Influence of Silver Nanoparticles on the Growth of Ascitic and Solid Ehrlich Adenocarcinoma: Focus on Copper Metabolism
Abstract
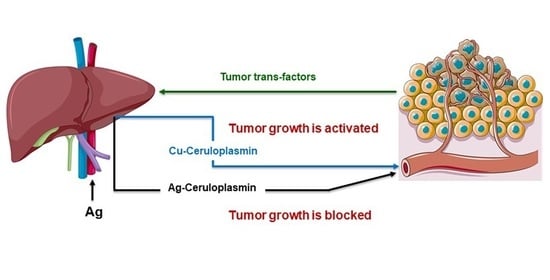
:1. Introduction
2. Materials and Methods
2.1. Fabrication of AgNPs and Their Physicochemical Characterization
2.2. Animals
2.3. Treatment of Mice with AgNPs and Ehrlich Ascites Carcinoma Cells
2.4. Gene Expression Analysis
2.5. Biochemical Methods
3. Results and Discussion
3.1. Physicochemical Specification of Fabricated Silver Nanoparticles
3.2. Design of AgNP Treatment of Mice with Growing Ascitic or Solid Ehrlich Adenocarcinomas
3.3. Influence of AgNPs on Survival of Mice with Growing Ascitic or Solid EAC
3.4. Characterization of Copper Status in Mice with Ascitic or Solid EAC Treated with AgNPs
3.5. Influence of EAC Tumors on Expression of Genes Encoding Copper Homeostasis Proteinsin the Liver
3.6. Changes of Copper-Related Genes Expression in Ascitic and Solid EAC Tumors Induced by AgNP Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walshe, J.M.; Waldenström, E.; Sams, V.; Nordlinder, H.; Westermark, K. Abdominal Malignancies in Patients with Wilson’s Disease. QJM 2003, 96, 657–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, C.J. Copper-overload causes cancer? The LEC rat: A model for human hepatitis, liver cancer, and much more. Hum. Exp. Toxicol. 1994, 13, 910–912. [Google Scholar] [PubMed]
- Huster, D.; Finegold, M.J.; Morgan, C.T.; Burkhead, J.L.; Nixon, R.; Vanderwerf, S.M.; Gilliam, C.T.; Lutsenko, S. Consequences of Copper Accumulation in the Livers of the Atp7b-/- (Wilson Disease Gene) Knockout Mice. Am. J. Pathol. 2006, 168, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupte, A.; Mumper, R.J. Elevated Copper and Oxidative Stress in Cancer Cells as a Target for Cancer Treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Krohn, K.A.; Link, J.M.; Mason, R.P. Molecular Imaging of Hypoxia. J. Nucl. Med. 2008, 49, 129S–148S. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Ye, F.; Xue, W.; Zhou, Z.; Kang, Y.J. Copper Regulation of Hypoxia-Inducible Factor-1 Activity. Mol. Pharmacol. 2009, 75, 174–182. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhang, W.; Kang, Y.J. Copper Affects the Binding of HIF-1α to the Critical Motifs of Its Target Genes. Metallomics 2019, 11, 429–438. [Google Scholar] [CrossRef]
- Gogvadze, V.; Zhivotovsky, B.; Orrenius, S. The Warburg Effect and Mitochondrial Stability in Cancer Cells. Mol. Asp. Med. 2010, 31, 60–74. [Google Scholar] [CrossRef]
- Gérard, C.; Bordeleau, L.J.; Barralet, J.; Doillon, C.J. The Stimulation of Angiogenesis and Collagen Deposition by Copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, L.; Lin, C.; Yang, H.; Fu, H.; Li, R.; Kang, Y.J. Copper-Dependent and -Independent Hypoxia-Inducible Factor-1 Regulation of Gene Expression. Metallomics 2014, 6, 1889–1893. [Google Scholar] [CrossRef]
- Rigiracciolo, D.C.; Scarpelli, A.; Lappano, R.; Pisano, A.; Santolla, M.F.; Marco, P.D.; Cirillo, F.; Cappello, A.R.; Dolce, V.; Belfiore, A.; et al. Copper Activates HIF-1α/GPER/VEGF Signalling in Cancer Cells. Oncotarget 2015, 6, 34158–34177. [Google Scholar] [CrossRef] [Green Version]
