An Overview of the Stability and Delivery Challenges of Commercial Nucleic Acid Therapeutics
Abstract
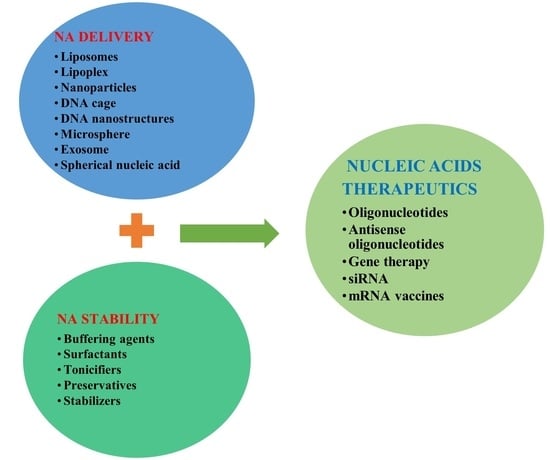
:1. Introduction
2. Lasting Challenges and Considerations of NA Therapeutics
2.1. NA Therapeutics Stability

2.2. NA Therapeutics Delivery
3. Commercially Approved NA Therapeutics
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bjeloevi, M.; Pobirk, A.; Planinšek, O.; Grabnar, P. Excipients in freeze-dried biopharmaceuticals: Contributions toward formulation stability and lyophilization cycle optimization. Int. J. Pharm. 2020, 576, 119029. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A.; Kim, J.J.; Patel, D.S.; Rains, K.; Estoll, C.R. A comprehensive scientific Survey of excipients used in currently marketed, therapeutic biological drug products. Pharm. Res. 2020, 37, 200. [Google Scholar] [CrossRef]
- Gervasi, V.; Agnol, R.D.; Cullen, S.; McCoy, T.; Vucen, S.; Crean, A. Parenteral protein formulations: An overview of approved products within the European Union. Eur. J. Pharm. Biopharm. 2018, 131, 8–24. [Google Scholar] [CrossRef]
- Cui, Y.; Cui, P.; Chen, B.; Li, S.; Guan, H. Monoclonal antibodies: Formulations of marketed products and recent advances in novel delivery system. Drug Dev. Ind. Pharm. 2017, 43, 519–530. [Google Scholar] [CrossRef]
- Wan, W.B.; Migawa, M.T.; Vasquez, G.; Murray, H.M.; Nichols, J.G.; Gaus, H.; Berdeja, A.; Lee, S.; Hart, C.E.; Lima, W.F.; et al. Synthesis, biophysical properties and biological activity of second-generation antisense oligonucleotides containing chiral phosphorothioate linkages. Nucleic Acids Res. 2014, 42, 13456–13468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Hope, M.J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [Green Version]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [Green Version]
- Walsh, G.; Walsh, E. Biopharmaceuticals benchmarks 2022. Nat. Biotechnol. 2022, 40, 1722–1760. [Google Scholar] [CrossRef]
- Cring, M.R.; Sheffield, V.C. Gene therapy and gene correction: Targets, progress, and challenges for treating human diseases. Gene Ther. 2020, 29, 3–12. [Google Scholar] [CrossRef]
- Ke, W.; Afonin, K.A. Exosomes as natural delivery carriers for programmable therapeutic nucleic acid nanoparticles (NANPs). Adv. Drug Deliv. Rev. 2021, 176, 113835. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V. Nusinersen for spinal muscular atrophy: Are we paying too much for too little? JAMA Pediatr. 2018, 172, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Mitchell, M.J.; Nie, G. Nanomaterials for therapeutic RNA delivery. Matter 2020, 3, 1948–1975. [Google Scholar] [CrossRef]
- Weng, Y.; Li, C.; Yang, T.; Hu, B.; Zhang, M.; Guo, S.; Xiao, H.; Liang, X.-J.; Huang, Y. The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 2020, 40, 107534. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Crommelin, D.J.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the cold reality of mRNA vaccine stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Morris, G.; O’Brien, D.; Henshall, D.C. Opportunities and challenges for microRNA-targeting therapeutics for epilepsy. Trends Pharmacol. Sci. 2021, 42, 605–616. [Google Scholar] [CrossRef]
- Crooke, S.T.; Liang, X.-H.; Baker, B.F.; Crooke, R.M. Antisense technology: A review. J. Biol. Chem. 2021, 296, 100416. [Google Scholar] [CrossRef]
- Nielsen, P.; Egholm, M.; Berg, R.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500. [Google Scholar] [CrossRef]
- Egholm, M.; Buchardt, O.; Christensen, L.; Behrens, C.; Freier, S.M.; Driver, D.A.; Berg, R.H.; Kim, S.K.; Norden, B.; Nielsen, P.E. PNA hybridizes to complementary oligonucleotides obeying the Watson–Crick hydrogen-bonding rules. Nature 1993, 365, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Zureigat, H.; Al Karaki, A.; Al-Kadash, A.; Gharaibeh, L.; Ma’mon, M.H.; Aljabali, A.A.; Awidi, A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef]
- Parashar, D.; Rajendran, V.; Shukla, R.; Sistla, R. Lipid-based nanocarriers for delivery of small interfering RNA for T therapeutic use. Eur. J. Pharm. Sci. 2020, 142, 105159. [Google Scholar] [CrossRef]
