Molecular Interactions between APIs and Enteric Polymeric Excipients in Solid Dispersion: Insights from Molecular Simulations and Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Simulation Models and Methods
2.2. Experimental Description
2.2.1. Powder X-ray Diffraction Analysis
2.2.2. FTIR Spectroscopy
2.2.3. Dissolution Study
- (1)
- In Simulated Gastric fluid (SGF) pH 1.2 for 2 h: The samples were placed in a basket and dilution was tested for 2 h in SGF pH 1.2 with sample collections at 15, 30, 60, 90 and 120 min. A 2 mL sample was withdrawn after each time interval and fresh media were replenished. The samples were filtered through a 0.45 µm syringe filter and analyzed by HPLC.
- (2)
- In Simulated Intestinal fluid (SIF) pH 6.5 for 2 h: After 2 h in SGF, the dissolution medium was changed to SIF and the basket was lowered to run the same sample again. Samples were collected at 5, 10, 15, 30, 60, 90 and 120 min and 2 mL of fresh SIF was replenished after each collection. The samples were filtered through a 0.45 µm syringe filter and analyzed by HPLC.
3. Results and Discussion
3.1. Force Field Validation of Drug API
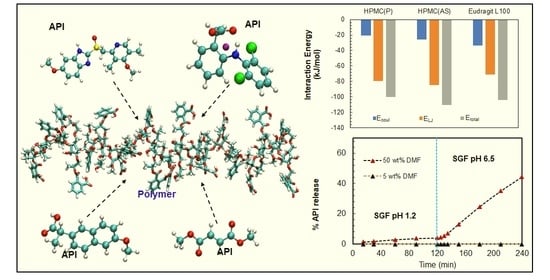
3.2. Evaluation of Drug-Polymer Pairs for Solid Dispersion Formulations
3.3. Evaluation of Drug-Polymer Pairs by Experiment
3.3.1. Solid Dispersion by Hot Melt Extruder Experiments
3.3.2. PXRD and FTIR
3.3.3. Dissolution Experiments
3.4. Effect of Drug Loading
3.5. Effect of Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di, L.; Kerns, E.; Carter, G. Drug-like property concepts in pharmaceutical design. Curr. Pharm. Des. 2009, 15, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Qian, F.; Huang, J.; Hussain, M. Drug-polymer solubility and miscibility: Stability consideration and practical challenges in amorphous solid dispersion development. J. Pharm. Sci. 2010, 99, 2941–2947. [Google Scholar] [CrossRef]
- Murdande, S.B.; Pikal, M.J.; Shanker, R.M.; Bogner, R.H. Solubility advantage of amorphous pharmaceuticals: I. A thermodynamic analysis. J. Pharm. Sci. 2010, 99, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Maddineni, S.; Lu, J.; Repka, M.A. Melt extrusion with poorly soluble drugs. Int. J. Pharm. 2013, 453, 233–252. [Google Scholar] [CrossRef]
- Dinunzio, J.; Zhang, F.; Martin, C.; Mcginity, J. Formulating Poorly Water Soluble Drugs; Springer: New York, NY, USA, 2012; pp. 311–362. [Google Scholar]
- Stanković, M.; Frijlink, H.; Hinrichs, W. Polymeric formulations for drug release prepared by hot melt extrusion: Application and characterization. Drug Discov. Today 2015, 20, 812–823. [Google Scholar] [CrossRef]
- Breitenbach, J. Melt extrusion: From process to drug delivery technology. Eur. J. Pharm. Biopharm. 2002, 54, 107–117. [Google Scholar] [CrossRef]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef]
- Al-Japairai, K.A.S.; Alkhalidi, H.M.; Mahmood, S.; Almurisi, S.H.; Doolaanea, A.A.; Al-Sindi, T.A.; Chatterjee, B. Lyophilized Amorphous Dispersion of Telmisartan in a Combined Carrier–Alkalizer System: Formulation Development and In Vivo Study. ACS Omega 2020, 5, 32466–32480. [Google Scholar] [CrossRef]
- Repka, M.A.; Battu, S.K.; Upadhye, S.B.; Thumma, S.; Crowley, M.M.; Zhang, F.; Martin, C.; McGinity, J.W. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev. Ind. Pharm. 2007, 33, 1043–1057. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Pang, H.; Guo, Z.; Lin, L.; Dong, Y.; Li, G.; Lu, M.; Wu, C. Interactions between drugs and polymers influencing hot melt extrusion. J. Pharm. Pharmacol. 2013, 66, 148–166. [Google Scholar] [CrossRef]
- Ahlneck, C.; Zografi, G. The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state. Int. J. Pharm. 1990, 62, 87–95. [Google Scholar] [CrossRef]