- Urso, E.; Maffia, M. Behind the Link between Copper and Angiogenesis: Established Mechanisms and an Overview on the Role of Vascular Copper Transport Systems. J. Vasc. Res. 2015, 52, 172–196. [Google Scholar] [CrossRef]
- Rucker, R.B.; Kosonen, T.; Clegg, M.S.; Mitchell, A.E.; Rucker, B.R.; Uriu-Hare, J.Y.; Keen, C.L. Copper, Lysyl Oxidase, and Extracellular Matrix Protein Cross-Linking. Am. J. Clin. Nutr. 1998, 67, 996S–1002S. [Google Scholar] [CrossRef] [Green Version]
- Payne, S.L.; Hendrix, M.J.; Kirschmann, D.A. Paradoxical Roles for Lysyl Oxidases in Cancer—A Prospect. J. Cell. Biochem. 2007, 101, 1338–1354. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, Y. The Function and Mechanisms of Action of LOXL2 in Cancer (Review). Int. J. Mol. Med. 2015, 36, 1200–1204. [Google Scholar] [CrossRef] [Green Version]
- Shanbhag, V.; Jasmer-McDonald, K.; Zhu, S.; Martin, A.L.; Gudekar, N.; Khan, A.; Ladomersky, E.; Singh, K.; Weisman, G.A.; Petris, M.J. ATP7A Delivers Copper to the Lysyl Oxidase Family of Enzymes and Promotes Tumorigenesis and Metastasis. Proc. Natl. Acad. Sci. USA 2019, 116, 6836–6841. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Ge, G. Lysyl Oxidase, Extracellular Matrix Remodeling and Cancer Metastasis. Cancer Microenviron. 2012, 5, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Pez, F.; Dayan, F.; Durivault, J.; Kaniewski, B.; Aimond, G.; Le Provost, G.S.; Deux, B.; Clézardin, P.; Sommer, P.; Pouysségur, J.; et al. The HIF-1-Inducible Lysyl Oxidase Activates HIF-1 via the Akt Pathway in a Positive Regulation Loop and Synergizes with HIF-1 in Promoting Tumor Cell Growth. Cancer Res. 2011, 71, 1647–1657. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Qian, Y.; Jin, W.; Tian, R.; Zhu, Y.; Wang, J.; Meng, X.; Wang, R. Hypoxia-Inducible Factor-1α Regulates Epithelial-To-Mesenchymal Transition in Paraquat-Induced Pulmonary Fibrosis by Activating Lysyl Oxidase. Exp. Ther. Med. 2018, 15, 2287–2294. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Penning, L.C. COMMD1, a Multi-Potent Intracellular Protein Involved in Copper Homeostasis, Protein Trafficking, Inflammation, and Cancer. J. Trace Elem. Med. Biol. 2021, 65, 126712. [Google Scholar] [CrossRef] [PubMed]
- Hanifeh, M.; Ataei, F. XIAP as a Multifaceted Molecule in Cellular Signaling. Apoptosis 2022, 27, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Burstein, E.; Ganesh, L.; Dick, R.D.; van De Sluis, B.; Wilkinson, J.C.; Klomp, L.W.; Wijmenga, C.; Brewer, G.J.; Nabel, G.J.; Duckett, C.S. A Novel Role for XIAP in Copper Homeostasis through Regulation of MURR1. EMBO J. 2004, 23, 244–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mufti, A.R.; Burstein, E.; Duckett, C.S. XIAP: Cell Death Regulation Meets Copper Homeostasis. Arch. Biochem. Biophys. 2007, 463, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.R.; Siraj, A.K.; Ahmed, M.; Bu, R.; Pratheeshkumar, P.; Alrashed, A.M.; Qadri, Z.; Ajarim, D.; Al-Dayel, F.; Beg, S.; et al. XIAP Over-Expression is an Independent Poor Prognostic Marker in Middle Eastern Breast Cancer and Can be Targeted to Induce Efficient Apoptosis. BMC Cancer 2017, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Nakanishi, H.; Li, Y.N.; Matsubara, H.; Yamamoto, K.; Sato, N.; Shiraishi, T.; Nakamura, T.; Mikami, K.; Okihara, K.; et al. Overexpression of XIAP Expression in Renal Cell Carcinoma Predicts a Worse Prognosis. Int. J. Oncol. 2007, 30, 919–925. [Google Scholar] [CrossRef] [Green Version]