- Bruno, K. Using drug-excipient interactions for siRNA delivery. Adv. Drug Deliv. Rev. 2011, 63, 1210–1226. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, F.; Everton, E.; Smith, A.R.; Liu, H.; Osota, E.; Beattie, M.; Tam, Y.; Pardi, N.; Weissman, D.; Gouon-Evans, V. Murine liver repair via transient activation of regenerative pathways in hepatocytes using lipid nanoparticle-complexed nucleoside-modified mRNA. Nat. Commun. 2021, 12, 613. [Google Scholar] [CrossRef]
- Yang, R.; Deng, Y.; Huang, B.; Huang, L.; Lin, A.; Li, Y.; Wang, W.; Liu, J.; Lu, S.; Zhan, Z.; et al. A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity. Signal Transduct. Target. Ther. 2021, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Gökirmak, T.; Nikan, M.; Wiechmann, S.; Prakash, T.P.; Tanowitz, M.; Seth, P.P. Overcoming the challenges of tissue delivery for oligonucleotide therapeutics. Trends Pharmacol. Sci. 2021, 42, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R. Key considerations in formulation development for gene therapy products. Drug Discov. Today 2021, 27, 292–303. [Google Scholar] [CrossRef]
- Amer, M.H.; White, L.J.; Shakesheff, K.M. The effect of injection using narrow-bore needles on mammalian cells: Administration and formulation considerations for cell therapies. J. Pharm. Pharmacol. 2015, 67, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Falconer, R.J. Advances in liquid formulations of parenteral therapeutic proteins. Biotechnol. Adv. 2019, 37, 107412. [Google Scholar] [CrossRef]
- Medley, C.D.; Muralidhara, B.K.; Chico, S.; Durban, S.; Mehelic, P.; Demarest, C. Quantitation of plasmid DNA deposited on gold particles for particle mediated epidermal delivery using ICP-MS. Anal. Bioanal. Chem. 2010, 398, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Boerner, L.J.; Zaleski, J.M. Metal complex-DNA interactions: From transcription inhibition to photoactivated cleavage. Curr. Opin. Chem. Biol. 2005, 9, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Muralidhara, B.K.; Baid, R.; Bishop, S.M.; Huang, M.; Wang, W.; Nema, S. Critical considerations for developing nucleic acid macromolecule based drug products. Drug Discov. Today 2016, 21, 430–444. [Google Scholar] [CrossRef]
- Daugherty, A.L.; Mrsny, R.J. Formulation and delivery issues for monoclonal B antibody therapeutics. Adv. Drug Del. Rev. 2006, 58, 686–706. [Google Scholar] [CrossRef] [PubMed]
- Pogocki, D.; Schoneich, C. Chemical stability of nucleic acid–derived drugs. J. Pharm. Sci. 2000, 89, 443–456. [Google Scholar] [CrossRef]
- Evans, R.K.; Xu, Z.; Bohannon, K.E.; Wang, B.; Bruner, M.W.; Volkin, D.B. Evaluation of degradation pathways for plasmid DNA in pharmaceutical formulations via accelerated stability studies. J. Pharm. Sci. 2000, 89, 76–87. [Google Scholar] [CrossRef]
- Frelon, S.; Douki, T.; Favier, A.; Cadet, J. Hydroxyl radical is not the main reactive species involved in the degradation of DNA bases by copper in the presence of hydrogen peroxide. Chem. Res. Toxicol. 2003, 16, 191–197. [Google Scholar] [CrossRef]
- Nugraheni, R.; Mulyadi, N.; Yusuf, H. Freeze-dried liposome formulation for small molecules, nucleic acid, and protein delivery. Sys. Rev. Pharm. 2020, 11, 143–151. [Google Scholar]
- Liang, W.; Chan, A.Y.; Chow, M.Y.; Lo, F.F.; Qiu, Y.; Kwok, P.C.L.; Lam, J.K. Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery. Asian J. Pharm. Sci. 2018, 13, 163–172. [Google Scholar] [CrossRef]
- Preston, K.B.; Randolph, T.W. Stability of lyophilized and spray dried vaccine formulations. Adv. Drug Deliv. Rev. 2021, 171, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Kubczak, M.; Michlewska, S.; Bryszewska, M.; Aigner, A.; Ionov, M. Nanoparticles for local delivery of siRNA in lung therapy. Adv. Drug Deliv. Rev. 2021, 179, 114038. [Google Scholar] [CrossRef] [PubMed]
- Zoulikha, M.; Xiao, Q.; Boafo, G.F.; Sallam, M.A.; Chen, Z.; He, W. Pulmonary delivery of siRNA against acute lung injury/acute respiratory distress syndrome. Acta Pharm. Sin. B 2021, 12, 600–620. [Google Scholar] [CrossRef]
- Uddin, M.; Roni, M. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Packer, M.; Gyawali, D.; Yerabolu, R.; Schariter, J. White. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat. Commun. 2021, 12, 6777. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Yadav, K.; Singh, M.R.; Rai, V.K.; Srivastava, N.; Yadav, N.P. Commercial aspects and market potential of novel delivery systems for bioactives and biological agents. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Academic Press: Cambridge, MA, USA, 2020; pp. 595–620. [Google Scholar]
