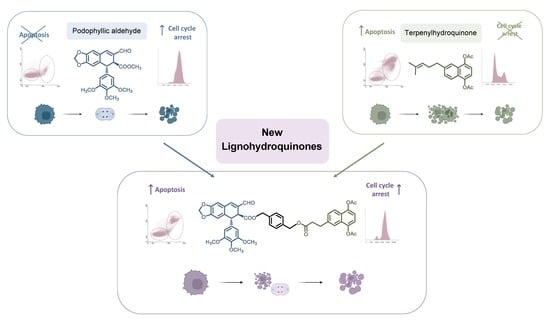
Improving Properties of Podophyllic Aldehyde-Derived Cyclolignans: Design, Synthesis and Evaluation of Novel Lignohydroquinones, Dual-Selective Hybrids against Colorectal Cancer Cells
Abstract
:1. Introduction
Chemistry Approach and Objective
2. Materials and Methods
2.1. Chemical Methods
2.1.1. Starting Materials
2.1.2. General Method for Formation of Esters
Acetonide 7
Acetonide 8
Acetonide 9
MHQ 10
MHQ 11
MHQ 12
Compound 13
Compound 14
Compound 15
2.1.3. General Method for the Synthesis of Hybrids Lignomonoterpenylnaphthohydroquinones (L-MHQs) 16–18
Aldehyde 16
Aldehyde 17
Aldehyde 18
2.2. Biological Methods
2.3. Docking Methods
3. Results
3.1. Synthesis of the New Lignohydroquinones
3.2. Biological Assays
3.2.1. Cytotoxicity Evaluation
3.2.2. Flow Cytometry Assays
3.3. Molecular Docking Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Shen, B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.H.; Li, S.; Zhang, J.H.; Huang, Y.Y.; Zhang, L.Q.; Zhao, F.; Du, X.; Hou, J.L.; Zhang, T.; Shi, C.J.; et al. Current state and future perspective of cardiovascular medicines derived from natural products. Pharmacol. Ther. 2020, 216, 107698. [Google Scholar] [CrossRef]
- Ouyang, L.; Luo, Y.; Tian, M.; Zhang, S.Y.; Lu, R.; Wang, J.H.; Kasimu, R.; Li, X. Plant natural products: From traditional compounds to new emerging drugs in cancer therapy. Cell Prolif. 2014, 47, 506–515. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T.; The International Natural Product Sciences Taskforce. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Li, G.; Lou, H.-X. Strategies to diversify natural products for drug discovery. Med. Res. Rev. 2018, 38, 1255–1294. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2016, 25 (Suppl. S2), 41–59. [Google Scholar] [CrossRef]
- Dutta, S.; Mahalanobish, S.; Saha, S.; Ghosh, S.; Sil, P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019, 128, 240–255. [Google Scholar] [CrossRef]
- Manoharan, S.; Perumal, E. Potential role of Marine Bioactive Compounds in cancer signaling pathways: A review. Eur. J. Pharmacol. 2022, 936, 175330. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Oliveira, C.; Reis, R.L.; Martins, A.; Silva, T.H. Marine-Inspired Drugs and Biomaterials in the Perspective of Pancreatic Cancer Therapies. Mar. Drugs 2022, 20, 689. [Google Scholar] [CrossRef]
- Carbone, D.; Vestuto, V.; Ferraro, M.R.; Ciaglia, T.; Pecoraro, C.; Sommella, E.; Cascioferro, S.; Salviati, E.; Novi, S.; Tecce, M.F.; et al. Metabolomics-assisted discovery of a new anticancer GLS-1 inhibitor chemotype from a nortopsentin-inspired library: From phenotype screening to target identification. Eur. J. Med. Chem. 2022, 234, 114233. [Google Scholar] [CrossRef]
- Pecoraro, C.; Parrino, B.; Cascioferro, S.; Puerta, A.; Avan, A.; Peters, G.J.; Diana, P.; Giovannetti, E.; Carbone, D. A New Oxadiazole-Based Topsentin Derivative Modulates Cyclin-Dependent Kinase 1 Expression and Exerts Cytotoxic Effects on Pancreatic Cancer Cells. Molecules 2022, 27, 19. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsalahat, I.; Daoud, S.; Abutayeh, R.F.; Mahmod, A.I. Plant-Derived Natural Products in Cancer Research: Extraction, Mechanism of Action, and Drug Formulation. Molecules 2020, 25, 5319. [Google Scholar] [CrossRef]
