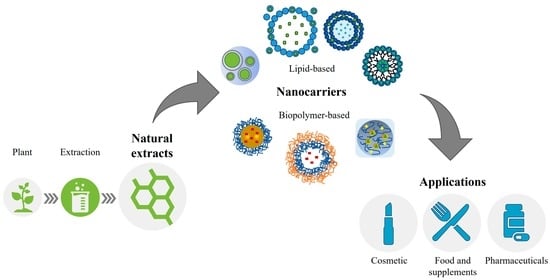
Biopolymer- and Lipid-Based Carriers for the Delivery of Plant-Based Ingredients
Abstract
:1. Introduction
2. Methods
3. Limitations in Using Plant-Derived Bioactive Molecules: The Reasons behind Encapsulation
4. Biopolymer-Based Encapsulation of Plant Extracts and Natural Ingredients
4.1. Formulation of Biopolymeric Nanoparticles
4.2. Fabrication of Biopolymeric Nanoparticles
4.3. Protein Nanoparticles: Stabilization under Different Environmental Conditions and through Complexation with Polysaccharides
4.3.1. Effect of pH
4.3.2. Effect of Ionic Strength
4.3.3. Effect of Temperature
4.3.4. Effect of Digestive Enzymes
- pH-sensitive coatings, such as polymers that undergo a conformational change at specific pH values, can be used to modify protein-based nanoparticles. These coatings can protect the nanoparticles from degradation by enzymes in the gastrointestinal tract and allow for the controlled release of the encapsulated materials in specific regions of the gastrointestinal tract. For example, in the stomach, the pH is acidic, while the pH is neutral in the small intestine; by using pH-sensitive coatings, nanoparticles can be designed to release the encapsulated material in the small intestine when the pH changes. For example, chitosan, characterized by excellent biocompatibility, non-toxicity, and mucoadhesive properties [94], can be chemically modified (e.g., via succinylation of the amino groups) such that it can be used to control the pharmaceutical potentialities released at varying pH levels. In this scenario, a novel pH-sensitive polymeric nanoparticle was proposed, with a core–shell-corona morphology using succinyl chitosan (SCS) and alginate (ALG) as a suitable nanocarrier for oral quercetin (1 mg/mL) delivery. As a result, the sphere-shaped nanoformulations, with a mean particle size ranging between 92 and 310 nm, were able to exert a pH-sensitive controlled release of quercetin following a non-Fickian anomalous trend in both in vitro and in vivo studies [95]. Other polymers can be rendered pH-sensitive through chemical modification or grafting with specific ligands, as reported for starch [96], alginate [97], and pectins [98].
- Enzyme inhibitors such as trypsin inhibitors can be added to protein-based nanoparticles to protect them from degradation by enzymes in the gastrointestinal tract [99] and thus contribute to improving the stability and efficacy of nanoparticles.
- Targeting moieties, such as antibodies or peptides, can be added to protein-based nanoparticles, with an 118 nm diameter, a polydispersity index of 0.37, −38.26 mV (at neutral pH), and 95% incorporation efficiency, to target specific cells or tissues in the body [100].
- Surface modification, such as the use of polyethylene glycol (PEG) or polyethyleneimine (PEI), can be used to modify protein-based nanoparticles. These modifications can help improve the stability and circulation time of nanoparticles in the body and reduce the potential toxicity of the nanoparticles [101,102].
- Combinations of different strategies, such as the use of pH-sensitive coatings and enzyme inhibitors, can be used to enhance the stability and efficacy of protein-based nanoparticles.
4.4. Stabilization through Protein/Polysaccharide Association
5. Lipid-Based Nano-Systems
5.1. Colloidal Emulsions
5.2. Solid-Lipid Nanoparticles and Nanostructured Lipid Carriers
5.3. Liposomes
5.4. Phytosomes
5.5. Niosomes
6. Combination of Nanoparticles and Emulsions: The Pickering Emulsions
7. Examples of Application of Plant Extracts and Bioactive Compounds Encapsulated in Colloidal Carriers in Foodstuff
8. Challenges of and Limitations to the Use of Biopolymeric and Lipid-Based Nanocarriers
9. Conclusions and Perspectives
- The development of new and improved biopolymers that are more effective at delivering plant-based ingredients and offer improved stability or better biocompatibility;
- The optimization of lipid-based carriers, such as liposomes and solid-lipid nanoparticles, to improve their ability to deliver plant-based ingredients through, e.g., the modification of the composition of the lipids or via novel processing methods for the better control of the size and shape of the particles;
- The combination of biopolymers and lipids to create hybrid carrier systems that have the advantages of both types of carriers;
- The exploration of different plant-based extracts, rather than specific plant-based ingredients, to exploit the potential contributions of unrefined extracts’ constituents (e.g., polysaccharides, proteins, and lipids) to stabilize, as in the original plant material, their bioactive ingredients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Vinha, A.F.; Rodrigues, F.; Nunes, M.A.; Oliveira, M.B.P.P. Natural pigments and colorants in foods and beverages. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 363–391. [Google Scholar] [CrossRef]
- Almeida, T.P.; Ramos, A.A.; Ferreira, J.; Azqueta, A.; Rocha, E. Bioactive Compounds from Seaweed with Anti-Leukemic Activity: A Mini-Review on Carotenoids and Phlorotannins. Mini-Rev. Med. Chem. 2020, 20, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of polyphenol-loaded nanoparticles in food industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef] [Green Version]
- Mosaddik, A.; Ravinayagam, V.; Elaanthikkal, S.; Fessi, H.; Badri, W.; Elaissari, A. Development and use of polymeric nanoparticles for the encapsulation and administration of plant extracts. In Natural Products as Source of Molecules with Therapeutic Potential: Research and Development, Challenges and Perspectives; Filho, V.C., Ed.; Springer: Cham, Switherland, 2018; pp. 391–463. [Google Scholar] [CrossRef]
- Gali, L.; Bedjou, F.; Ferrari, G.; Donsì, F. Formulation and characterization of zein/gum arabic nanoparticles for the encapsulation of a rutin-rich extract from Ruta chalepensis L. Food Chem. 2021, 367, 129982. [Google Scholar] [CrossRef]
- Leiva-Vega, J.; Villalobos-Carvajal, R.; Ferrari, G.; Donsì, F.; Zúñiga, R.N.; Shene, C.; Beldarraín-Iznaga, T. Influence of interfacial structure on physical stability and antioxidant activity of curcumin multilayer emulsions. Food Bioprod. Process. 2020, 121, 65–75. [Google Scholar] [CrossRef]
- Mauriello, E.; Ferrari, G.; Donsì, F. Effect of formulation on properties, stability, carvacrol release and antimicrobial activity of carvacrol emulsions. Colloids Surf. B Biointerfaces 2020, 197, 111424. [Google Scholar] [CrossRef]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Campos, F.; Peixoto, A.F.; Fernandes, P.A.R.; Coimbra, M.A.; Mateus, N.; de Freitas, V.; Fernandes, I.; Fernandes, A. The Antidiabetic Effect of Grape Pomace Polysaccharide-Polyphenol Complexes. Nutrients 2021, 13, 4495. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy: Fundamentals, Applications and Strategy; Academic Press: Cambridge, MA, USA, 2017; pp. 233–266. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.; Giri, A. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Ferrari, G. Changing the Vision in Smart Food Design Utilizing the Next Generation of Nanometric Delivery Systems for Bioactive Compounds. Foods 2020, 9, 1100. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Król-Kilińska, Z.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V.; Donsì, F. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2020, 38, 1735–1790. [Google Scholar] [CrossRef]