- Mufti, A.R.; Burstein, E.; Csomos, R.A.; Graf, P.C.; Wilkinson, J.C.; Dick, R.D.; Challa, M.; Son, J.K.; Bratton, S.B.; Su, G.L.; et al. XIAP is a Copper Binding Protein Deregulated in Wilson’s Disease and Other Copper Toxicosis Disorders. Mol. Cell 2006, 21, 775–785. [Google Scholar] [CrossRef]
- Cobine, P.A.; Moore, S.A.; Leary, S.C. Getting out What You Put in: Copper in Mitochondria and Its Impacts on Human Disease. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118867. [Google Scholar] [CrossRef]
- Yang, F.; Liao, J.; Yu, W.; Qiao, N.; Guo, J.; Han, Q.; Li, Y.; Hu, L.; Pan, J.; Tang, Z. Exposure to Copper Induces Mitochondria-Mediated Apoptosis by Inhibiting Mitophagy and the PINK1/Parkin Pathway in Chicken (Gallus Gallus) Livers. J. Hazard. Mater. 2021, 408, 124888. [Google Scholar] [CrossRef]
- Cobine, P.A.; Brady, D.C. Cuproptosis: Cellular and Molecular Mechanisms Underlying Copper-Induced Cell Death. Mol. Cell 2022, 82, 1786–1787. [Google Scholar] [CrossRef]
- Polishchuk, E.V.; Merolla, A.; Lichtmannegger, J.; Romano, A.; Indrieri, A.; Ilyechova, E.Y.; Concilli, M.; De Cegli, R.; Crispino, R.; Mariniello, M.; et al. Activation of Autophagy, Observed in Liver Tissues From Patients with Wilson Disease and From ATP7B-Deficient Animals, Protects Hepatocytes from Copper-Induced Apoptosis. Gastroenterology 2019, 156, 1173–1189. [Google Scholar] [CrossRef] [Green Version]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper Induces Cell Death by Targeting Lipoylated TCA Cycle Proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper Homeostasis and Cuproptosis in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting Copper and Cancer: From Transition Metal Signalling to Metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef]
- Brewer, G.J.; Dick, R.D.; Yuzbasiyan-Gurkin, V.; Tankanow, R.; Young, A.B.; Kluin, K.J. Initial Therapy of Patients with Wilson’s Disease with Tetrathiomolybdate. Arch. Neurol. 1991, 48, 42–47. [Google Scholar] [CrossRef]
- Brewer, G.J.; Dick, R.D.; Grover, D.K.; LeClaire, V.; Tseng, M.; Wicha, M.; Pienta, K.; Redman, B.G.; Jahan, T.; Sondak, V.K.; et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin. Cancer Res. 2000, 6, 1–10. [Google Scholar]
- Ogra, Y.; Komada, Y.; Suzuki, K.T. Comparative Mechanism and Toxicity of Tetra- and Dithiomolybdates in the Removal of Copper. J. Inorg. Biochem. 1999, 75, 199–204. [Google Scholar] [CrossRef]
- Brewer, G.J. Copper-Lowering Therapy with Tetrathiomolybdate for Cancer and Diseases of Fibrosis and Inflammation. J. Trace Elem. Exp. Med. 2003, 16, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Henry, N.L.; Dunn, R.; Merjaver, S.; Pan, Q.; Pienta, K.J.; Brewer, G.; Smith, D.C. Phase II Trial of Copper Depletion with Tetrathiomolybdate as an Antiangiogenesis Strategy in Patients with Hormone-Refractory Prostate Cancer. Oncology 2006, 71, 168–175. [Google Scholar] [CrossRef]
