Efficient mRNA Delivery with mRNA Lipoplexes Prepared Using a Modified Ethanol Injection Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. mRNAs
2.3. Cell Culture
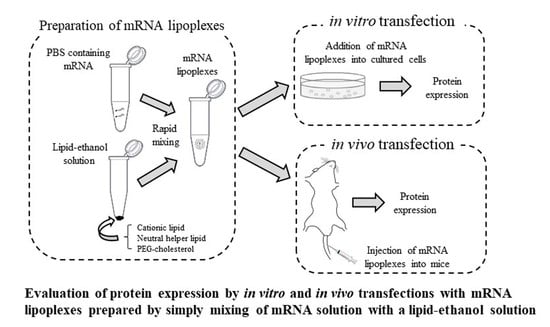
2.4. Preparation of mRNA Lipoplexes for In Vitro Transfection
2.5. Size and ζ-Potential Measurements of mRNA Lipoplexes
2.6. Evaluation of Luciferase Expression in Cells after Transfection with FLuc mRNA Lipoplexes
2.7. Evaluation of EGFP Expression in Cells after Transfection with EGFP mRNA Lipoplexes
2.8. Cellular Uptake of mRNA Lipoplexes
2.9. Cytotoxicity of mRNA Lipoplexes
2.10. Preparation of mRNA Lipoplexes for In Vivo Transfection
2.11. Biodistribution of mRNA after Injection of mRNA Lipoplexes into Mice
2.12. Luciferase Expression after Injection of mRNA Lipoplexes into Mice
2.13. Measurement of Ovalbumin (OVA) Antibody in Mice Post OVA mRNA Injection
2.14. Statistical Analysis
3. Results
3.1. Size of mRNA Lipoplexes
3.2. In Vitro Protein Expression and Cell Viability after Transfection with mRNA Lipoplexes
3.3. Biodistribution and Protein Expression of mRNA after Intramuscular Injection of mRNA Lipoplexes
3.4. Biodistribution and Protein Expression of mRNA after Systemic Injection of mRNA Lipoplexes
3.5. Induction of Anti-OVA Antibody by Intravenous Injections of OVA mRNA Lipoplexes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Van Driessche, A.; Ponsaerts, P.; Van Bockstaele, D.R.; Van Tendeloo, V.F.; Berneman, Z.N. Messenger RNA electroporation: An efficient tool in immunotherapy and stem cell research. Folia Histochem. Cytobiol. 2005, 43, 213–216. Available online: https://journals.viamedica.pl/folia_histochemica_cytobiologica/article/view/4599 (accessed on 1 March 2023). [PubMed]
- Ponsaerts, P.; Van Tendeloo, V.F.; Berneman, Z.N. Cancer immunotherapy using RNA-loaded dendritic cells. Clin. Exp. Immunol. 2003, 134, 378–384. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Liang, Y.; Huang, L. Development and delivery systems of mRNA vaccines. Front. Bioeng. Biotechnol. 2021, 9, 718753. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery strategies for mRNA vaccines. Pharm. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Lorentzen, C.L.; Haanen, J.B.; Met, O.; Svane, I.M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Yen, A.; Cheng, Y.; Sylvestre, M.; Gustafson, H.H.; Puri, S.; Pun, S.H. Serum nuclease susceptibility of mRNA cargo in condensed polyplexes. Mol. Pharm. 2018, 15, 2268–2276. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.Y.; Lu, A.; Wang, X.Y.; Jiang, L.X.; Wang, J.C. Non-viral vectors for RNA delivery. J. Control. Release 2022, 342, 241–279. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial delivery systems for mRNA vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ren, X.; Xu, S.; Zhang, D.; Han, T. Optimization of lipid nanoformulations for effective mRNA delivery. Int. J. Nanomed. 2022, 17, 2893–2905. [Google Scholar] [CrossRef]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Di, J.; Du, Z.; Wu, K.; Jin, S.; Wang, X.; Li, T.; Xu, Y. Biodistribution and non-linear gene expression of mRNA LNPs affected by delivery route and particle size. Pharm. Res. 2022, 39, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, C.; Wang, X.; Zhao, D.; Zhang, M.; Cao, H.; Liang, Z.; Xiao, H.; Liang, X.J.; Weng, Y.; et al. Efficient hepatic delivery and protein expression enabled by optimized mRNA and ionizable lipid nanoparticle. Bioact. Mater. 2020, 5, 1053–1061. [Google Scholar] [CrossRef]