- Hu, K.; McClements, D.J. Fabrication of biopolymer nanoparticles by antisolvent precipitation and electrostatic deposition: Zein-alginate core/shell nanoparticles. Food Hydrocoll. 2014, 44, 101–108. [Google Scholar] [CrossRef]
- Armendariz-Barragan, B.; Zafar, N.; Badri, W.; Galindo-Rodríguez, S.A.; Kabbaj, D.; Fessi, H.; Elaissari, A. Plant extracts: From encapsulation to application. Expert Opin. Drug Deliv. 2016, 13, 1165–1175. [Google Scholar] [CrossRef]
- Schillaci, C.; Nepravishta, R.; Bellomaria, A. Antioxidants in food and pharmaceutical research. Albanian J. Pharm. Sci. 2014, 1, 9–15. [Google Scholar]
- Zhong, Y.; Shahidi, F. Lipophilized Epigallocatechin Gallate (EGCG) Derivatives as Novel Antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef]
- Danihelova, M.; Viskupicova, J.; Šturdík, E. Lipophilization of flavonoids for their food, therapeutic and cosmetic applications. Acta Chim. Slovaca 2012, 5, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Perera, N.; Ambigaipalan, P.; Shahidi, F. Epigallocatechin gallate (EGCG) esters with different chain lengths fatty acids and their antioxidant activity in food and biological systems. J. Food Bioact. 2018, 1, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A translational perspective. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2023–2050. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Flanagan, J.; Matia-Merino, L.; Awati, A.; Omri, A.; Suntres, Z.E.; Singh, H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J. Sci. Food Agric. 2006, 86, 2038–2045. [Google Scholar] [CrossRef]
- Chang, C.-C.; Liu, D.-Z.; Lin, S.-Y.; Liang, H.-J.; Hou, W.-C.; Huang, W.-J.; Chang, C.-H.; Ho, F.-M.; Liang, Y.-C. Liposome encapsulation reduces cantharidin toxicity. Food Chem. Toxicol. 2008, 46, 3116–3121. [Google Scholar] [CrossRef]
- Alexander, A.; Ajazuddin; Patel, R.J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef]
- Falcão, D.Q.; Oliveira, A.P.; Lima, B.G.; Cardoso, A.C.A.; Almeida, K.B.; Santos, T.C.; Nascimento, L.M.; Desmarais, G.C.; Sanches, P.S.; Araújo, E.M.; et al. Nanotechnology in phytotherapy: Current challenges of lipid-based nanocarriers for the delivery of natural products. In Lipid Nanocarriers for Drug Targeting; Grumezescu, A.M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; pp. 139–174. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, X.; Liu, H.; Tian, J.; Yang, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. Antibacterial peptide encapsulation and sustained release from chitosan-based delivery system. Eur. Polym. J. 2022, 181, 111640. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Donsì, F.; McClements, D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, A.; Jafari, S.M.; Assadpour, E.; Esfanjani, A.F. Nano-encapsulation of olive leaf phenolic compounds through WPC-pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 2015, 82, 816–822. [Google Scholar] [CrossRef]
- Umerska, A.; Gaucher, C.; Oyarzun-Ampuero, F.; Fries-Raeth, I.; Colin, F.; Villamizar-Sarmiento, M.G.; Maincent, P.; Sapin-Minet, A. Polymeric Nanoparticles for Increasing Oral Bioavailability of Curcumin. Antioxidants 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, N.Ć.; Šavikin, K.; Bigović, D.; Trifković, K.; Đorđević, V.; Bugarski, B. Potential of encapsulated phytochemicals in hydrogel particles. In Nanomaterials for Drug Delivery and Therapy; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 305–342. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Shanmugam, R.; Lee, J.-K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef]
- Prakash, P.; Lee, W.-H.; Loo, C.-Y.; Wong, H.S.J.; Parumasivam, T. Advances in Polyhydroxyalkanoate Nanocarriers for Effective Drug Delivery: An Overview and Challenges. Nanomaterials 2022, 12, 175. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of Natural Polymer Gum Arabic: A Review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Dai, L.; Li, R.; Wei, Y.; Sun, C.; Mao, L.; Gao, Y. Fabrication of zein and rhamnolipid complex nanoparticles to enhance the stability and in vitro release of curcumin. Food Hydrocoll. 2018, 77, 617–628. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Rodríguez-Felix, F.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Robles-García, M.A.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-polysaccharide nanoparticles as matrices for antioxidant compounds: A strategy for prevention of chronic degenerative diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef]
- Donsì, F.; Voudouris, P.; Veen, S.J.; Velikov, K.P. Zein-based colloidal particles for encapsulation and delivery of epigallocatechin gallate. Food Hydrocoll. 2017, 63, 508–517. [Google Scholar] [CrossRef]
- Fernandez-Avila, C.; Hebishy, E.; Donsì, F.; Arranz, E.; Trujillo, A.J. Chapter Six—Production of food bioactive-loaded nanostructures by high-pressure homogenization. In Nanoencapsulation in the Food Industry; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 251–340. [Google Scholar] [CrossRef]
- Donsì, F.; Sessa, M.; Ferrari, G. Encapsulation of Bioactive Compounds. In Handbook of Encapsulation and Controlled Release; Mishra, M., Ed.; CRC Press Book: Boca Raton, FL, USA, 2015; pp. 765–799. [Google Scholar]
- Assadpour, E.; Jafari, S.M. An overview of biopolymer nanostructures for encapsulation of food ingredients. Nanoencapsulation Food Ind. 2019, 1, 1–35. [Google Scholar] [CrossRef]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Kovačević, D.B.; Putnik, P.; Donsì, F.; Barba, F.J.; Jambrak, A.R. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Liu, C.; Zhu, J.; Fan, M.; Sun, X.; Wang, T.; Xu, Y.; Cao, Y. Fabrication of stable zein nanoparticles coated with soluble soybean polysaccharide for encapsulation of quercetin. Food Hydrocoll. 2018, 87, 342–351. [Google Scholar] [CrossRef]
- Lima, A.C.; Sher, P.; Mano, J.F. Production methodologies of polymeric and hydrogel particles for drug delivery applications. Expert Opin. Drug Deliv. 2012, 9, 231–248. [Google Scholar] [CrossRef]
- Villalva, D.G.; França, C.G.; Loh, W. Characterization of cubosomes immobilized in hydrogels of hyaluronic acid and their use for diclofenac controlled delivery. Colloids Surf. B Biointerf. 2022, 212, 112352. [Google Scholar] [CrossRef]
