; et al. Sequential Phase II clinical trials evaluating CRLX101 as monotherapy and in combination with bevacizumab in recurrent ovarian cancer. Gynecol. Oncol. 2021, 162, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Keefe, S.; Hoffman-Censits, J.; Cohen, R.; Mamtani, R.; Heitjan, D.; Eliasof, S.; Nixon, A.; Turnbull, B.; Garmey, E.; Gunnarsson, O.; et al. Efficacy of the nanoparticle–drug conjugate CRLX101 in combination with bevacizumab in metastatic renal cell carcinoma: Results of an investigator-initiated phase I–IIa clinical trial. Ann. Oncol. 2016, 27, 1579–1585. [Google Scholar] [CrossRef]
- Pfisterer, J.; Shannon, C.M.; Baumann, K.; Rau, J.; Harter, P.; Joly, F.; Sehouli, J.; Canzler, U.; Schmalfeldt, B.; Dean, A.P.; et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 699–709. [Google Scholar] [CrossRef] [PubMed]




| Antivascular Therapy | Immunotherapy | Disease | Outcomes | Ref. |
|---|---|---|---|---|
| Axitinib (VEGFR-TKI) | IMT (IDO inhibitor) | Melanoma | Increased proportion of tumor- infiltrating T lymphocytes (CTLs and Th cells); inhibition of Tregs and TAMs | [37] |
| Bevacizumab (targets VEGF-A/VEGFR-2) | aPD-1 mAb | Colon adenocarcinoma | Increased infiltration by CD8+ T cells; upregulated IFN-γ expression; increased amount of aPD-1 mAb delivered to the tumor | [38] |
| CA4P (ECs) | aPD-1 mAb | Breast cancer | Increases the efficacy of aPD-1 mAb; increase infiltration by CD4+ and CD8+ T cells | [39] |
| Tetrathiomolybdate (Inhibits NF-KB signaling.) | Breast cancer | Enhances immune activation | [40] | |
| Endostar (recombinant human endostatin) | NSCLC | Increased IFN-γ and IL-17 expression; decreased TGF-β1 expression | [41] | |
| FSEC (anti-angiogenic peptide) | DPPA (immune checkpoint block peptides) | Breast cancer | Increased infiltration by CD8+ T cells | [42] |
| Gold nanoparticles (inhibit endothelial Smad2/3 signaling) | Gastric carcinoma and breast adenocarcinoma | Increased infiltration by CD3+ and CD8+ T cells | [43] | |
| pshVEGF-A(VEGF-A) | PshPD-L1 | Melanoma | Remission of ICB-induced adaptive resistance | [44] |
| Sorafenib (multi-target kinase inhibitors) | PD-L1 antibody | HCC | Increases the efficacy of anti-PD-L1 antibodies | [45] |
| Antivascular Therapy | Chemotherapy | Disease | Outcomes | Ref. |
|---|---|---|---|---|
| CA-4 (targets ECs) | Dox | Melanoma/breast cancer | Assists chemotherapeutic drugs in eradicating the tumor cells | [68] |
| CA-4 (targets ECs) | MMP9-DOX | Breast cancer | Induces hypoxia to amplify MMP9 signaling in tumors | [69] |
| CA-4 (targets ECs) | CDDP | Breast cancer | Increases the retention time to improve the accumulation of drugs within the tumor | [70] |
| cRGD-folate-heparin nanoparticles (targets endothelium-dependent vessels/antivascular mimicry) | CDDP | Ovarian cancer | Promotes CDDP to effectively inhibit the development and metastasis of cancer | [71] |
| Curcumin (VEGF) combretastatin A-4 phosphate (VEGFR2) | HCC | Inhibits tumor metastasis | [72] | |
| DA (targets ANG1/VEGF/KL2) | DOX | Breast cancer | Increases blood flow perfusion and reduces IFP | [28] |
| DMXAA (targets ECs) | DOX | Cervical cancer | Enhances tumor suppression | [26] |
| Erlotinib (EGFR TKI) | Topotecan | Breast cancer | Prolongs TVN and increases drug delivery efficiency | [73] |