- Huang, C.; Powell, C.T.; Sun, Y.; Cai, T.; Yu, L. Effect of Low-Concentration Polymers on Crystal Growth in Molecular Glasses: A Controlling Role for Polymer Segmental Mobility Relative to Host Dynamics. J. Phys. Chem. B 2017, 121, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Vo, C.L.-N.; Park, C.; Lee, B.-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Konno, H.; Handa, T.; Alonzo, D.E.; Taylor, L.S. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur. J. Pharm. Biopharm. 2008, 70, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, W.; Nightingale, J.; Herbig, S. Utility of Hydroxypropylmethylcellulose Acetate Succinate (HPMCAS) for Initiation and Maintenance of Drug Supersaturation in the GI Milieu. Pharm. Res. 2009, 26, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Larson, R.G. Assessing the Efficiency of Polymeric Excipients by Atomistic Molecular Dynamics Simulations. Mol. Pharm. 2014, 11, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.; Chauhan, H.; Atef, E. Role of Molecular Interactions for Synergistic Precipitation Inhibition of Poorly Soluble Drug in Supersaturated Drug–Polymer–Polymer Ternary Solution. Mol. Pharm. 2016, 13, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Punčochová, K.; Heng, J.Y.; Beránek, J.; Stěpánek, F. Investigation of drug-polymer interaction in solid dispersions by vapour sorption methods. Int. J. Pharm. 2014, 469, 159–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Inbar, P.; Chokshi, H.P.; Malick, A.W.; Choi, D.S. Prediction of the thermal phase diagram of amorphous solid dispersions by Flory–Huggins theory. J. Pharm. Sci. 2011, 100, 3196–3207. [Google Scholar] [CrossRef]
- Marsac, P.J.; Shamblin, S.; Taylor, L. Theoretical and practical approaches for prediction of drug–polymer miscibility and solubility. Pharm. Res. 2006, 23, 2417. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Pang, J.; Morgan, D.J.; Douroumis, D. Molecular Modeling as a Predictive Tool for the Development of Solid Dispersions. Mol. Pharm. 2015, 12, 1040–1049. [Google Scholar] [CrossRef] [Green Version]
- Yani, Y.; Kanaujia, P.; Chow, P.S.; Tan, R.B.H. Effect of API-Polymer Miscibility and Interaction on the Stabilization of Amorphous Solid Dispersion: A Molecular Simulation Study. Ind. Eng. Chem. Res. 2017, 56, 12698–12707. [Google Scholar] [CrossRef]
- Meng, F.; Trivino, A.; Prasad, D.; Chauhan, H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. [Google Scholar] [CrossRef]
- Meng, F.; Dave, V.; Chauhan, H. Qualitative and quantitative methods to determine miscibility in amorphous drug–polymer systems. Eur. J. Pharm. Sci. 2015, 77, 106–111. [Google Scholar] [CrossRef]
- Lu, X.; Li, M.; Huang, C.; Lowinger, M.B.; Xu, W.; Yu, L.; Byrn, S.R.; Templeton, A.C.; Su, Y. Atomic-Level Drug Substance and Polymer Interaction in Posaconazole Amorphous Solid Dispersion from Solid-State NMR. Mol. Pharm. 2020, 17, 2585–2598. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Nunes, C.; Vyas, S.; Jonnalagadda, S. Prediction of Solubility Parameters and Miscibility of Pharmaceutical Compounds by Molecular Dynamics Simulations. J. Phys. Chem. B 2011, 115, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.; Wagner, K.G.; Kornherr, A.; Zifferer, G. Molecular dynamics simulations for drug dosage form development: Thermal and solubility characteristics for hot-melt extrusion. Mol. Simul. 2008, 34, 1197–1207. [Google Scholar] [CrossRef]
- Walden, D.M.; Bundey, Y.; Jagarapu, A.; Antontsev, V.; Chakravarty, K.; Varshney, J. Molecular Simulation and Statistical Learning Methods toward Predicting Drug–Polymer Amorphous Solid Dispersion Miscibility, Stability, and Formulation Design. Molecules 2021, 26, 182. [Google Scholar] [CrossRef]
- Liu, S.; Jia, L.; Xu, S.; Chen, Y.; Tang, W.; Gong, J. Insight into the State Evolution of Norfloxacin as a Function of Drug Concentration in Norfloxacin-Vinylpyrrolidone/Hydroxypropyl Methylcellulose/Hydroxypropyl Methylcellulose Phthalate Solid Dispersions. Cryst. Growth Des. 2019, 19, 6239–6251. [Google Scholar] [CrossRef]
- Kabedev, A.; Zhuo, X.; Leng, D.; Foderà, V.; Zhao, M.; Larsson, P.; Bergström, C.A.S.; Löbmann, K. Stabilizing Mechanisms of β-Lactoglobulin in Amorphous Solid Dispersions of Indomethacin. Mol. Pharm. 2022, 19, 3922–3933. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.M.; Liu, J.; Jiang, J. A molecular simulation study for efficient separation of 2,5-furandiyldimethanamine by a microporous polyarylate membrane. Polymer 2019, 175, 8–14. [Google Scholar] [CrossRef]
- Batwa, A.; Norrman, A. Blockchain Technology and Trust in Supply Chain Management: A Literature Review and Research Agenda. Oper. Supply Chain. Manag. Int. J. 2021, 14, 203–220. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Ribeiro, A.A.; Horta, B.; Alencastro, R. MKTOP: A program for automatic construction of molecular topologies. J. Braz. Chem. Soc. 2008, 19, 1433–1435. [Google Scholar] [CrossRef]
- Available online: http://erg.biophys.msu.ru/wordpress/archives/32 (accessed on 23 February 2023).