- Wen, J.; Galloni, M.; Al, Y.N. Filled hydrogel particles. In Emulsion-Based Systems for Delivery of Food Active Compounds: Formation, Application, Health and Safety; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 161–180. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, Z.; Zhang, G. Delivery of Bioactive Conjugated Linoleic Acid with Self-Assembled Amylose—CLA Complex. J. Agric. Food Chem. 2009, 57, 7125–7130. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.R.; Quintans, J.D.S.S.; Gandhi, G.R.; Araújo, A.A.D.S.; Júnior, L.J.Q. The use of cyclodextrin inclusion complexes to improve anticancer drug profiles: A systematic review. Expert Opin. Drug Deliv. 2020, 17, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Hamman, J.H. Chitosan Based Polyelectrolyte Complexes as Potential Carrier Materials in Drug Delivery Systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [Green Version]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2018, 121, 1276–1286. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Dos Santos, L.P.; Caon, T.; Battisti, M.A.; da Silva, C.H.B.; Simões, C.M.O.; Reginatto, F.H.; de Campos, A.M. Antioxidant polymeric nanoparticles containing standardized extract of Ilex paraguariensis A. St.-Hil. for topical use. Ind. Crop. Prod. 2017, 108, 738–747. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Chen, Z.; Wang, T.; Wang, L.; Zhong, Q. Biological macromolecule delivery system fabricated using zein and gum arabic to control the release rate of encapsulated tocopherol during in vitro digestion. Food Res. Int. 2018, 114, 251–257. [Google Scholar] [CrossRef]
- Gagliardi, A.; Paolino, D.; Costa, N.; Fresta, M.; Cosco, D. Zein- vs. PLGA-based nanoparticles containing rutin: A comparative investigation. Mater. Sci. Eng. C 2020, 118, 111538. [Google Scholar] [CrossRef]
- Pereira, F.; Baptista, R.; Ladeiras, D.; Madureira, A.M.; Teixeira, G.; Rosado, C.; Fernandes, A.S.; Ascensão, L.; Silva, C.O.; Reis, C.P.; et al. Production and characterization of nanoparticles containing methanol extracts of Portuguese Lavenders. Measurement 2015, 74, 170–177. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Cyclodextrin encapsulated catechin: Effect of pH, relative humidity and various food models on antioxidant stability. LWT-Food Sci. Technol. 2017, 85, 232–239. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion Complexes of Lycopene and β-Cyclodextrin: Preparation, Characterization, Stability and Antioxidant Activity. Antioxidants 2019, 8, 314. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Celli, G.B.; Selig, M.J.; Abbaspourrad, A. Catechin modulates the copigmentation and encapsulation of anthocyanins in polyelectrolyte complexes (PECs) for natural colorant stabilization. Food Chem. 2018, 264, 342–349. [Google Scholar] [CrossRef]
- Xu, W.; Huang, L.; Jin, W.; Ge, P.; Shah, B.R.; Zhu, D.; Jing, J. Encapsulation and release behavior of curcumin based on nanoemulsions-filled alginate hydrogel beads. Int. J. Biol. Macromol. 2019, 134, 210–215. [Google Scholar] [CrossRef]
- Outuki, P.M.; de Francisco, L.M.B.; Hoscheid, J.; Bonifácio, K.L.; Barbosa, D.S.; Cardoso, M.L.C. Development of arabic and xanthan gum microparticles loaded with an extract of Eschweilera nana Miers leaves with antioxidant capacity. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 103–112. [Google Scholar] [CrossRef]
- Torkamani, A.E.; Syahariza, Z.A.; Norziah, M.H.; Wan, A.K.M.; Juliano, P. Encapsulation of polyphenolic antioxidants obtained from Momordica charantia fruit within zein/gelatin shell core fibers via coaxial electrospinning. Food Biosci. 2018, 21, 60–71. [Google Scholar] [CrossRef]
- Verma, M.L.; Dhanya, B.; Sukriti; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and protein based biopolymeric nanoparticles: Current status and biotechnological applications. Int. J. Biol. Macromol. 2020, 154, 390–412. [Google Scholar] [CrossRef]
- Cabral, B.R.P.; de Oliveira, P.M.; Gelfuso, G.M.; Quintão, T.D.S.C.; Chaker, J.A.; Karnikowski, M.G.D.O.; Gris, E.F. Improving stability of antioxidant compounds from Plinia cauliflora (jabuticaba) fruit peel extract by encapsulation in chitosan microparticles. J. Food Eng. 2018, 238, 195–201. [Google Scholar] [CrossRef]
- Lamoudi, L.; Chaumeil, J.-C.; Daoud, K. Effet des paramètres du procédé de microencapsulation du piroxicam par coacervation complexe. Ann. Pharm. Françaises 2015, 73, 37–42. [Google Scholar] [CrossRef]
- Donsì, F.; Sessa, M.; Ferrari, G. Nanometric-Size delivery systems for bioactive compounds for the nutraceutical and food industries. In Bio-Nanotechnology: A Revolution in Food, Biomedical and Health Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Rivas, C.J.M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Rodríguez, S.A.G.; Román, R.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Chavoshpour-Natanzi, Z.; Sahihi, M. Encapsulation of quercetin-loaded β-lactoglobulin for drug delivery using modified anti-solvent method. Food Hydrocoll. 2019, 96, 493–502. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Joye, I.J.; McClements, D.J. Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 1: Preparation and characterization. Food Hydrocoll. 2014, 45, 309–316. [Google Scholar] [CrossRef]
- Luz, P.P.; Magalhães, L.G.; Pereira, A.C.; Cunha, W.R.; Rodrigues, V.; E Silva, M.L.A. Curcumin-loaded into PLGA nanoparticles: Preparation and in vitro schistosomicidal activity. Parasitol. Res. 2011, 110, 593–598. [Google Scholar] [CrossRef]
- Peltonen, L.; Aitta, J.; Hyvönen, S.; Karjalainen, M.; Hirvonen, J. Improved Entrapment Efficiency of Hydrophilic Drug Substance During Nanoprecipitation of Poly(l)lactide Nanoparticles. AAPS PharmSciTech 2004, 5, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Bilati, U.; Allémann, E.; Doelker, E. Nanoprecipitation versus emulsion-based techniques for the encapsulation of proteins into biodegradable nanoparticles and process-related stability issues. AAPS PharmSciTech 2005, 6, E594–E604. [Google Scholar] [CrossRef] [Green Version]
- Saad, W.S.; Prud’Homme, R.K. Principles of nanoparticle formation by flash nanoprecipitation. Nano Today 2016, 11, 212–227. [Google Scholar] [CrossRef]
- Leung, M.H.M.; Shen, A.Q. Microfluidic Assisted Nanoprecipitation of PLGA Nanoparticles for Curcumin Delivery to Leukemia Jurkat Cells. Langmuir 2018, 34, 3961–3970. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practice and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Pujara, N.; Giri, R.; Wong, K.Y.; Qu, Z.; Rewatkar, P.; Moniruzzaman; Begun, J.; Ross, B.P.; McGuckin, M.; Popat, A. pH—Responsive colloidal carriers assembled from β-lactoglobulin and Epsilon poly-L-lysine for oral drug delivery. J. Colloid Interface Sci. 2020, 589, 45–55. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef]
- Jiang, F.; Yang, L.; Wang, S.; Ying, X.; Ling, J.; Ouyang, X. Fabrication and characterization of zein-alginate oligosaccharide complex nanoparticles as delivery vehicles of curcumin. J. Mol. Liq. 2021, 342, 116937. [Google Scholar] [CrossRef]
- Fang, Y.; Corredig, M. Whey protein nanoparticles prepared with desolvation with ethanol: Characterization, thermal stability and interfacial behavior. Food Hydrocoll. 2012, 29, 258–264. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2019, 99, 105334. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2009, 62, 3–11. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Maity, S.; Mandal, S.; Chakraborti, A.S.; Prajapati, A.; Kundu, P. Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr. Polym. 2018, 182, 42–51. [Google Scholar] [CrossRef]