- Megahed, M.; El-Sawy, H.; El-Say, K. The promising expedition of the delivery systems for monoclonal antibodies. In Advances in the Development of Novel Carriers for Bioactives and Biological Agents; Academic Press: Cambridge, MA, USA, 2020; pp. 69–103. [Google Scholar]
- Herkt, M.; Thum, T. Pharmacokinetics and proceedings in clinical application of nucleic acid therapeutics. Mol. Ther. 2021, 29, 521–539. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, W.; Cole, J.; Zhu, G. Delivery of nucleic acid therapeutics for cancer immunotherapy. Med. Drug Discov. 2020, 6, 100023. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Lin, Z.; Zhang, Z.; Mao, M.; Wu, J.; Li, Q.; Zhang, Y.; Fan, C. Pharmaceutical applications of framework nucleic acids. Acta Pharm. Sin. B 2021, 12, 76–91. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA nanotechnology-enabled drug delivery systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef] [PubMed]
- Scheideler, M.; Vidakovic, I.; Prassl, R. Lipid nanocarriers for microRNA delivery. Chem. Phys. Lipids 2020, 226, 104837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Davis, D.A.; AboulFotouh, K.; Wang, J.; Williams, D.; Bhambhani, A.; Zakrewsky, M.; Maniruzzaman, M.; Cui, Z.; Williams, R.O. Williams. Novel formulations and drug delivery systems to administer biological solids. Adv. Drug Deliv. Rev. 2021, 172, 183–210. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Siegmund, M.; Aigner, A. Nucleic acid delivery with extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 173, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Silva, M.; Jarak, I.; Alvarez-Lorenzo, C.; Concheiro, A.; Santos, A.C.; Veiga, F.; Figueiras, A. Micelleplexes as nucleic acid delivery systems for cancer-targeted therapies. J. Control. Release 2020, 323, 442–462. [Google Scholar] [CrossRef]
- Asami, Y.; Yoshioka, K.; Nishina, K.; Nagata, T.; Yokota, T. Drug delivery system of therapeutic oligonucleotides. Drug Discov. Ther. 2016, 10, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Andresen, J.L.; Manan, R.S.; Langer, R. Nucleic acid delivery for therapeutic applications. Adv. Drug Deliv. Rev. 2021, 178, 113834. [Google Scholar] [CrossRef]
- Fattal, E.; Fay, F. Nanomedicine-based delivery strategies for nucleic acid gene inhibitors in inflammatory diseases. Adv. Drug Deliv. Rev. 2021, 175, 113809. [Google Scholar] [CrossRef]
- Patel, P.; Agrawal, Y. Targeting nanocarriers containing antisense oligonucleotides to cancer cell. J. Drug Deliv. Sci. Technol. 2017, 37, 97–114. [Google Scholar] [CrossRef]
- Zafar, A.; Alruwaili, N.; Imam, S.; Alharbi, K.; Afzal, M.; Alotaibi, N.; Yasir, M.; Elmowafy, M.; Alshehri, S. Novel nanotechnology approaches for diagnosis and therapy of breast, ovarian and cervical cancer in female: A review. J. Drug Del. Sci. Tech. 2021, 61, 102198. [Google Scholar] [CrossRef]
- Subhan, M.; Torchilin, V. siRNA based drug design, quality, delivery and clinical translation. Nanomed. Nanotech. Bio. Med. 2020, 29, 102239. [Google Scholar] [CrossRef] [PubMed]
- Maurer, V.; Altin, S.; Seleci, D.A.; Zarinwall, A.; Temel, B.; Vogt, P.; Strauß, S.; Stahl, F.; Scheper, T.; Bucan, V. In-vitro application of magnetic hybrid niosomes: Targeted siRNA-delivery for enhanced breast cancer therapy. Pharmaceutics 2021, 13, 394. [Google Scholar] [CrossRef]
- Aparajay, P.; Dev, A. Functionalized niosomes as a smart delivery device in cancer and fungal infection. Eur. J. Pharm. Sci. 2022, 168, 106052. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Chandler, M.; Afonin, K.A. Nucleic acid nanoparticles (NANPs) as molecular tools to direct desirable and avoid undesirable immunological effects. Adv. Drug Deliv. Rev. 2021, 173, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Salaita, K. Smart nucleic acids as future therapeutics. Trends Biotechnol. 2021, 39, 1289–1307. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal–organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Magar, K.T.; Boafo, G.F.; Li, X.; Chen, Z.; He, W. Liposome-based delivery of biological drugs. Chin. Chem. Lett. 2021, 33, 587–596. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, W.; Lin, L.; Chen, A.; Feng, J.; Qu, X.; Zhang, H.; Yue, J. Delivery of therapeutic oligonucleotides in nanoscale. Bioact. Mater. 2022, 7, 292–323. [Google Scholar] [CrossRef]