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Kim, Y.B.; Song, H.; Park, I. Refinement of the structure of naproxen, (+)-6-methoxy-α-methyl-2-naphthaleneacetic acid. Arch. Pharmacal Res. 1987, 10, 232–238. [Google Scholar] [CrossRef]
- Llinàs, A.; Burley, J.C.; Box, K.J.; Glen, R.C.; Goodman, J.M. Diclofenac Solubility: Independent Determination of the Intrinsic Solubility of Three Crystal Forms. J. Med. Chem. 2007, 50, 979–983. [Google Scholar] [CrossRef]
- Kooijman, H.; Sprengers, J.W.; Agerbeek, M.J.; Elsevier, C.J.; Spek, A.L. Di-methyl fumarate. Acta Crystallogr. Sect. E 2004, 60, o917–o918. [Google Scholar] [CrossRef]
- Deng, J.; Chi, Y.; Fu, F.; Cui, X.; Yu, K.; Zhu, J.; Jiang, Y. Resolution of omeprazole by inclusion complexation with a chiral host BINOL. Tetrahedron Asymmetry 2000, 11, 1729–1732. [Google Scholar] [CrossRef]
- Cadden, J.; Gupta, K.M.; Kanaujia, P.; Coles, S.J.; Aitipamula, S. Cocrystal Formulations: Evaluation of the Impact of Excipients on Dissolution by Molecular Simulation and Experimental Approaches. Cryst. Growth Des. 2021, 21, 1006–1018. [Google Scholar] [CrossRef]
- Gupta, K.M.; Jiang, J. Systematic Investigation of Nitrile Based Ionic Liquids for CO2 Capture: A Combination of Molecular Simulation and ab Initio Calculation. J. Phys. Chem. C 2014, 118, 3110–3118. [Google Scholar] [CrossRef]
- Gupta, K.M. Tetracyanoborate based ionic liquids for CO2 capture: From ab initio calculations to molecular simulations. Fluid Phase Equilibria 2016, 415, 34–41. [Google Scholar] [CrossRef]
- Gupta, K.M.; Das, S.; Wong, A.B.H.; Chow, P.S. Formulation and Skin Permeation of Active-Loaded Lipid Nanoparticles: Evaluation and Screening by Synergizing Molecular Dynamics Simulations and Experiments. Langmuir 2023, 39, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.-X.; Le, Y.; Chen, J.-F. Studies of Bicalutamide–Excipients Interaction by Combination of Molecular Docking and Molecular Dynamics Simulation. Mol. Pharm. 2013, 10, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Luzar, A.; Chandler, D. Hydrogen-bond kinetic in liquid water. Nature 1996, 379, 55–57. [Google Scholar] [CrossRef]
- Sovizi, M.R. Thermal behavior of drugs. J. Therm. Anal. Calorim. 2010, 102, 285–289. [Google Scholar] [CrossRef]
- Vynckier, A.K.; De Beer, M.; Monteyne, T.; Voorspoels, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Enteric protection of naproxen in a fixed-dose combination product produced by hot-melt co-extrusion. Int. J. Pharm. 2015, 491, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Riedel, A.; Leopold, C.S. Degradation of Omeprazole Induced by Enteric Polymer Solutions and Aqueous Dispersions: HPLC Investigations. Drug Dev. Ind. Pharm. 2005, 31, 151–160. [Google Scholar] [CrossRef]
- Stroyer, A.; McGinity, J.; Leopold, C. Solid state interactions between the proton pump inhibitor omeprazole and various enteric coating polymers. J. Pharm. Sci. 2006, 95, 1342–1353. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.D.; Akocak, S.; Ilies, M.A.; Fassihi, R. Solid-State Interactions at the Core-Coat Interface: Physicochemical Characterization of Enteric-Coated Omeprazole Pellets without a Protective Sub-Coat. AAPS PharmSciTech 2015, 16, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, M.; Josefsson, L. Pharmaceutical Formulation of Omeprazole. U.S. Patent 6090827, 18 May 1998. [Google Scholar]