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L.T.; Pinzon, M.I.; Villa, C.C. Development of native and modified banana starch nanoparticles as vehicles for curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Anila, M.M.; Franklin, S. Synthesis characterization and biological evaluation of alginate nanoparticle for the targeted delivery of curcumin. Mater. Sci. Eng. C 2017, 78, 1125–1134. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, K.; Li, X.; Xiao, S.; Zheng, D.; Zhu, P.; Li, C.; Liu, J.; He, J.; Lei, J.; et al. A novel self-assembled nanoparticle platform based on pectin-eight-arm polyethylene glycol-drug conjugates for co-delivery of anticancer drugs. Mater. Sci. Eng. C 2018, 86, 28–41. [Google Scholar] [CrossRef]
- Costa, R.O.D.A.; Matias, L.L.R.; Passos, T.S.; de Queiroz, J.L.C.; de Carvalho, F.M.C.; Maciel, B.L.L.; Uchôa, A.F.; Amado, I.R.; Gonçalves, C.; Pastrana, L.; et al. Safety and potential functionality of nanoparticles loaded with a trypsin inhibitor isolated from tamarind seeds. Futur. Foods 2020, 1–2, 100001. [Google Scholar] [CrossRef]
- Shargh, V.H.; Hondermarck, H.; Liang, M. Antibody-targeted biodegradable nanoparticles for cancer therapy. Nanomedicine 2016, 11, 63–79. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, Y.; Zhang, L.; Zhang, Y.; Liu, J.; Yu, P. Poly ethylene glycol (PEG)-Related controllable and sustainable antidiabetic drug delivery systems. Eur. J. Med. Chem. 2021, 217, 113372. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.-J.; Yu, H.-Y.; Hsia, J.-C.; Chen, Y.-H.; Hung, T.-T.; Chao, H.-M.; Chern, E.; Huang, Y.-Y. Highly Efficient Intracellular Protein Delivery by Cationic Polyethyleneimine-Modified Gelatin Nanoparticles. Materials 2018, 11, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Yang, Y.; Ju, X.; Udenigwe, C.C.; He, R. Polyelectrolyte Complex Nanoparticles from Chitosan and Acylated Rapeseed Cruciferin Protein for Curcumin Delivery. J. Agric. Food Chem. 2018, 66, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Lall, A.; Tamo, A.K.; Doench, I.; David, L.; De Oliveira, P.N.; Gorzelanny, C.; Osorio-Madrazo, A. Nanoparticles and Colloidal Hydrogels of Chitosan–Caseinate Polyelectrolyte Complexes for Drug-Controlled Release Applications. Int. J. Mol. Sci. 2020, 21, 5602. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Zou, Y.; Liang, X.; Peng, Y.; McClements, D.J.; Hu, K. Encapsulation of resveratrol in zein/pectin core-shell nanoparticles: Stability, bioaccessibility, and antioxidant capacity after simulated gastrointestinal digestion. Food Hydrocoll. 2019, 93, 261–269. [Google Scholar] [CrossRef]
- Aiqian, Y.; Flanagan, J.; Harjinder, S. Formation of Stable Nanoparticles via Electrostatic Complexation Between Sodium Caseinate and Gum Arabic. Biopolymers 2006, 82, 121–133. [Google Scholar] [CrossRef]
- Wu, W.; Kong, X.; Zhang, C.; Hua, Y.; Chen, Y. Improving the stability of wheat gliadin nanoparticles—Effect of gum arabic addition. Food Hydrocoll. 2018, 80, 78–87. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, Y.; Luo, S.; Mu, D.; Li, X.; Zhong, X.; Jiang, S.; Zheng, Z. Encapsulation of curcumin in soluble soybean polysaccharide-coated gliadin nanoparticles: Interaction, stability, antioxidant capacity, and bioaccessibility. J. Sci. Food Agric. 2022, 102, 5121–5131. [Google Scholar] [CrossRef]
- Fathi, M.; Mozafari, M.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.; Neumann, D.; Lamprecht, A. Lipid nanocapsules for dermal application: A comparative study of lipid-based versus polymer-based nanocarriers. Eur. J. Pharm. Biopharm. 2011, 79, 36–42. [Google Scholar] [CrossRef]
- Rao, S.; Prestidge, C.A. Polymer-lipid hybrid systems: Merging the benefits of polymeric and lipid-based nanocarriers to improve oral drug delivery. Expert Opin. Drug Deliv. 2016, 13, 691–707. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Donsì, F.; Velikov, K.P. Encapsulation of food ingredients by single O/W and W/O nanoemulsions. In Lipid-Based Nanostructures for Food Encapsulation Purposes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 37–87. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef] [Green Version]
- Donsì, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Sanhueza, L.; García, P.; Giménez, B.; Benito, J.M.; Matos, M.; Gutiérrez, G. Encapsulation of Pomegranate Peel Extract (Punica granatum L.) by Double Emulsions: Effect of the Encapsulation Method and Oil Phase. Foods 2022, 11, 310. [Google Scholar] [CrossRef]
- Jayari, A.; Donsì, F.; Ferrari, G.; Maaroufi, A. Nanoencapsulation of Thyme Essential Oils: Formulation, Characterization, Storage Stability, and Biological Activity. Foods 2022, 11, 1858. [Google Scholar] [CrossRef]
- Ganesan, P.; Narayanasamy, D. Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain. Chem. Pharm. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Panigrahi, S.S.; Syed, I.; Sivabalan, S.; Sarkar, P. Nanoencapsulation strategies for lipid-soluble vitamins. Chem. Pap. 2018, 73, 1–16. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Nemati, S.; Rahimi, H.M.; Hesari, Z.; Sharifdini, M.; Aghdam, N.J.; Mirjalali, H.; Zali, M.R. Formulation of Neem oil-loaded solid lipid nanoparticles and evaluation of its anti-Toxoplasma activity. BMC Complement. Med. Ther. 2022, 22, 122. [Google Scholar] [CrossRef]
- Sabapati, M.; Palei, N.N.; Ashok Kumar, C.K.; Molakpogu, R.B. Solid lipid nanoparticles of Annona muricata fruit extract: Formulation, optimization and in vitro cytotoxicity studies. Drug Dev. Ind. Pharm. 2019, 45, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Khezri, K.; Farahpour, M.R.; Rad, S.M. Efficacy of Mentha pulegium essential oil encapsulated into nanostructured lipid carriers as an in vitro antibacterial and infected wound healing agent. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124414. [Google Scholar] [CrossRef]
- Karimi, N.; Ghanbarzadeh, B.; Hamishehkar, H.; Mehramuz, B.; Kafil, H.S. Antioxidant, Antimicrobial and Physicochemical Properties of Turmeric Extract-Loaded Nanostructured Lipid Carrier (NLC). Colloid Interface Sci. Commun. 2018, 22, 18–24. [Google Scholar] [CrossRef]
- Garg, T.; Goyal, A.K. Liposomes: Targeted and Controlled Delivery System. Drug Deliv. Lett. 2014, 4, 62–71. [Google Scholar] [CrossRef]
- Milani, D.; Athiyah, U.; Hariyadi, D.M.; Pathak, Y.V. Surface Modifications of Liposomes for Drug Targeting. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, Y.V., Ed.; Springer: Cham, Switzerland, 2019; pp. 207–220. [Google Scholar] [CrossRef]
- Gorjian, H.; Amiri, Z.R.; Milani, J.M.; Khaligh, N.G. Preparation and characterization of the encapsulated myrtle extract nanoliposome and nanoniosome without using cholesterol and toxic organic solvents: A comparative study. Food Chem. 2020, 342, 128342. [Google Scholar] [CrossRef]
- Ng, Z.Y.; Wong, J.-Y.; Panneerselvam, J.; Madheswaran, T.; Kumar, P.; Pillay, V.; Hsu, A.; Hansbro, N.; Bebawy, M.; Wark, P.; et al. Assessing the potential of liposomes loaded with curcumin as a therapeutic intervention in asthma. Colloids Surf. B Biointerf. 2018, 172, 51–59. [Google Scholar] [CrossRef]