- Jain, S.; Cohen, J.; Ward, M.M.; Kornhauser, N.; Chuang, E.; Cigler, T.; Moore, A.; Donovan, D.; Lam, C.; Cobham, M.V.; et al. Tetrathiomolybdate-Associated Copper Depletion Decreases Circulating Endothelial Progenitor Cells in Women with Breast Cancer at High Risk of Relapse. Ann. Oncol. 2013, 24, 1491–1498. [Google Scholar] [CrossRef]
- Sammons, S.; Brady, D.; Vahdat, L.; Salama, A.K. Copper Suppression as Cancer Therapy: The Rationale for Copper Chelating Agents in BRAFV600 Mutated Melanoma. Melanoma Manag. 2016, 3, 207–216. [Google Scholar] [CrossRef]
- Li, Y. Copper Homeostasis: Emerging Target for Cancer Treatment. IUBMB Life 2020, 72, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Briehl, M.M.; Mazar, A.P.; Batinic-Haberle, I.; Reboucas, J.S.; Glinsmann-Gibson, B.; Rimsza, L.M.; Tome, M.E. The Copper Chelator ATN-224 Induces Peroxynitrite-Dependent Cell Death in Hematological Malignancies. Free. Radic. Biol. Med. 2013, 60, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Zahurak, M.; Beer, T.M.; Ryan, C.J.; Wilding, G.; Mathew, P.; Morris, M.; Callahan, J.A.; Gordon, G.; Reich, S.D.; et al. A Non-Comparative Randomized Phase II Study of 2 Doses of ATN-224, a Copper/Zinc Superoxide Dismutase Inhibitor, in Patients with Biochemically Recurrent Hormone-Naïve Prostate Cancer. Urol. Oncol. 2013, 31, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Naggar, N.E.; Hussein, M.H.; El-Sawah, A.A. Bio-Fabrication of Silver Nanoparticles by Phycocyanin, Characterization, in Vitro Anticancer Activity Against Breast Cancer Cell Line and in Vivo Cytotxicity. Sci. Rep. 2017, 7, 10844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rageh, M.M.; El-Gebaly, R.H.; Afifi, M.M. Antitumor Activity of Silver Nanoparticles in Ehrlich Carcinoma-Bearing Mice. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 1421–1430. [Google Scholar] [CrossRef]
- Kabir, S.R.; Dai, Z.; Nurujjaman, M.; Cui, X.; Asaduzzaman, A.K.M.; Sun, B.; Zhang, X.; Dai, H.; Zhao, X. Biogenic Silver/Silver Chloride Nanoparticles Inhibit Human Glioblastoma Stem Cells Growth in Vitro and Ehrlich Ascites Carcinoma Cell growth in Vivo. J. Cell Mol. Med. 2020, 24, 13223–13234. [Google Scholar] [CrossRef]
- Kabir, S.R.; Islam, F.; Asaduzzaman, A.K.M. Biogenic Silver/Silver Chloride Nanoparticles Inhibit Human Cancer Cells Proliferation in Vitro and Ehrlich Ascites Carcinoma Cells Growth in Vivo. Sci. Rep. 2022, 12, 8909. [Google Scholar] [CrossRef]
- Ghose, R.; Asaduzzaman, A.K.M.; Hasan, I.; Kabir, S.R. Hypnea Musciformis-Mediated Ag/AgCl-NPs Inhibit Pathogenic Bacteria, HCT-116 and MCF-7 Cells’ Growth in Vitro and Ehrlich Ascites Carcinoma Cells in Vivo in Mice. IET Nanobiotechnology 2022, 16, 49–60. [Google Scholar] [CrossRef]
- Puchkova, L.V.; Broggini, M.; Polishchuk, E.V.; Ilyechova, E.Y.; Polishchuk, R.S. Silver Ions as a Tool for Understanding Different Aspects of Copper Metabolism. Nutrients 2019, 11, 1364. [Google Scholar] [CrossRef] [Green Version]