| Fruquintinib (targets VEGFR-1, -2, and -3) | DOX | Breast cancer | Achieves deep delivery of drugs into tumor tissue | [29] |
| LMWH (targets bFGF/VEGF) | GA | Breast cancer | Increases blood flow perfusion and reduces IFP | [74] |
| LMWH (targets bFGF/VEGF) | Paclitaxel/Gemcitabine | HCC | Induces simultaneous drug delivery and normalization of tumor vessels | [75] |
| Silybin (targets the NF-κB signaling pathway) | Paclitaxel | A549 lung cancer | Chemosensitization | [76] |
| Thrombin (tumor vessel) | DOX | HCC | Blocks the blood supply to tumors and inhibits cancer cell proliferation | [77] |
| Antivascular Therapy | Phototherapy | Disease | Outcomes | Ref. |
|---|---|---|---|---|
| Bevacizumab (targets VEGF-A/VEGFR-2) | PDT | Colorectal cancer | Inhibits tumor growth and recurrence | [106] |
| Candesartan (Ang II receptor blocker) | PDT | HCC | Reduces the secretion of VEGF and restores a normal oxygen microenvironment | [107] |
| CA4 (targets ECs) | PDT | Breast cancer | Disrupts the vasculature | [108] |
| CA4 (targets ECs) | PTT | Breast cancer | Restricts the nutrient supply of tumor cells to achieve the “attack + guard” strategy | [109] |
| Cetuximab (targets EGFR) | PTT | Breast cancer | Decreases the requirement for laser energy and reduces damage to normal tissue | [110] |
| Celecoxib (targets cyclooxygenase-2) | PTT | Colorectal cancer | Reduces the risk of tumor metastasis after PTT treatment | [111] |
| cRGD-CSOSA (targets neovascular ECs) | PDT | Glioblastoma | Promotes the production of ROS | [112] |
| DMXAA (targets ECs) | PDT | Breast cancer | Overcomes the challenges related to hypoxia of traditional type II PDT and inhibits tumor metastasis | [113] |
| DMXAA (targets ECs) | PTT/PDT | Cervical cancer | Achieves complete tumor ablation | [114] |
| DMXAA (targets ECs) | PTT | Colon cancer | DMXAA promotes aggregation of gold nanoparticles with NIR absorption to increase absorption and enhance the photothermal ablation of PTT | [115] |
| HBA (targets VEGF) | PTT/PDT | Colorectal cancer | Reduces the secretion of VEGF | [116] |
| Infrared laser irradiation (ECs) | PTT | Cervical cancer | Induces avascular necrosis of tumors | [117] |
| Sorafenib (VEGFR/PDGFR TKI) | PTT/PDT | OSCC | Increases photothermal conversion efficiency and ROS production | [118] |
| TNP-470 (VEGF) | PDT | Prostate carcinoma | Effectively reduces tumor growth and metastasis | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Fang, W.; Wang, D.; Shao, N.; Chen, J.; Nie, T.; Huang, C.; Huang, Y.; Luo, L.; Xiao, Z. Nanomaterial-Based Antivascular Therapy in the Multimodal Treatment of Cancer. Pharmaceutics 2023, 15, 1207. https://doi.org/10.3390/pharmaceutics15041207
Ma X, Fang W, Wang D, Shao N, Chen J, Nie T, Huang C, Huang Y, Luo L, Xiao Z. Nanomaterial-Based Antivascular Therapy in the Multimodal Treatment of Cancer. Pharmaceutics. 2023; 15(4):1207. https://doi.org/10.3390/pharmaceutics15041207
Chicago/Turabian StyleMa, Xiaocong, Weimin Fang, Duo Wang, Ni Shao, Jifeng Chen, Tianqi Nie, Cuiqing Huang, Yanyu Huang, Liangping Luo, and Zeyu Xiao. 2023. "Nanomaterial-Based Antivascular Therapy in the Multimodal Treatment of Cancer" Pharmaceutics 15, no. 4: 1207. https://doi.org/10.3390/pharmaceutics15041207