- Sasaki, K.; Sato, Y.; Okuda, K.; Iwakawa, K.; Harashima, H. mRNA-loaded lipid nanoparticles targeting dendritic cells for cancer immunotherapy. Pharmaceutics 2022, 14, 1572. [Google Scholar] [CrossRef]
- Hamada, E.; Kurosaki, T.; Hashizume, J.; Harasawa, H.; Nakagawa, H.; Nakamura, T.; Kodama, Y.; Sasaki, H. Anionic complex with efficient expression and good safety profile for mRNA delivery. Pharmaceutics 2021, 13, 126. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Yang, L.; Gong, L.; Wang, P.; Zhao, X.; Zhao, F.; Zhang, Z.; Li, Y.; Huang, W. Recent advances in lipid nanoparticles for delivery of mRNA. Pharmaceutics 2022, 14, 2682. [Google Scholar] [CrossRef]
- Maeki, M.; Okada, Y.; Uno, S.; Niwa, A.; Ishida, A.; Tani, H.; Tokeshi, M. Production of siRNA-loaded lipid nanoparticles using a microfluidic device. J. Vis. Exp. 2022, 181, e62999. [Google Scholar] [CrossRef]
- Hattori, Y.; Saito, H.; Nakamura, K.; Yamanaka, A.; Tang, M.; Ozaki, K.I. In vitro and in vivo transfections using siRNA lipoplexes prepared by mixing siRNAs with a lipid-ethanol solution. J. Drug Deliv. Sci. Technol. 2022, 75, 103635. [Google Scholar] [CrossRef]
- Hattori, Y.; Tamaki, K.; Ozaki, K.I.; Kawano, K.; Onishi, H. Optimized combination of cationic lipids and neutral helper lipids in cationic liposomes for siRNA delivery into the lung by intravenous injection of siRNA lipoplexes. J. Drug Deliv. Sci. Technol. 2019, 52, 1042–1050. [Google Scholar] [CrossRef]
- Hattori, Y.; Tang, M.; Torii, S.; Tomita, K.; Sagawa, A.; Inoue, N.; Yamagishi, R.; Ozaki, K.I. Optimal combination of cationic lipid and phospholipid in cationic liposomes for gene knockdown in breast cancer cells and mouse lung using siRNA lipoplexes. Mol. Med. Rep. 2022, 26, 253. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Tamaki, K.; Sakasai, S.; Ozaki, K.I.; Onishi, H. Effects of PEG anchors in pegylated siRNA lipoplexes on in vitro gene-silencing effects and siRNA biodistribution in mice. Mol. Med. Rep. 2020, 22, 4183–4196. [Google Scholar] [CrossRef] [PubMed]
- Koulov, A.V.; Vares, L.; Jain, M.; Smith, B.D. Cationic triple-chain amphiphiles facilitate vesicle fusion compared to double-chain or single-chain analogues. Biochim. Biophys. Acta 2002, 1564, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Grant-Serroukh, D.; Hunter, M.R.; Maeshima, R.; Tagalakis, A.D.; Aldossary, A.M.; Allahham, N.; Williams, G.R.; Edbrooke, M.; Desai, A.; Hart, S.L. Lipid-peptide nanocomplexes for mRNA delivery in vitro and in vivo. J. Control. Release 2022, 348, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Kelava, T.; Ćavar, I.; Čulo, F. Biological actions of drug solvents. Period. Biol. 2011, 113, 311–320. Available online: https://hrcak.srce.hr/74090 (accessed on 1 March 2023).