- Ajazuddin; Saraf, S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar] [CrossRef]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef]
- Wanjiru, J.; Gathirwa, J.; Sauli, E.; Swai, H.S. Formulation, Optimization, and Evaluation of Moringa oleifera Leaf Polyphenol-Loaded Phytosome Delivery System against Breast Cancer Cell Lines. Molecules 2022, 27, 4430. [Google Scholar] [CrossRef]
- Murugesan, M.P.; Ratnam, M.V.; Mengitsu, Y.; Kandasamy, K. Evaluation of anti-cancer activity of phytosomes formulated from aloe vera extract. Mater. Today Proc. 2020, 42, 631–636. [Google Scholar] [CrossRef]
- Rabbani, M.; Pezeshki, A.; Ahmadi, R.; Mohammadi, M.; Tabibiazar, M.; Azar, F.A.N.; Ghorbani, M. Phytosomal nanocarriers for encapsulation and delivery of resveratrol- Preparation, characterization, and application in mayonnaise. LWT 2021, 151, 112093. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Thabet, Y.; Elsabahy, M.; Eissa, N.G. Methods for preparation of niosomes: A focus on thin-film hydration method. Methods 2021, 199, 9–15. [Google Scholar] [CrossRef]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef]
- Elmowafy, E.; El-Derany, M.O.; Biondo, F.; Tiboni, M.; Casettari, L.; Soliman, M.E. Quercetin Loaded Monolaurate Sugar Esters-Based Niosomes: Sustained Release and Mutual Antioxidant—Hepatoprotective Interplay. Pharmaceutics 2020, 12, 143. [Google Scholar] [CrossRef] [Green Version]
- Vilela, J.D.M.V.; Moghassemi, S.; Dadashzadeh, A.; Dolmans, M.-M.; Azevedo, R.B.; Amorim, C.A. Safety of Lavender Oil-Loaded Niosomes for In Vitro Culture and Biomedical Applications. Nanomaterials 2022, 12, 1999. [Google Scholar] [CrossRef]
- Poorani, V.; Vigneswaran; Kumar, G.V. Nano-Niosomal Formulation of Alkaloids from Vinca rosea for Improved Oral Delivery. J. Pharm. Med. Res. 2020, 5, 102–105. [Google Scholar] [CrossRef]
- Al Saqr, A.; Annaji, M.; Poudel, I.; Rangari, S.; Boddu, S.H.S.; Tiwari, A.K.; Babu, R.J. Niosomal formulation of hydroxytyrosol, a polyphenolic antioxidant, for enhancing transdermal delivery across human cadaver skin. Pharm. Dev. Technol. 2022, 27, 155–163. [Google Scholar] [CrossRef]
- Chaari, M.; Theochari, I.; Papadimitriou, V.; Xenakis, A.; Ammar, E. Encapsulation of carotenoids extracted from halophilic Archaea in oil-in-water (O/W) micro- and nano-emulsions. Colloids Surf. B Biointerfaces 2018, 161, 219–227. [Google Scholar] [CrossRef]
- Weigel, F.; Weiss, J.; Decker, E.A.; McClements, D.J. Lutein-enriched emulsion-based delivery systems: Influence of emulsifiers and antioxidants on physical and chemical stability. Food Chem. 2018, 242, 395–403. [Google Scholar] [CrossRef]
- Nishad, J.; Dutta, A.; Saha, S.; Rudra, S.G.; Varghese, E.; Sharma, R.; Tomar, M.; Kumar, M.; Kaur, C. Ultrasound-assisted development of stable grapefruit peel polyphenolic nano-emulsion: Optimization and application in improving oxidative stability of mustard oil. Food Chem. 2020, 334, 127561. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Argin, S.; Ozilgen, M.; McClements, D.J. Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural biopolymers: Whey protein isolate and gum arabic. Food Chem. 2015, 188, 256–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharat, M.; Skrzynski, M.; Decker, E.A.; McClements, D.J. Enhancement of chemical stability of curcumin-enriched oil-in-water emulsions: Impact of antioxidant type and concentration. Food Chem. 2020, 320, 126653. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ju, X.; Aluko, R.E.; Zou, Y.; Wang, Z.; Liu, M.; He, R. Rice bran protein-based nanoemulsion carrier for improving stability and bioavailability of quercetin. Food Hydrocoll. 2020, 108, 106042. [Google Scholar] [CrossRef]
- Zardini, A.A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2017, 55, 287–298. [Google Scholar] [CrossRef]
- Shtay, R.; Keppler, J.K.; Schrader, K.; Schwarz, K. Encapsulation of (─)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J. Food Eng. 2018, 244, 91–100. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Souza, C.R.; Oliveira, W.P. Encapsulation of eugenol rich clove extract in solid lipid carriers. J. Food Eng. 2014, 127, 34–42. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: Preparation and in vitro characterization studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Moreno-Sastre, M.; López-Méndez, T.B.; Gainza, E.; Pedraz, J.L. Encapsulation of Oleuropein in Nanostructured Lipid Carriers: Biocompatibility and Antioxidant Efficacy in Lung Epithelial Cells. Pharmaceutics 2020, 12, 429. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, R.; Liu, L.; Chi, J.; Huang, F.; Dong, L.; Ma, Q.; Jia, X.; Zhang, M. Preparation, stability and antioxidant capacity of nano liposomes loaded with procyandins from lychee pericarp. J. Food Eng. 2020, 284, 110065. [Google Scholar] [CrossRef]
- Ben-Fadhel, Y.; Maherani, B.; Salmieri, S.; Lacroix, M. Preparation and characterization of natural extracts-loaded food grade nanoliposomes. LWT 2021, 154, 112781. [Google Scholar] [CrossRef]
- Vu, H.T.; Hook, S.M.; Siqueira, S.D.; Müllertz, A.; Rades, T.; McDowell, A. Are phytosomes a superior nanodelivery system for the antioxidant rutin? Int. J. Pharm. 2018, 548, 82–91. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Phosphatidylcholine-rutin complex as a potential nanocarrier for food applications. J. Funct. Foods 2017, 33, 134–141. [Google Scholar] [CrossRef]
- Huang, Z.; Brennan, C.S.; Zhao, H.; Liu, J.; Guan, W.; Mohan, M.S.; Stipkovits, L.; Zheng, H.; Kulasiri, D. Fabrication and assessment of milk phospholipid-complexed antioxidant phytosomes with vitamin C and E: A comparison with liposomes. Food Chem. 2020, 324, 126837. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, Q.; Liu, T.; Wusigale; Liang, L. Co-encapsulation of α-tocopherol and resveratrol in oil-in-water emulsion stabilized by sodium caseinate: Impact of polysaccharide on the stability and bioaccessibility. J. Food Eng. 2019, 264, 109685. [Google Scholar] [CrossRef]
- Linke, C.; Drusch, S. Pickering emulsions in foods—Opportunities and limitations. Crit. Rev. Food Sci. Nutr. 2017, 58, 1971–1985. [Google Scholar] [CrossRef]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent advances of characterization techniques for the formation, physical properties and stability of Pickering emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef]
- Wu, J.; Shi, M.; Li, W.; Zhao, L.; Wang, Z.; Yan, X.; Norde, W.; Li, Y. Pickering emulsions stabilized by whey protein nanoparticles prepared by thermal cross-linking. Colloids Surf. B Biointerf. 2015, 127, 96–104. [Google Scholar] [CrossRef]
- Schröder, A.; Corstens, M.N.; Ho, K.K.H.Y.; Schroën, K. Pickering Emulsions. In Emulsion-Based Systems for Delivery of Food Active Compounds: Formation, Application, Health and Safety; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 29–67. [Google Scholar] [CrossRef]
- Pirozzi, A.; Capuano, R.; Avolio, R.; Gentile, G.; Ferrari, G.; Donsì, F. O/W Pickering Emulsions Stabilized with Cellulose Nanofibrils Produced through Different Mechanical Treatments. Foods 2021, 10, 1886. [Google Scholar] [CrossRef]