- Skvortsov, A.N.; Ilyechova, E.Y.; Puchkova, L.V. Chemical Background of Silver Nanoparticles Interfering with Mammalian Copper Metabolism. J. Hazard. Mater. 2023, 451, 131093. [Google Scholar] [CrossRef]
- Ilyechova, E.Y.; Saveliev, A.N.; Skvortsov, A.N.; Babich, P.S.; Zatulovskaia, Y.A.; Pliss, M.G.; Korzhevskii, D.E.; Tsymbalenko, N.V.; Puchkova, L.V. The Effects of Silver Ions on Copper Metabolism in Rats. Metallomics 2014, 6, 1970–1987. [Google Scholar] [CrossRef]
- Orlov, I.A.; Sankova, T.P.; Babich, P.S.; Sosnin, I.M.; Ilyechova, E.Y.; Kirilenko, D.A.; Brunkov, P.N.; Ataev, G.L.; Romanov, A.E.; Puchkova, L.V. New Silver Nanoparticles Induce Apoptosis-Like Process in E.coli and Interfere with Mammalian Copper Metabolism. Int. J. Nanomed. 2016, 11, 6561–6574. [Google Scholar] [CrossRef] [Green Version]
- Owen, J.; Smith, H. Detection of ceruloplasmin after zone electrophoresis. Clin. Chim. Acta 1961, 6, 441–444. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Kostevich, V.A.; Romanico, D.N.; Zakharova, E.T.; Vasilyev, V.B. Two-Stage Method for Purification of Ceruloplasmin Based on Its Interaction with Neomycin. Biochemistry 2012, 77, 631–638. [Google Scholar] [CrossRef]
- Skomorokhova, E.A.; Sankova, T.P.; Orlov, I.A.; Savelev, A.N.; Magazenkova, D.N.; Pliss, M.G.; Skvortsov, A.N.; Sosnin, I.M.; Kirilenko, D.A.; Grishchuk, I.V.; et al. Size-Dependent Bioactivity of Silver Nanoparticles: Antibacterial Properties, Influence on Copper Status in Mice, and Whole-Body Turnover. Nanotechnol. Sci. Appl. 2020, 13, 137–157. [Google Scholar] [CrossRef]
- Pratsinis, A.; Hervella, P.; Leroux, J.C.; Pratsinis, S.E.; Sotiriou, G.A. Toxicity of Silver Nanoparticles in Macrophages. Small 2013, 9, 2576–2584. [Google Scholar] [CrossRef]
- Puchkova, L.V.; Sankova, T.P.; Magazenkova, D.N.; Skomorokhova, E.A.; Orlov, I.A.; Sakhenberg, E.I.; Sosnin, I.M.; Al Farroukh, M.; Romanov, A.E.; Ilyechova, E.Y. Shape-Dependent Biological Activity of Spherical and Quasi-Spherical Silver Nanoparticles in E. coli, A549 Cells and Mice. Environ. Sci. Nano 2022, 9, 3581–3598. [Google Scholar] [CrossRef]
- Ilyechova, E.Y.; Skvortsov, A.N.; Zatulovsky, E.; Tsymbalenko, N.V.; Shavlovsky, M.M.; Broggini, M.; Puchkova, L.V. Experimental Switching of Copper Status in Laboratory Rodents. J. Trace Elem. Med. Biol. 2011, 25, 27–35. [Google Scholar] [CrossRef]
- Babich, P.S.; Skvortsov, A.N.; Rusconi, P.; Tsymbalenko, N.V.; Mutanen, M.; Puchkova, L.V.; Broggini, M. Non-Hepatic Tumors Change the Activity of Genes Encoding Copper Trafficking Proteins in the Liver. Cancer Biol. Ther. 2013, 14, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Rosenthal, D.T.; Bao, L.; Kleer, C.G.; Merajver, S.D. Antiangiogenic Tetrathiomolybdate Protects against Her2/Neu-Induced Breast Carcinoma by Hypoplastic Remodeling of the Mammary Gland. Clin. Cancer Res. 2009, 15, 7441–7446. [Google Scholar] [CrossRef] [Green Version]