- Shivakumar, H.N.; Desai, B.; Deshmukh, G. Design and optimization of diclofenac sodium controlled release solid dispersions by response surface methodology. Indian J. Pharm. Sci. 2008, 70, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.W.; Lee, T. Round Granules of Dimethyl Fumarate by Three-in-One Intensified Process of Reaction, Crystallization, and Spherical Agglomeration in a Common Stirred Tank. Org. Process Res. Dev. 2017, 21, 1326–1339. [Google Scholar] [CrossRef]
- Aielo, P.B.; Borges, F.A.; Romeira, K.M.; Miranda, M.C.R.; Arruda, L.B.d.; Filho, P.N.L.; Drago, B.d.C.; Herculano, R.D. Evaluation of sodium diclofenac release using natural rubber latex as carrier. Mater. Res. 2014, 17, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Garg, V.; Sonali; Singh, R.P.; Dutt, R. Chitosan-alginate core-shell-corona shaped nanoparticles of dimethyl fumarate in orodispersible film to improve bioavailability in treatment of multiple sclerosis: Preparation, characterization and biodistribution in rats. J. Drug Deliv. Sci. Technol. 2021, 64, 102645. [Google Scholar] [CrossRef]
- Adeyeye, C.M.; Li, P.-K. Diclofenac Sodium. Anal. Profiles Drug Subst. 1990, 19, 123–144. [Google Scholar]
- Gupta, K.M.; Das, S.; Chow, P.S.; Macbeath, C. Encapsulation of Ferulic Acid in Lipid Nanoparticles as Antioxidant for Skin: Mechanistic Understanding through Experiment and Molecular Simulation. ACS Appl. Nano Mater. 2020, 3, 5351–5361. [Google Scholar] [CrossRef]
- Gupta, K.M.; Yani, Y.; Poornachary, S.K.; Chow, P.S. Atomistic Simulation to Understand Anisotropic Growth Behavior of Naproxen Crystal in the Presence of Polymeric Additives. Cryst. Growth Des. 2019, 19, 3768–3776. [Google Scholar] [CrossRef]
- Metzler, R.; Klafter, J. The random walk’s guide to anomalous diffusion: A fractional dynamics approach. Phys. Rep. 2000, 339, 1–77. [Google Scholar] [CrossRef]














| Barrel Zone | 1 | 2 | 3 | 4 | 5 | 6 | Rod Die |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | |||||||
| NaDLO-HPMC(P) | Feeding | 110 | 140 | 140 | 145 | 145 | 145 |
| DMF-HPMC(AS) | Feeding | 75 | 100 | 110 | 120 | 120 | 115 |
| NPX-Eudragit L100 | Feeding | 130 | 200 | 215 | 220 | 220 | 215 |
| OPZ-HPMC(AS) | Feeding | 75 | 100 | 110 | 120 | 120 | 115 |
| API | Temp (K) | Density (g/cc) | Lattice Parameters | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | ||||
| Naproxen | 300 (283–303) | 1.27 (1.25) | 13.30 (13.38) | 5.76 (5.79) | 7.87 (7.91) | 90.0 (90.0) | 93.9 (93.9) | 90.0 (90.0) | [41] |
| Diclofenac sodium | 150 (150) | 1.48 (1.44) | 9.48 (9.55) | 39.19 (39.49) | 9.77 (9.84) | 90.0 (90.0) | 90.7 (90.7) | 90.0 (90.0) | [42] |
| Dimethyl fumarate | 150 (150) | 1.43 (1.43) | 3.87 (3.87) | 5.64 (5.64) | 8.36 (8.36) | 100.8 (100.8) | 100.3 (100.3) | 105.7 (105.7) | [43] |
| Omeprazole | 300 (283–303) | 1.31 (1.33) | 9.81 (9.70) | 10.49 (10.29) | 10.45 (10.62) | 90.0 (90.4) | 111.5 (112.1) | 116.5 (115.9) | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, K.M.; Chin, X.; Kanaujia, P. Molecular Interactions between APIs and Enteric Polymeric Excipients in Solid Dispersion: Insights from Molecular Simulations and Experiments. Pharmaceutics 2023, 15, 1164. https://doi.org/10.3390/pharmaceutics15041164
Gupta KM, Chin X, Kanaujia P. Molecular Interactions between APIs and Enteric Polymeric Excipients in Solid Dispersion: Insights from Molecular Simulations and Experiments. Pharmaceutics. 2023; 15(4):1164. https://doi.org/10.3390/pharmaceutics15041164
Chicago/Turabian StyleGupta, Krishna M., Xavier Chin, and Parijat Kanaujia. 2023. "Molecular Interactions between APIs and Enteric Polymeric Excipients in Solid Dispersion: Insights from Molecular Simulations and Experiments" Pharmaceutics 15, no. 4: 1164. https://doi.org/10.3390/pharmaceutics15041164