| Liposome | Formulation (mol%) |
|---|---|
| LP-DOTAP/DOPE | DOTAP/DOPE/PEG-Chol (49.5/49.5/1) |
| LP-DOTAP/DOPC | DOTAP/DOPC/PEG-Chol (49.5/49.5/1) |
| LP-DOTAP/Chol | DOTAP/Chol/PEG-Chol (49.5/49.5/1) |
| LP-DC-1-14/DOPE | DC-1-14/DOPE/PEG-Chol (49.5/49.5/1) |
| LP-DC-1-14/DOPC | DC-1-14/DOPC/PEG-Chol (49.5/49.5/1) |
| LP-DC-1-14/Chol | DC-1-14/Chol/PEG-Chol (49.5/49.5/1) |
| LP-DC-1-16/DOPE | DC-1-16/DOPE/PEG-Chol (49.5/49.5/1) |
| LP-DC-1-16/DOPC | DC-1-16/DOPC/PEG-Chol (49.5/49.5/1) |
| LP-DC-1-16/Chol | DC-1-16/Chol/PEG-Chol (49.5/49.5/1) |
| LP-DDAB/DOPE | DDAB/DOPE/PEG-Chol (49.5/49.5/1) |
| LP-DDAB/DOPC | DDAB/DOPC/PEG-Chol (49.5/49.5/1) |
| LP-DDAB/Chol | DDAB/Chol/PEG-Chol (49.5/49.5/1) |
| LP-DC-6-14/DOPE | DC-6-14/DOPE/PEG-Chol (49.5/49.5/1) |
| LP-DC-6-14/DOPC | DC-6-14/DOPC/PEG-Chol (49.5/49.5/1) |
| LP-DC-6-14/Chol | DC-6-14/Chol/PEG-Chol (49.5/49.5/1) |
| LP-TC-1-12/DOPE | TC-1-12/DOPE/PEG-Chol (49.5/49.5/1) |
| LP-TC-1-12/DOPC | TC-1-12/DOPC/PEG-Chol (49.5/49.5/1) |
| LP-TC-1-12/Chol | TC-1-12/Chol/PEG-Chol (49.5/49.5/1) |
| Liposome | Size (nm) | PDI |
|---|---|---|
| LP-DOTAP/DOPE | 136.3 ± 0.1 | 0.09 ± 0.02 |
| LP-DOTAP/DOPC | 284.0 ± 7.1 | 0.25 ± 0.00 |
| LP-DOTAP/Chol | 112.1 ± 0.9 | 0.10 ± 0.02 |
| LP-DC-1-14/DOPE | 197.9 ± 0.5 | 0.08 ± 0.01 |
| LP-DC-1-14/DOPC | 657.9 ± 19.4 | 0.30 ± 0.01 |
| LP-DC-1-14/Chol | 178.2 ± 2.7 | 0.07 ± 0.02 |
| LP-DC-1-16/DOPE | 211.6 ± 4.3 | 0.05 ± 0.03 |
| LP-DC-1-16/DOPC | 156.9 ± 3.5 | 0.14 ± 0.01 |
| LP-DC-1-16/Chol | 196.5 ± 2.9 | 0.11 ± 0.02 |
| LP-DDAB/DOPE | 217.7 ± 4.0 | 0.08 ± 0.03 |
| LP-DDAB/DOPC | 120.9 ± 1.8 | 0.11 ± 0.02 |
| LP-DDAB/Chol | 265.1 ± 6.5 | 0.14 ± 0.01 |
| LP-DC-6-14/DOPE | 314.4 ± 5.4 | 0.23 ± 0.02 |
| LP-DC-6-14/DOPC | 207.7 ± 1.2 | 0.18 ± 0.01 |
| LP-DC-6-14/Chol | 313.4 ± 6.7 | 0.15 ± 0.02 |
| LP-TC-1-12/DOPE | 137.0 ± 2.5 | 0.12 ± 0.01 |
| LP-TC-1-12/DOPC | 97.6 ± 1.3 | 0.09 ± 0.01 |
| LP-TC-1-12/Chol | 133.7 ± 0.4 | 0.14 ± 0.01 |
| Liposome | Size (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|
| LP-DC-1-16/DOPE | 243.4 ± 1.3 | 0.20 ± 0.01 | 28.3 ± 0.9 |
| LP-TC-1-12/DOPE | 170.8 ± 0.9 | 0.12 ± 0.01 | 35.9 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Sagawa, A.; Inoue, N.; Torii, S.; Tomita, K.; Hattori, Y. Efficient mRNA Delivery with mRNA Lipoplexes Prepared Using a Modified Ethanol Injection Method. Pharmaceutics 2023, 15, 1141. https://doi.org/10.3390/pharmaceutics15041141
Tang M, Sagawa A, Inoue N, Torii S, Tomita K, Hattori Y. Efficient mRNA Delivery with mRNA Lipoplexes Prepared Using a Modified Ethanol Injection Method. Pharmaceutics. 2023; 15(4):1141. https://doi.org/10.3390/pharmaceutics15041141
Chicago/Turabian StyleTang, Min, Ayane Sagawa, Nodoka Inoue, Satomi Torii, Kana Tomita, and Yoshiyuki Hattori. 2023. "Efficient mRNA Delivery with mRNA Lipoplexes Prepared Using a Modified Ethanol Injection Method" Pharmaceutics 15, no. 4: 1141. https://doi.org/10.3390/pharmaceutics15041141