- Anwar, M.; Muhammad, F.; Akhtar, B. Biodegradable nanoparticles as drug delivery devices. Drug Deliv. Sci. Technol. 2021, 64, 1026388. [Google Scholar] [CrossRef]
- Subhan, M.; Torchilin, V. Efficient nanocarriers of siRNA therapeutics for cancer treatment. Transl. Res. 2019, 214, 62–91. [Google Scholar] [CrossRef] [PubMed]
- Aminu, N.; Bello, I.; Umar, N.M.; Tanko, N.; Aminu, A.; Audu, M.M. The influence of nanoparticulate drug delivery systems in drug therapy. J. Drug Deliv. Sci. Technol. 2020, 60, 101961. [Google Scholar] [CrossRef]
- Jin, J.-O.; Kim, G.; Hwang, J.; Han, K.H.; Kwak, M.; Lee, P.C. Nucleic acid nanotechnology for cancer treatment. BBA-Rev. Cancer 2020, 1874, 188377. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Gorain, B.; Padhi, S.; Choudhury, H.; Gabr, G.A.; Shadab; Mishra, D.K.; Kesharwani, P. Recent update of toxicity aspects of nanoparticulate systems for drug delivery. Eur. J. Pharm. Biopharm. 2021, 161, 100–119. [Google Scholar] [CrossRef]
- Barani, M.; Bilal, M.; Sabir, F.; Rahdar, A.; Kyzas, G.Z. Nanotechnology in ovarian cancer: Diagnosis and treatment. Life Sci. 2021, 266, 118914. [Google Scholar] [CrossRef]
- Ickenstein, L.M.; Garidel, P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin. Drug Deliv. 2019, 16, 1205–1226. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in liposomal drug delivery to cancer: An overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Springer, A.; Dowdy, S. GalNAc-siRNA conjugates: Leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Ponti, F.; Campolungo, M.; Melchiori, C.; Bono, N.; Candiani, G. Cationic lipids for gene delivery: Many players, one goal. Chem. Phys. Lipids 2021, 235, 105032. [Google Scholar] [CrossRef]
- Piotrowski-Daspit, A.S.; Kauffman, A.C.; Bracaglia, L.G.; Saltzman, W.M. Polymeric vehicles for nucleic acid delivery. Adv. Drug Deliv. Rev. 2020, 156, 119–132. [Google Scholar] [CrossRef]
- Blakney, A.K.; McKay, P.F.; Hu, K.; Samnuan, K.; Jain, N.; Brown, A.; Thomas, A.; Rogers, P.; Polra, K.; Sallah, H.; et al. Polymeric and lipid nanoparticles for delivery of self-amplifying RNA vaccines. J. Control. Release 2021, 338, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Aftab, S.; Nisar, J.; Naeem, M.; Faiza, A.; Iftikhar, J. Nanocarriers for targeted drug delivery. J. Drug Del. Sci. Tech. 2021, 62, 102426. [Google Scholar] [CrossRef]
- Junquera, E.; Aicart, E. Recent progress in gene therapy to deliver nucleic acids with multivalent cationic vectors. Adv. Colloid Interface Sci. 2016, 233, 161–175. [Google Scholar] [CrossRef]
- Hayat, S.M.G.; Farahani, N.; Safdarian, E.; Roointan, A.; Sahebkar, A. Gene delivery using lipoplexes and polyplexes: Principles, limitations and solutions. Crit. Rev. Eukaryot. Gene Expr. 2019, 29, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Jia, F.; Wang, P.; Zhang, K. Nucleic acid-based drug delivery strategies. J. Control. Release 2020, 323, 240–252. [Google Scholar] [CrossRef]
- Ndwandwe, D.; Wiysonge, C. COVID-19 vaccines. Curr. Opin. Immunol. 2021, 71, 111–116. [Google Scholar] [CrossRef]
- Begum, A.A.; Toth, I.; Hussein, W.M.; Moyle, P.M. Advances in targeted gene delivery. Curr. Drug Deliv. 2019, 16, 588–608. [Google Scholar] [CrossRef]
- Swingle, K.L.; Hamilton, A.G.; Mitchell, M.J. Lipid nanoparticle-mediated delivery of mRNA therapeutics and vaccines. Trends Mol. Med. 2021, 27, 616–617. [Google Scholar] [CrossRef]
- Gupta, V.; Lourenço, S.P.; Hidalgo, I.J. Development of gene therapy vectors: Remaining challenges. J. Pharm. Sci. 2021, 110, 1915–1920. [Google Scholar] [CrossRef]
- Zbacnik, T.J.; Holcomb, R.E.; Katayama, D.S.; Murphy, B.M.; Payne, R.W.; Coccaro, R.C.; Evans, G.J.; Matsuura, J.E.; Henry, C.S.; Manning, M.C. Role of buffers in protein formulations. J. Pharm. Sci. 2017, 106, 713–733. [Google Scholar] [CrossRef]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.K.; Paris, C.; Khan, K.S.; Robson, F.; Ng, W.-L.; Rocchi, P. Nucleic acid-based technologies targeting coronaviruses. Trends Biochem. Sci. 2021, 46, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Roncarolo, M.G. Gene Therapy. N. Eng. J. Med. 2019. Available online: https://www.xianjichina.com/news/details_141521.html (accessed on 14 November 2022).