- Kaneda, A.; Wakazono, K.; Tsukamoto, T.; Watanabe, N.; Yagi, Y.; Tatematsu, M.; Kaminishi, M.; Sugimura, T.; Ushijima, T. Lysyl Oxidase is a Tumor Suppressor Gene Inactivated by Methylation and Loss of Heterozygosity in Human Gastric Cancers. Cancer Res. 2004, 64, 6410–6415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vavilova, T.P.; Gusarova, I.N.; Koroleva, O.V.; Medvedev, A.E. The role of ceruloplasmin in the development of neoplastic processes. Review. Biomeditsinskaia Khimiia 2005, 51, 263–275. [Google Scholar] [PubMed]
- Harvey, L.J.; Ashton, K.; Hooper, L.; Casgrain, A.; Fairweather-Tait, S.J. Methods of Assessment of Copper Status in Humans: A Systematic Review. Am. J. Clin. Nutr. 2009, 89, 2009S–2024S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platonova, N.A.; Orlov, I.A.; Klotchenko, S.A.; Babich, V.S.; Ilyechova, E.Y.; Babich, P.S.; Garmai, Y.P.; Vasin, A.V.; Tsymbalenko, N.V.; Puchkova, L.V. Ceruloplasmin Gene Expression Profile Changes in the Rat Mammary Gland during Pregnancy, Lactation and Involution. J. Trace Elem. Med. Biol. 2017, 43, 126–134. [Google Scholar] [CrossRef]
- Maximchik, P.V.; Kulikov, A.V.; Zhivotovsky, B.D.; Gogvadze, V.G. Cellular Energetics as a Target for Tumor Cell Elimination. Biochemistry 2016, 81, 65–79. [Google Scholar] [CrossRef]
- Kim, B.E.; Nevitt, T.; Thiele, D.J. Mechanisms for Copper Acquisition, Distribution and Regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef]
- Meijers, W.C.; Maglione, M.; Bakker, S.J.L.; Oberhuber, R.; Kieneker, L.M.; de Jong, S.; Haubner, B.J.; Nagengast, W.B.; Lyon, A.R.; van der Vegt, B.; et al. Heart Failure Stimulates Tumor Growth by Circulating Factors. Circulation 2018, 138, 678–691. [Google Scholar] [CrossRef]
- Giurgea, N.; Constantinescu, M.I.; Stanciu, R.; Suciu, S.; Muresan, A. Ceruloplasmin—acute-phase reactant or endogenous antioxidant? The case of cardiovascular disease. Med. Sci. Monit. 2005, 11, RA48–RA51. [Google Scholar]
- Kim, H.; Son, H.Y.; Bailey, S.M.; Lee, J. Deletion of Hepatic Ctr1 Reveals Its Function in Copper Acquisition and Compensatory Mechanisms for Copper Homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G356–G364. [Google Scholar] [CrossRef] [Green Version]
- Ilyechova, E.Y.; Tsymbalenko, N.V.; Puchkova, L.V. The Role of Subcutaneous Adipose Tissue in Supporting the Copper Balance in Rats with a Chronic Deficiency in Holo-Ceruloplasmin. PLoS ONE 2017, 12, e0175214. [Google Scholar] [CrossRef] [Green Version]
- Bielli, P.; Calabrese, L. Structure to Function Relationships in Ceruloplasmin: A ‘Moonlighting’ Protein. Cell. Mol. Life Sci. 2002, 59, 1413–1427. [Google Scholar] [CrossRef]
- Das, S.; Sahoo, P.K. Ceruloplasmin, a Moonlighting Protein in Fish. Fish Shellfish. Immunol. 2018, 82, 460–468. [Google Scholar] [CrossRef]
- Zaitseva, I.; Zaitsev, V.; Card, G.; Moshkov, K.; Bax, B.; Ralph, A.; Lindley, P. The X-ray Structure of Human Serum Ceruloplasmin at 3.1 Angstrom: Nature of the Copper Centres. J. Biol. Inorg. Chem. 1996, 1, 15–23. [Google Scholar] [CrossRef]
- Card, G.L.; Zaitsev, V.N.; Lindley, P.F. X-ray Crystal Structure of Human Ceruloplasmin at 3.0 Angstroms. PDB. 2011. Available online: https://www.wwpdb.org/pdb?id=pdb_00001kcw (accessed on 10 January 2023).