- Hunter, S.J.; Cornel, E.J.; Mykhaylyk, O.O.; Armes, S.P. Effect of Salt on the Formation and Stability of Water-in-Oil Pickering Nanoemulsions Stabilized by Diblock Copolymer Nanoparticles. Langmuir 2020, 36, 15523–15535. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhou, D.; Mackie, A.; Yang, S.; Dai, L.; Zhang, L.; Mao, L.; Gao, Y. Stability, Interfacial Structure, and Gastrointestinal Digestion of β-Carotene-Loaded Pickering Emulsions Co-stabilized by Particles, a Biopolymer, and a Surfactant. J. Agric. Food Chem. 2021, 69, 1619–1636. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, W.; Zhang, Z.; Zhu, Y.; Wang, L.; Fu, J. A nanoparticle/oil double epigallocatechin gallate-loaded Pickering emulsion: Stable and delivery characteristics. LWT 2020, 130, 109369. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, Y.; Wu, Z.; Miao, J.; Gao, H.; Ma, L.; Zou, L.; Peng, S.; Liu, C.; Liu, W. Gliadin Nanoparticles Pickering Emulgels for β-Carotene Delivery: Effect of Particle Concentration on the Stability and Bioaccessibility. Molecules 2020, 25, 4188. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, H.; Zheng, T.; Liu, Q.; Zhu, J.; Huang, Q. Evaluation of Oral Bioaccessibility of Aged Citrus Peel Extracts Encapsulated in Different Lipid-Based Systems: A Comparison Study Using Different In Vitro Digestion Models. J. Agric. Food Chem. 2019, 68, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Doost, A.S.; Nasrabadi, M.N.; Kassozi, V.; Dewettinck, K.; Stevens, C.V.; Van der Meeren, P. Pickering stabilization of thymol through green emulsification using soluble fraction of almond gum—Whey protein isolate nano-complexes. Food Hydrocoll. 2018, 88, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Chen, F.; Gao, C.; Zhang, Y.; Tang, X. Environmental stability and curcumin release properties of Pickering emulsion stabilized by chitosan/gum arabic nanoparticles. Int. J. Biol. Macromol. 2020, 157, 202–211. [Google Scholar] [CrossRef]
- Estévez, M.; Güell, C.; De Lamo-Castellví, S.; Ferrando, M. Encapsulation of grape seed phenolic-rich extract within W/O/W emulsions stabilized with complexed biopolymers: Evaluation of their stability and release. Food Chem. 2018, 272, 478–487. [Google Scholar] [CrossRef]
- Zhang, B.; Lei, M.; Huang, W.; Liu, G.; Jiang, F.; Peng, D.; Huang, Q.; Jin, W. Improved Storage Properties and Cellular Uptake of Casticin-Loaded Nanoemulsions Stabilized by Whey Protein-Lactose Conjugate. Foods 2021, 10, 1640. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Erdagi, S.I.; Yildiz, U. Pickering emulsions stabilized nanocellulosic-based nanoparticles for coumarin and curcumin nanoencapsulations: In vitro release, anticancer and antimicrobial activities. Carbohydr. Polym. 2018, 201, 317–328. [Google Scholar] [CrossRef]
- Donsì, F. Applications of Nanoemulsions in Foods. In Nanoemulsions; Academic Press: Cambridge, MA, USA, 2018; pp. 349–377. [Google Scholar] [CrossRef]
- Nejatian, M.; Darabzadeh, N.; Bodbodak, S.; Saberian, H.; Rafiee, Z.; Kharazmi, M.S.; Jafari, S.M. Practical application of nanoencapsulated nutraceuticals in real food products; a systematic review. Adv. Colloid Interface Sci. 2022, 305, 102690. [Google Scholar] [CrossRef]
- Tripathy, S.; Verma, D.K.; Thakur, M.; Patel, A.R.; Srivastav, P.P.; Singh, S.; Chávez-González, M.L.; Aguilar, C.N. Encapsulated Food Products as a Strategy to Strengthen Immunity Against COVID-19. Front. Nutr. 2021, 8, 673174. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Tupuna-Yerovi, D.S.; González, Z.; Ruales, J. Encapsulation of bioactive compounds from fruit and vegetable by-products for food application—A review. Trends Food Sci. Technol. 2021, 116, 11–23. [Google Scholar] [CrossRef]
- Fathi, M.; Martín, A.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Ershadi, A.; Parastouei, K.; Khaneghah, A.; Hadian, Z.; Lorenzo, J. Encapsulation of Curcumin in Persian Gum Nanoparticles: An Assessment of Physicochemical, Sensory, and Nutritional Properties. Coatings 2021, 11, 841. [Google Scholar] [CrossRef]
- Fathi, M.; Vinceković, M.; Jurić, S.; Viskić, M.; Jambrak, A.R.; Donsì, F. Food-Grade Colloidal Systems for the Delivery of Essential Oils. Food Rev. Int. 2019, 37, 1–45. [Google Scholar] [CrossRef]
- Serna, C.M.B.; Dacanal, G.; Fernandes, A.; Pinho, S.C. Antifungal activity of nanoemulsions encapsulating oregano (Origanum vulgare) essential oil: In vitro study and application in Minas Padrão cheese. Braz. J. Microbiol. 2018, 49, 929–935. [Google Scholar] [CrossRef]
- Dima, I.G.; Aprodu, I.; Cîrciumaru, A.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Microencapsulation of lycopene from tomatoes peels by complex coacervation and freeze-drying: Evidences on phytochemical profile, stability and food applications. J. Food Eng. 2020, 288, 110166. [Google Scholar] [CrossRef]
- Waraho, T.; McClements, D.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- Gomes, A.; Costa, A.L.R.; Cardoso, D.D.; Náthia-Neves, G.; Meireles, M.A.A.; Cunha, R.L. Interactions of β-carotene with WPI/Tween 80 mixture and oil phase: Effect on the behavior of O/W emulsions during in vitro digestion. Food Chem. 2020, 341, 128155. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, X.; Chen, X. Polymer-Modified Liposomes for Drug Delivery: From Fundamentals to Applications. Pharmaceutics 2022, 14, 778. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Sobral, P.J.D.A. Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds. Molecules 2021, 27, 60. [Google Scholar] [CrossRef]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of active ingredients in polysaccharide–protein complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef]








| Antioxidant Agent | Biopolymer | Method | Particle Type | Purpose/Result | Reference | |
|---|---|---|---|---|---|---|
| Bottom-up approach | Curcumin (Curcuminoids) | PLGA, PCL, Eudragit® RLPO (ERL) | Single-emulsion solvent evaporation | Biopolymeric particles (245 nm diameter) | Increase oral bioavailability (91% released over 1 h) | [41] |
| Extract of Ilex Paraguariensis | Polycaprolactone (PCL) and PLGA | Double-emulsion solvent evaporation | Biopolymeric particles (150–160 nm average particle size) | Enhance skin permeation (80 μg cm−2 of skin permeated by chlorogenic acid) | [66] | |
| Epigallocatechin gallate (Flavanol) | Zein | Antisolvent precipitation | Biopolymeric particles (mean particle size was between 170 and 250 nm) | Sustain release during digestion (84% of epigallocatechin gallate released after 30 min) | [49] | |
| α-tocopherol (Vitamin) | Zein/Gum Arabic | Antisolvent precipitation | Core–shell particles (130–170 nm average particle size) | Controlled release during digestion (82% at 10 min, 90% at 30 min, and 97% at 240 min during intestinal digestion) | [67] | |
| Extract of Ruta chalepensis L. | Zein/Gum Arabic | Antisolvent precipitation | Polyelectrolyte complex (mean particle size was between 150 and 250 nm) | Increase in encapsulation efficiency (67%) and promotion of rutin release (of about 99% after the first 120 min in simulated gastric fluids) | [9] | |