- van den Berg, A.I.S.; Yun, C.O.; Schiffelers, R.M.; Hennink, W.E. Polymeric delivery systems for nucleic acid therapeutics: Approaching the clinic. J. Control. Release 2021, 331, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Srivastava, A.; Mallela, K.M.; Deorkar, N.; Brophy, G. Manufacturing challenges and rational formulation development for AAV viral vectors. J. Pharm. Sci. 2021, 110, 2609–2624. [Google Scholar] [CrossRef]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Pilkington, E.H.; Suys, E.J.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Buschmann, M.; Carrasco, M.; Alishetty, S.; Paige, M.; Alameh, M.; Weissman, D. Nanomaterial delivery systems for mRNA vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Dong, Y. Nanoscale platforms for messenger RNA delivery. WIREs Nanomed. Nanobiotechnol. 2019, 18, e1530. [Google Scholar] [CrossRef]
- Quemener, A.; Bachelot, L.; Forestier, A.; Donnou-Fournet, E.; Gilot, D.; Galibert, M. The powerful world of antisense oligonucleotides: From bench to bedside. Wiley Interdiscip. Rev. RNA 2020, 11, e1594. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Almalki, W.H.; Khatoon, F.; Alharbi, K.S.; Alghamdi, S.; Akhter, H.; Khalilullah, H.; Baothman, A.A.; Hafeez, A.; Rahman, M.; et al. Lipid and polymer-based nanocomplexes in nucleic acid delivery as cancer vaccines. Drug Discov. Today 2021, 26, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Mukalel, A.; Riley, R.; Zhang, R.; Mitchell, M. Nanoparticles for nucleic acid delivery: Applications in cancer immunotherapy. Cancer Lett. 2019, 458, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Rafael, D.; Andrade, F.; Arranja, A.; Luís, S.; Videira, M. Lipoplexes and polyplexes: Gene therapy. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; CRC Press: Boca Raton, FL, USA, 2015; pp. 4335–4347. [Google Scholar]
- Yang, Y.; Qin, Z.; Zeng, W.; Yang, T.; Cao, Y.; Mei, C.; Kuang, Y. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol. Rev. 2017, 6, 279–289. [Google Scholar] [CrossRef]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Machhi, J.; Shahjin, F.; Das, S.; Patel, M.; Abdelmoaty, M.; Cohen, J. Nanocarrier vaccines for SARS-CoV-2. Adv. Drug Deliv. Rev. 2021, 171, 215–239. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Nwagwu, C.; Anyim, O.; Ekweremadu, C.; Kim, S. COVID-19 and cancer: From basic mechanisms to vaccine development using nanotechnology. Int. Immunopharmacol. 2021, 90, 107247. [Google Scholar] [CrossRef]
- Hassett, K.J.; Higgins, J.; Woods, A.; Levy, B.; Xia, Y.; Hsiao, C.J.; Acosta, E.; Almarsson, Ö.; Moore, M.J.; Brito, L.A. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J. Control. Release 2021, 335, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Piyush, R.; Rajarshi, K.; Chatterjee, A.; Khan, R.; Ray, S. Nucleic acid-based therapy for coronavirus disease. Heliyon 2020, 6, e05007. [Google Scholar] [CrossRef]
- Available online: https://go.drugbank.com (accessed on 10 December 2022).
- Yamada, Y. Nucleic acid drugs—Current status, issues, and expectations for exosomes. Cancers 2021, 13, 5002. [Google Scholar] [CrossRef]
- Geary, R.S. Antisense oligonucleotide pharmacokinetics and metabolism. Expert Opin. Drug Metab. Toxicol. 2009, 5, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Nema, S.; Brendel, R.J.; Chan, E.; Maa, Y.-F.; Overcashier, D.; Hsu, C.C. Excipients and their role in approved injectable products: Current usage and future directions. PDA J. Pharm. Sci. Technol. 2011, 65, 287–332. [Google Scholar] [CrossRef]
- Kamerzell, T.; Esfandiary, R.; Joshi, S.; Middaugh, C.; Volkin, D. Protein–excipient interactions: Mechanisms and biophysical characterization applied to protein formulation development. Adv. Drug Deliv. Rev. 2011, 63, 1118–1159. [Google Scholar] [CrossRef]
- Rayaprolu, B.M.; Strawser, J.J.; Anyarambhatla, G. Excipients in parenteral formulations: Selection considerations and effective utilization with small molecules and biologics. Drug Dev. Ind. Pharm. 2018, 44, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://USFDA.org (accessed on 12 December 2022).
- Poecheim, J.; Graeser, K.A.; Hoernschemeyer, J.; Becker, G.; Storch, K.; Printz, M. Development of stable liquid formulations for oligonucleotides. Eur. J. Pharm. Biopharm. 2018, 129, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.; Klein, R.S. The deamination of cytidine and cytosine by acidic buffer solutions-mutagenic implications. Biochemistry 1966, 5, 2358–2362. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Okamoto, A. Degradation of DNA by bisulfite treatment. Bioorganic Med. Chem. Lett. 2007, 17, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Thorat, A.; Munjal, B.; Geders, T.; Suryanarayanan, R. Freezing-induced protein aggregation—Role of pH shift and potential T mitigation strategies. J. Control. Release 2020, 323, 591–599. [Google Scholar] [CrossRef]