- Zatulovskiy, E.; Samsonov, S.; Skvortsov, A. Docking Study on Mammalian CTR1 Copper Importer Motifs. BMC Syst. Biol. 2007, 1, 54. [Google Scholar] [CrossRef] [Green Version]
- Ramos, D.; Mar, D.; Ishida, M.; Vargas, R.; Gaite, M.; Montgomery, A.; Linder, M.C. Mechanism of Copper Uptake from Blood Plasma Ceruloplasmin by Mammalian Cells. PLoS ONE 2016, 11, e0149516. [Google Scholar] [CrossRef] [Green Version]
- Besold, A.N.; Shanbhag, V.; Petris, M.J.; Culotta, V.C. Ceruloplasmin as a Source of Cu for a Fungal Pathogen. J. Inorg. Biochem. 2021, 219, 111424. [Google Scholar] [CrossRef]












| AgNPs IP | AgNPs IT | aEAC | sEAC | |
|---|---|---|---|---|
Group 1 | − | − | − | − |
Group 2 | + | − | − | − |
Group 3 | − | − | + | − |
Group 4 | + | − | + | − |
Group 5 | − | − | − | + |
Group 6 | + | − | − | + |
Group 7 | − | + | − | − |
Group 8 | − | + | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magazenkova, D.N.; Skomorokhova, E.A.; Farroukh, M.A.; Zharkova, M.S.; Jassem, Z.M.; Rekina, V.E.; Shamova, O.V.; Puchkova, L.V.; Ilyechova, E.Y. Influence of Silver Nanoparticles on the Growth of Ascitic and Solid Ehrlich Adenocarcinoma: Focus on Copper Metabolism. Pharmaceutics 2023, 15, 1099. https://doi.org/10.3390/pharmaceutics15041099
Magazenkova DN, Skomorokhova EA, Farroukh MA, Zharkova MS, Jassem ZM, Rekina VE, Shamova OV, Puchkova LV, Ilyechova EY. Influence of Silver Nanoparticles on the Growth of Ascitic and Solid Ehrlich Adenocarcinoma: Focus on Copper Metabolism. Pharmaceutics. 2023; 15(4):1099. https://doi.org/10.3390/pharmaceutics15041099
Chicago/Turabian StyleMagazenkova, Daria N., Ekaterina A. Skomorokhova, Mohammad Al Farroukh, Maria S. Zharkova, Zena M. Jassem, Valeria E. Rekina, Olga V. Shamova, Ludmila V. Puchkova, and Ekaterina Y. Ilyechova. 2023. "Influence of Silver Nanoparticles on the Growth of Ascitic and Solid Ehrlich Adenocarcinoma: Focus on Copper Metabolism" Pharmaceutics 15, no. 4: 1099. https://doi.org/10.3390/pharmaceutics15041099