| Rutin (Flavonol) | Zein or PLGA | Antisolvent precipitation | Biopolymeric particles (mean size of about 120 nm) | Controlled release (~88% of the bioactive compound retained by the nanosystems) | [68] | |
| Lavender extracts | PLGA | Spontaneous emulsification and desolvation | Biopolymeric particles (particle mean sizes 302 nm) | No measurable amounts of added extracts were detected during epidermal permeation and evaluation of in vitro cytotoxicity, indicating low permeation, low risk of toxicity, and increased stability in cosmetics | [69] | |
| Catechin (Flavanol) | β-cyclodextrin | Solvent evaporation | Inclusion complex | Stabilization and masking of the astringent taste in foods (60–90% of antioxidant retention in food models) | [70] | |
| Lycopene (Carotene) | β-cyclodextrin | Co-precipitation | Inclusion complex (~180 nm) | Preservation of lycopene activity (yield and the entrapment efficiency of the inclusion complexes were 83% and 72%, respectively) | [71] | |
| Anthocyanin and catechin (Flavonoids) | Chondroitin sulfate and chitosan | Self-assembly | Polyelectrolyte complex (particle size ranged between 400 and 600 nm) | Preservation of color and stability (70–80% anthocyanins remaining) | [72] | |
| Curcumin (Curcuminoids) | Tea oil seed/Sodium alginate | Ionic gelatinization | Filled hydrogel particles (particle size of 460 µm) | Improved bioavailability (equilibrium state reached after about 29 h with about 85% of curcumin released) | [73] | |
| Top-down approach | Extract of Eschweilera nana Miers leaves | Arabic gum and xanthan gum | Spray drying | Biopolymeric microparticles (~4 nm) | Increased solubility (95–98% of encapsulation efficiency), bioavailability, and stability (maximum rutin release of 84% over 8 h) | [74] |
| Extract of Momordica charantia fruit | Zein/gelatin | Coaxial electrospinning | Polyelectrolyte complex (core–shell fibers) (average fiber dimeter of about 311–380 nm) | Stabilization in supplement production (70% (FRAP) and 80% (RSA%) of initial antioxidant properties of the encapsulated extract were conserved over 105 days) | [75] | |
| Coffee grounds extracts | Gum Arabic, maltodextrin | Spray drying | Biopolymeric microparticles | Preservation of antioxidant activity | [76] | |
| Jabuticaba (Plinia cauliflora) fruit peel extract | Chitosan | Spray drying | Biopolymeric microparticles (mean diameter of ~9 μm) | Enhanced stability of the polyphenolic compounds during storage at both refrigerated and room temperature for 60 days (979% and 83%, respectively) for their use in food and cosmetic products | [77] |
| Lipid System | Antioxidant | Preparation Method | Purposes | Outcomes | References |
|---|---|---|---|---|---|
| Emulsions | Carotenoids from halophilic Archaea | High-pressure homogenization (emulsifiers: Triton X-100/Tween-80) | Improve solubility, stability, and bio-accessibility | The nano-emulsion’s diameter was about 180 nm greater than that of the microemulsion (15 nm) due to interactions between the encapsulated molecules and the surfactant monolayer. Emulsions showed the ability to retain the carotenoids’ antioxidant capacity (43–98% Tempol Inhibition) | [141] |
| Lutein (carotenoid) | High-shear mixing/microfluidization (emulsifier: Quillaja saponins) | Improve solubility, stability, and bioavailability | Quillaja saponin emulsifiers are effective at producing physically stable emulsions over 10 d of storage with no significant change in mean particle diameter (d32 = 0.23–0.25 μm) or ζ-potential (−55 to −62 mV) while reducing carotenoid degradation | [142] | |
| Grapefruit peel polyphenols | High-speed homogenization followed by sonication (emulsifier: sorbitan monooleate) | Improve stability of mustard oil in W/O emulsions | The nano-emulsion protected phenols against degradation in the capacity to prevent rancidity of mustard oil, achieving a concentration of 84.84 µgGAE/mL with respect to 60.13 and 75.52 µgGAE/mL of mustard oil and mixture of oil and emulsion, respectively | [143] | |
| Vitamin E | Homogenization using a high-speed blender (emulsifiers: whey protein isolate and gum arabic) | Improve stability and protection against environmental conditions | Whey protein isolate produced small droplets of d32 = 0.11 μm at 1% concentration of emulsifier. Conversely, the gum arabic was much more effective at stabilizing the droplets against environmental stresses | [144] | |
| Curcumin | Mixing/sonication (emulsifier: Quillaja saponins) | Increase bioavailability | Antioxidants improved the chemical stability of curcumin in O/W emulsions, with no change in the droplet size (100–130 nm) with respect to blank emulsions (130 nm) | [145] | |
| Quercetin | High-pressure homogenization (emulsifier: rice bran proteins) | Increase stability, bioavailability, and reduced toxicity | The addition of quercetin at 3 mg/mL led to the formation of nano-emulsions with a smaller droplet size (216 nm), high incorporation rate (inclusion rate of 98%), and good stability | [146] | |
| SLNs | Lycopene | High-shear homogenization and ultrasound (emulsifiers: glycerol monostearate, soybean phosphatidylcholine, tween 80) | Protection against environmental conditions; increase solubility and bioavailability | Lycopene-loaded nanoparticles presented particle size between 75 and 183 nm and an encapsulation efficiency between 65 and 89% | [147] |
| (─)-epigallocatechin-3-gallate (EGCG) | Hot, high-pressure homogenization (emulsifiers: mono- and diglycerides of fatty acids and sodium stearoyl-2-lactylate) | Provide protection from light and heat | The nanoparticles loaded with different concentrations of EGCG had an average particle size in the range of 108–122 nm with a maximal encapsulation efficiency of 69% | [148] | |
| Eugenol-rich clove extract | Hot homogenization followed by sonication (emulsifiers: Tween 80, Poloxamer 188) | Preserve antioxidant activity | Solid-lipid systems promote the encapsulation of the active substance, with a maximum retention efficiency of 60% and mean particle size that ranged between 11 and 22 µm | [149] | |
| NLCs | Turmeric Extract | High-shear homogenization (emulsifier: Poloxamer 407) | Increase stability and solubility; preserve bioactivity | The average size of turmeric-extract-loaded nanostructured lipid was 112.4 nm and it showed good physical stability, high encapsulation efficiency over time (98% at beginning and 92% after 40 days of storage), and a sustained release pattern | [124] |
| Quercetin/Linseed oil | Melting emulsification and high-pressure homogenization (emulsifiers: glyceryl monostearate, Tween 80) | Increase biological activity | Quercetin/linseed-oil-co-loaded nanoparticles were less than 100 nm in diameter, which allowed them to exhibit significantly better inhibition against oxidation compared with the linseed O/W emulsion over storage (10 and 25 µM/gOIL for quercetin/linseed oil and linseed emulsion, respectively, after 10 days) | [150] | |