- Garidel, P.; Pevestorf, B.; Bahrenburg, S. Stability of buffer-free freeze-dried formulations: A feasibility study of a monoclonal antibody at high protein concentrations. Eur. J. Pharm. Biopharm. 2015, 97, 125–139. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Wong-Arce, A.; de Lourdes Betancourt-Mendiola, M. RNA-based vaccines against SARS-CoV-2. In Biomedical Innovations to Combat COVID-19; Academic Press: Cambridge, MA, USA, 2022; pp. 129–152. [Google Scholar]
- Hovorka, S.; Schoneich, C. Oxidative degradation of pharmaceuticals: Theory, mechanisms and inhibition. J. Pharm. Sci. 2001, 90, 253–269. [Google Scholar] [CrossRef]
- Ball, R.; Bajaj, P.; Whitehead, K. Achieving long-term stability of lipid nano particles: Examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 2017, 12, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Ishihara, H. Difference in the lipid nanoparticle technology employed in three approved siRNA (patisiran) and mRNA (COVID-19 vaccine) drugs. Drug Metab. Pharmacokinet. 2021, 41, 100424. [Google Scholar] [CrossRef]
- Kon, E.; Elia, U.; Peer, D. Principles for designing an optimal mRNA lipid nanoparticle vaccine. Curr. Opin. Biotechnol. 2022, 73, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Venkataraman, A.; Wechsler, M.E.; Peppas, N.A. Peppas. Messenger RNA-based vaccines: Past, present, and future directions in the context of the COVID-19 pandemic. Adv. Drug Deliv. Rev. 2021, 179, 114000. [Google Scholar] [CrossRef]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.; Petsch, B. mRNA- based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralizing antibodies and mediates protection in rodents. NPJ Vaccine 2021, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. CureVac COVID vaccine let-down spotlights mRNA design challenges. Nature 2021, 594, 483. [Google Scholar] [CrossRef]
- de Alwis, R.; Gan, E.; Chen, S.; Leong, Y.; Tan, H.; Zhang, S.; Yau, C.; Low, J.; Kalimuddin, S.; Matsuda, D. A single dose of self-transcribing and replicating RNA-based SARS-CoV-2 vaccine produces protective adaptive immunity in mice. Mol. Ther. 2021, 29, 1970–1983. [Google Scholar] [CrossRef]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A thermostable mRNA vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef]
- Kobiyama, K.; Imai, M.; Jounai, N.; Nakayama, M.; Hioki, K.; Iwatsuki-Horimoto, K.; Yamayoshi, S.; Tsuchida, J.; Niwa, T.; Suzuki, T.; et al. Optimization of an LNP-mRNA vaccine candidate targeting SARS-CoV-2 receptor-binding domain. bioRxiv 2021, 2021. [Google Scholar] [CrossRef]
- McKay, P.F.; Hu, K.; Blakney, A.K.; Samnuan, K.; Brown, J.C.; Penn, R.; Zhou, J.; Bouton, C.R.; Rogers, P.; Polra, K.; et al. Self amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020, 11, 3523. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Q.; Zhu, F.; Zhao, M.; Yan, Y. mRNA delivery via non-viral carriers for biomedical applications. Int. J. Pharm. 2021, 607, 121020. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Villanueva, D.; El-Sherbiny, I.M.; Herrera-Ruiz, D.; Vlassov, A.V.; Smyth, H.D. Formulation approaches to short interfering RNA and MicroRNA: Challenges and implications. J. Pharm. Sci. 2012, 101, 4046–4066. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.xenon-pharma.com/glybera/ (accessed on 15 October 2022).
- Available online: http://www.drugs.com/uk/glybera.html (accessed on 10 December 2022).
- Muralidhara, B.K.; Wong, M. Critical considerations in the formulation development of parenteral biologic drugs. Drug Discov. Today 2020, 25, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Mastrobattista, E. Formulation and delivery solutions for the next generation biotherapeutics. J. Control. Release 2021, 336, 583–597. [Google Scholar] [CrossRef]

| Biologic Classification | Drug Name (Brand Name) | Subunits | Mol Formula | Target | Indication | Drug Delivery | Approving Agency | Approval Year |
|---|---|---|---|---|---|---|---|---|
| Oligonucleotides | Pegaptanib (Macugen) | 27 | C294H342F13N107Na28O188P28[C2H4O]2n | Selectively binds to VEGF165 | Neovascular (wet) age-related macular degeneration. | Naked | FDA | 2004 |
| Mipomersen (Kynamro) | 20 | C230H324N67O122P19S19 | mRNA of apoB-100 | Familial hypercholesterolemia | Naked | FDA | 2013 | |
| Defibrotide (Defitelio) | - | C20H21N4O6P | Adenosine receptors A1, A2a, A2b | Severe hepatic veno-occlusive disease | Naked | FDA | 2016 | |
| Antisense oligonucleotides | Fomivirsen (Vitravene) | 21 | C204H243N63O114P20S20Na20 | 30 kDa and 54 kDa immediate-early protein 2 | Cytomegalovirus (CMV) retinitis in patients with AIDS | Naked | FDA | 1998 |
| Eteplirsen (Exondys 51) | 30 | C364H569N177O122P30 | Forcing the exclusion of exon 51 from the mature DMD mRNA | Duchenne muscular dystrophy | Naked | FDA | 2016 | |
| Nusinersen (Spinraza) | 18 | C234H323N61O128P17S17Na17 | Survival motor neuron-2 protein | Spinal muscular atrophy | Naked | FDA | 2016 | |
| Inotersen (Tegsedi) | 20 | C230H318N69O121P19S19 | Transthyretin mRNA | Polyneuropathy | Naked | FDA | 2018 | |
| Volanesorsen (Waylivra) | - | C230H320N63O125P19S19 | Binds to apo C-III mRNA | Familial chylomicronemia syndrome | Naked | EMA | 2019 | |
| Golodirsen (Vyondys 53) | 25 | C305H481N138O112P25 | Dystrophin | Duchenne muscular dystrophy | Naked | FDA | 2019 | |
| Viltolarsen (VILTEPSO) | 21 | C244H381N113O88P20 | DMD gene (exon 53 viltolarsen target site) | Duchenne muscular dystrophy | Naked | FDA | 2020 | |
| Casimersen (Amondys 45) | 20 | C268H424N124O95P22 | DMD gene (exon 45) | Duchenne muscular dystrophy | Naked | FDA | 2021 | |
| Short interfering RNA (siRNA) | Patisiran (Onpattro) | 21 | C412H480N148Na40O290P40 | Transthyretin mRNA | Polyneuropathy | LNP | FDA | 2018 |
| Givosiran (Givlaari) | 21 | C524H694F16N173O316P43S6 | ALAS1 mRNA | Acute hepatic porphyria | N-acetylgalactosamine | FDA | 2019 | |
| Lumasiran (Oxlumo) | - | C530H669F10N173O320P43S6Na43 | hydroxyacid oxidase-1 (HAO1) mRNA in hepatocytes | Primary hyperoxaluria type 1 | N-acetylgalactosamine | FDA | 2020 | |
| Inclisiran (Leqvio) | - | C520H679 F21N175O309P43S6 | Inhibit hepatic translation proprotein convertase subtilisin-Kexin type 9 (PCSK9) | Primary hypercholesterolemia | N-acetylgalactosamine | EMA/FDA | 2020/2021 | |
| AMVUTTRA (Vutrisiran) | C530H715F9N171O323P43S6 | Transthyretin mRNA | Amyloidogenic Transthyretin Amyloidosis | Naked | FDA | 2022 | ||
| Gene therapy | Voretigene neparvovecrzyl (Luxturna) | - | - | Human retinal pigment epithelial 65 kDa protein (RPE65) encoded gene | Retinal dystrophy | Adeno-associated virus vector | FDA/EMA | 2017/2018 |
| Onasemnogene abeparvovec-xioi (Zolgensma) | - | - | Gene encoding copy delivery to the human SMN protein | Spinal muscular atrophy | Adeno-associated virus | FDA | 2019 | |
| Alipogene tiparvovec (Glybera) | - | - | - | Severe pancreatitis | Naked | EMA | 2012 | |
| Talimogene laherparepvec (Imlygic) | - | - | For the production of immune response stimulatory protein, human GM-CSF | In recurrent melanoma | Herpes simplex virus 1 | FDA/EMA | 2015 | |
| Strimvelis | - | - | Activate ADA enzyme | Adenosine deaminase-severe combined immunodeficiency (ADA-SCID) | Gamma retroviral vector | EMA | 2016 | |
| mRNA vaccines | Tozinameran (Comirnaty) (BNT162b2) | 4284 | - | SARS-CoV-2S glycoprotein | COVID-19 | LNP | FDA/EMA | 2021 |
| Elasomeran (Spikevax) (mRNA-1273) | 4284 | - | SARS-CoV-2S antigen | COVID-19 | LNP | FDA/EMA | 2021 |
| API | Dosage Form | Excipients | Strength | Dosage | pH Range | Route of Administration |
|---|---|---|---|---|---|---|
| Pegaptanib (0.3 mg) | Injection/solution | Dibasic sodium phosphate heptahydrate, monobasic sodium phosphate monohydrate, sodium chloride sodium hydroxide, and hydrochloric acid | 0.4 mg/mL, 3.47 mg/mL | 0.3 mg/90 µL | 6.0 to 7.0 | IVI |
| Mipomersen sodium (200 mg) | Injection/solution | Hydrochloric acid and sodium hydroxide | 200 mg/mL | 200 mg/mL solution | 7.5 to 8.5 | SC |
| Eteplirsen (50 mg) | Injection/solution | 0.2 mg potassium phosphate monobasic, 0.2 mg potassium chloride, 8 mg sodium chloride, and 1.14 mg sodium phosphate dibasic, anhydrous hydrochloric acid, and sodium hydroxide | 50 mg/mL | 100 mg/2 mL, 500 mg/10 mL | 7.5 | IV |
| Nusinersen (2.4 mg) | Injection/solution | 0.16 mg magnesium chloride hexahydrate USP, 0.22 mg potassium chloride USP, 0.21 mg calcium chloride dihydrate USP, 8.77 mg sodium chloride USP, 0.10 mg sodium phosphate dibasic anhydrous USP, 0.05 mg sodium phosphate monobasic dihydrate USP, hydrochloric acid and sodium hydroxide | 2.4 mg/mL | 12 mg/5 mL | ~7.2 | IT |