| Oleuropein (secoiridoids) | Hot melt homogenization (emulsifier: Poloxamer 188) | Increase stability during use conditions | Oleuropein-loaded nanostructured lipid showed a mean size of 150 nm, a zeta potential of −21 mV, an encapsulation efficiency of 99.12%, sustained release profile, and improved radical scavenging activity | [151] | |
| Liposomes | Procyanidins (Condensed tannins) from Litchi chinensis Sonn | Thin-film dispersion method (Yolk lecithin, cholesterol, Tween 80) | Provide protection against degradation (pH, temperature, light) | Oligomeric procyanidins encapsulated in liposomes showed particles with a size in the range of 80–100 nm, and the highest encapsulation efficiency (91%) was achieved when the oligomeric procyanidins’ load rate was 2% | [152] |
| Citrus extract and oregano, cinnamon, and citronella essential oils | Sonication (deoiled and fluidic sunflower lecithin) | Determine how liposome formulation and preparation conditions affect size and encapsulation efficiency | Citrus extract (CE) and essential oils (EO) encapsulated in liposomes achieved high stability during storage, with particle size values ranging from 97 to 95 nm for blanks, from 96 to 98 nm for CE-loaded-liposomes, and 87 to 102 nm for EO-loaded liposomes | [153] | |
| Phytosomes | Rutin (glycosylated flavonol) | Thin-film hydration method (soybean phosphatidylcholine + rutin) | Enable controlled release; improve stability and solubility | Phosphatidylcholine–rutin complex showed the greatest physical and chemical stability (during 30 days of storage) with fine particle sizes (<100 nm) and encapsulation efficiency of 99% | [154,155] |
| Vitamin E and C | Solvent evaporation method (milk phospholipids + ascorbic acid) | Enable sustainable release during intestinal digestion | Ascorbic acid and α-tocopherol encapsulated in phytosomes yielded an optimal complexing index of 99% at a molar ratio of 1:1. Moreover, cellular uptake studies demonstrated that phospholipid-based substances were more readily absorbed than liposomes | [156] | |
| Niosomes | Lavender (Lavandula angustifolia) oil | Reverse-phase evaporation method (Span 60 + cholesterol) | Improve the delivery of bioactive compounds without affecting their activity | Toxicity tests revealed that lavender oil niosomes (size of 1216 nm and ζ-potential of −22 mV) have cell viability rates similar to the normal culture medium ranging from 83 to 101% | [138] |
| Vinca rosea extracts enriched in alkaloids | Thin-film hydration method (Span 60 + cholesterol) | Demonstrate two-fold increase in bioavailability when encapsulated in niosomes | The niosomes with a size in the range of 400 to 800 nm increased the bioavailability of Vinca rosea alkaloid extract by two fold compared to the total extract | [139] | |
| Quercetin | Solvent evaporation method (Span 20, glucose monolaurate, sucrose monolaurate, trehalose monolaurate + cholesterol) | Demonstrate higher (in vivo) hepatoprotective effect of niosomes than for free quercetin | Nanosized quercetin-loaded niosomes presented a spherical shape and a particle size of 161 nm, with a drug encapsulation efficiency as high as 83.6 ± 3.7% and sustained quercetin release | [137] |
| Extract/Molecule | Formulation | Characteristics | Reference | |
|---|---|---|---|---|
| Proteins | Epigallocatechin gallate (EGCG) | Co-encapsulation of EGCG Oil phase: 30% v/v sunflower oil Stabilizer: 0.2% w/v zein loaded with EGCG | Increased stability over time in a 3–9 pH range, controlled EGCG release (20.1%, 2 h gastric), and superior bioaccessibility (65.2%) compared to that of free EGC) | [166] |
| Carvacrol | Oil phase: 1% w/w carvacrol/sunflower oil Stabilizer: 1.95% w/w whey protein microgels | Submicrometric droplet size (~284 nm), controlled release (maximum release of about 2.5 mg carvacrol during 80 h), and sustained antimicrobial activity (always ≤ 500 mg/L independently in the test microorganisms) | [11] | |
| β-carotene | Oil phase: 70% w/w corn oil Stabilizer: 0.5–1.5%, w/v gliadin | Emulgel with high loading, improved stability upon pasteurization (minimum β-carotene content of 83% and 94% after 28 days at 4 °C and after pasteurization, respectively) | [167] | |
| Polysaccharides | Curcumin and coumarin | Oil phase: 5 w/w% MCT Stabilizer: 0.2 w/w% cellulose | Submicrometric size (≤150 nm) and improved antimicrobial and anticancer activity (>50% percentage viability with respect to the normal cells) | [173] |
| Curcumin | Oil phase: 33% v/v MCT Stabilizers: 0.15–0.75% w/v chitosan–gum arabic particles | Higher curcumin encapsulation efficiency (>83% of curcumin) and stability and controlled release (lower than 47% during 120 min) | [170] | |
| Citrus peel extract | Oil phase: 50% w/w MCT Stabilizer: 1% w/w Cellulose | Submicrometric size (average droplet size of 264 nm) and improved bioaccessibility of a mean of 10% and 93% compared to nano-emulsion and bulk oil, respectively | [168] | |
| Protein/ polysaccharides | Phenolic-rich grape seed extract | Oil phase: 40% w/w sunflower oil Stabilizer: 0.5% w/w sodium caseinate and 0.375% w/w carboxymethyl cellulose or 0.5% w/w gum arabic | Efficient polyphenol encapsulation with values ranging from 77 to 79% and controlled release ranging from 20 to 26% over 14 days | [171] |
| Thymol | Oil phase: 4% w/w tricaprylin oil Stabilizer: 0.24% w/v whey protein, 0.16% w/v soluble fraction of almond gum | Submicrometric size (between 300 and 400 nm) and improved emulsion stabilization with negatively charged soluble complexes (−36.5 mV) through complexation | [169] | |
| Resveratrol and α-tocopherol | Co-encapsulation of α -tocopherol and resveratrol Oil phase: 5% w/w sunflower oil Stabilizer: 0.5% w/v caseinate+resveratrol, 0.1–1.0% w/v pectin or gum arabic | Submicrometric size (the peaks around 300 and 860 nm), improved digestive stability through complexation, and improved bioaccessibility of both tocopherol and resveratrol by about 90% after 42 days | [158] | |
| Casticin | Oil phase: 10% w/w MCT, Stabilizer: 1.0% w/w WPI-lactose Maillard conjugate | Submicrometric size with droplet size ranging from 15.4 (fresh) to 58.3 μm (100 mmol/L NaCl, 8 weeks), effective delivery, and improved uptake and biological activity | [172] | |
| Protein/ polysaccharides/ surfactant | β-carotene | Oil phase: 50% w/w MCT Stabilizer: 0.7% w/w zein, 0.27% w/w PGA+rhamnolipid | Enhanced stability and delayed lipid digestion (reduced from 19% to 3%) and improved bioaccessibility of β-carotene by about 21% | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gali, L.; Pirozzi, A.; Donsì, F. Biopolymer- and Lipid-Based Carriers for the Delivery of Plant-Based Ingredients. Pharmaceutics 2023, 15, 927. https://doi.org/10.3390/pharmaceutics15030927
Gali L, Pirozzi A, Donsì F. Biopolymer- and Lipid-Based Carriers for the Delivery of Plant-Based Ingredients. Pharmaceutics. 2023; 15(3):927. https://doi.org/10.3390/pharmaceutics15030927
Chicago/Turabian StyleGali, Lynda, Annachiara Pirozzi, and Francesco Donsì. 2023. "Biopolymer- and Lipid-Based Carriers for the Delivery of Plant-Based Ingredients" Pharmaceutics 15, no. 3: 927. https://doi.org/10.3390/pharmaceutics15030927