| Defibrotide sodium (80 mg) | Injection/solution | 10 mg sodium citrate USP, hydrochloric acid, and sodium hydroxide | 80 mg/mL | 200 mg/2.5 mL | 6.8–7.8 | IV |
| Inotersen (284 mg) | Injection/solution | Phosphate buffer, hydrochloric acid, and sodium hydroxide | 284 mg/1.5 mL | 284 mg/1.5 mL | 7.5 to 8.5 | SC |
| Patisiran (2.0 mg) | Injection/liposome | 13.0 mg (6Z,9Z,28Z,31Z)-heptatriaconta-6,9,28,31 tetraen-19-yl-4-(dimethylamino) butanoate (DLin-MC3-DMA), 3.3 mg 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 6.2 mg cholesterol USP, 1.6 mg α-(3′-{[1,2-di(myristyloxy)propanoxy] carbonylamino}propyl)-ω-methoxy, polyoxyethylene (PEG 2000 C-DMG), 0.2 mg potassium phosphate monobasic anhydrous NF, 2.3 mg sodium phosphate dibasic heptahydrate USP, and 8.8 mg sodium chloride USP | 2 mg/mL | 10 mg/5 mL | ~7.0 | IV |
| Givosiran (189 mg) | Injection/solution | Water for injection | 189 mg/mL | 189 mg/mL | - | SC |
| Volanesorsen sodium (200 mg) | Solution | Sodium hydroxide and hydrochloric acid | 200 mg of Volanesorsen sodium/mL | 285 mg of Volanesorsen/1.5 ml | 8.0 | SC |
| Golodirsen (50 mg) | Injection | 0.2 mg potassium phosphate monobasic, 0.2 mg potassium chloride, 8 mg sodium chloride, and 1.14 mg sodium phosphate dibasic anhydrous, sodium hydroxide, and hydrochloric acid | 50 mg/mL | 100 mg/2 mL | 7.5 | IV |
| Viltolarsen (50 mg) | Injection/solution | 9 mg (0.9%) sodium chloride, sodium hydroxide, and hydrochloric acid | 50 mg/mL | 250 mg/5 mL | 7.0 to 7.5 | IV |
| Casimersen (50 mg) | Injection/solution | 0.2 mg potassium chloride, 0.2 mg potassium phosphate monobasic, 8 mg sodium chloride, and 1.14 mg sodium phosphate dibasic | 50 mg/mL | 100 mg/2 mL | 7.5 | IV |
| Voretigene neparvovecrzyl | Solution/suspension | 10 mM sodium phosphate, 180 mM sodium chloride, and 0.001% poloxamer 188 | 5 × 1012 vg/mL | 0.3 mL, 0.5 mL in 2 mL | 7.3 | IOI |
| Onasemnogene abeparvovec-xioi | Suspension | Onasemnogene abeparvovec (20000000000000 1/1 mL) + Onasemnogene abeparvovec (20000000000000 1/1 mL) + isopropyl alcohol (0.7 mL/1 mL) | 2.0 × 1013 vg/mL | - | - | IV |
| Talimogene laherparepvec | Injection/suspension | 2.44 mg sodium dihydrogen phosphate dihydrate, 15.4 mg disodium hydrogen phosphate dihydrate, 8.5 mg sodium chloride, 40 mg myoinositol, and 20 mg sorbitol | 106(PFU)/1 mL | 106(PFU)/1 mL (For initial dose), 108(PFU)/1 mL (For subsequent dose) | - | SC/IL |
| Alipogene tiparvovec | Injection/solution | Potassium dihydrogen phosphate, potassium chloride, sodium chloride, disodium phosphate, and sucrose | 3 × 10 12-genome copies/mL | 3 × 10 12-genome copies/mL | - | IM |
| Lumasiran sodium | Solution | Sodium hydroxide and phosphoric acid | 94.5 mg/0.5 mL | 94.5 mg/0.5 mL | 7.0 | SC |
| Inclisiran sodium (284 mg) | Solution | Sodium hydroxide and con. phosphoric acid | 189 mg/mL | 284 mg/1.5 mL | - | SC |
| AMVUTTRA (Vutrisiran sodium) | Injection | 0.7 mg sodium phosphate dibasic dihydrate, 0.2 mg sodium phosphate monobasic dihydrate, 3.2 mg sodium chloride | 26.5 mg/0.5 mL | 26.5 mg/0.5 mL | 7.0 | SC |
| Tozinameran | Suspension | 0.09 mg 1,2-distearoyl-sn-glycero-3-phosphocholine, 0.05 mg 2[(polyethylene glycol)-2000]- N,N-ditetradecylacetamide, 0.43 mg (4-hydroxybutyl)azanediyl)-bis(hexane-6,1-diyl)bis(2-hexyldecanoate), 0.2 mg cholesterol), 0.01 mg potassium chloride, 0.01 mg monobasic potassium phosphate, 0.07 mg dibasic sodium phosphate dihydrate, 6 mg sucrose, and 0.36 mg sodium chloride | 30 mcg of mRNA | 0.5 mg/1 mL | 6.9 to 7.9 | IM |
| Elasomeran | Suspension | Lipids, cholesterol, 1.93 mg (SM-102, polyethylene glycol-2000, 1,2-distearoyl-sn-glycero-3-phosphocholine), 0.31 mg tromethamine, 1.18 mg tromethamine HCl, 0.043 mg acetic acid, 0.12 mg sodium acetate, dimyristoyl glycerol, and 43.5 mg sucrose | 100 mcg of mRNA | 0.2 mg/1 mL | 7.0 to 8.0 | IM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingle, R.G.; Fang, W.-J. An Overview of the Stability and Delivery Challenges of Commercial Nucleic Acid Therapeutics. Pharmaceutics 2023, 15, 1158. https://doi.org/10.3390/pharmaceutics15041158
Ingle RG, Fang W-J. An Overview of the Stability and Delivery Challenges of Commercial Nucleic Acid Therapeutics. Pharmaceutics. 2023; 15(4):1158. https://doi.org/10.3390/pharmaceutics15041158
Chicago/Turabian StyleIngle, Rahul G., and Wei-Jie Fang. 2023. "An Overview of the Stability and Delivery Challenges of Commercial Nucleic Acid Therapeutics" Pharmaceutics 15, no. 4: 1158. https://doi.org/10.3390/pharmaceutics15041158