- Guo, Z. The modification of natural products for medical use. Acta Pharm. Sin. B 2017, 7, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef]
- Berube, G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef]
- Sflakidou, E.; Leonidis, G.; Foroglou, E.; Siokatas, C.; Sarli, V. Recent Advances in Natural Product-Based Hybrids as Anti-Cancer Agents. Molecules 2022, 27, 6632. [Google Scholar] [CrossRef]
- Kumar, H.M.S.; Herrmann, L.; Tsogoeva, S.B. Structural hybridization as a facile approach to new drug candidates. Bioorganic Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef]
- Niro, G.; Weck, S.C.; Ducho, C. Merging Natural Products: Muraymycin-Sansanmycin Hybrid Structures as Novel Scaffolds for Potential Antibacterial Agents. Chem.-A Eur. J. 2020, 26, 16875–16887. [Google Scholar] [CrossRef]
- Zheng, L.-L.; Wen, G.; Yao, Y.-X.; Li, X.-H.; Gao, F. Design, Synthesis, and Anticancer Activity of Natural Product Hybrids With Paclitaxel Side Chain Inducing Apoptosis in Human Colon Cancer Cells. Nat. Prod. Commun. 2020, 15, 1934578X20917298. [Google Scholar] [CrossRef]
- Ding, C.; Chen, H.; Liang, B.; Jiao, M.; Liang, G.; Zhang, A. Biomimetic synthesis of the natural product salviadione and its hybrids: Discovery of tissue-specific anti-inflammatory agents for acute lung injury. Chem. Sci. 2019, 10, 4667–4672. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.H.E.; Choi, E.; Yoon, M.Y.; Lee, K.W.; Yoo, S.Y.; Cho, M.C.; Yang, J.S.; Kim, H.I.; Hong, J.Y.; Shin, J.-S.; et al. Natural products hybrids: 3,5,4 ′-Trimethoxystilbene-5,6,7-trimethoxyflavone chimeric analogs as potential cytotoxic agents against diverse human cancer cells. Eur. J. Med. Chem. 2019, 161, 559–580. [Google Scholar] [CrossRef]
- Gargantilla, M.; Persoons, L.; Kauerova, T.; del Rio, N.; Daelemans, D.; Priego, E.M.; Kollar, P.; Perez-Perez, M.J. Hybridization Approach to Identify Salicylanilides as Inhibitors of Tubulin Polymerization and Signal Transducers and Activators of Transcription 3 (STAT3). Pharmaceuticals 2022, 15, 835. [Google Scholar] [CrossRef]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Developments of Molecular Hybrids Targeting Tubulin Polymerization. Int. J. Mol. Sci. 2022, 23, 4001. [Google Scholar] [CrossRef]
- Garcia, P.A.; Hernandez, A.P.; San Feliciano, A.; Castro, M.Á. Bioactive Prenyl- and Terpenyl-Quinones/Hydroquinones of Marine Origin. Mar. Drugs 2018, 16, 292. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.Y.; Tong, Y.R.; Luo, Y.F.; Huang, L.Q.; Gao, W. Biosynthesis, total synthesis, and pharmacological activities of aryltetralin-type lignan podophyllotoxin and its derivatives. Nat. Prod. Rep. 2022, 39, 1856–1875. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M.; García, P.A.; Miguel del Corral, J.M.; Castro, M.A.; Gómez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef]
- Guerram, M.; Jiang, Z.-Z.; Zhang, L.-Y. Podophyllotoxin, a medicinal agent of plant origin: Past, present and future. Chin. J. Nat. Med. 2012, 10, 161–169. [Google Scholar] [CrossRef]
- Medrado, H.H.S.; David, J.M.; David, J.P.; Brandao, H.N. Distribution, Biological Activities, Synthesis, and Purification Methods for Podophyllotoxin and its Derivatives. Quim. Nova 2015, 38, 243–258. [Google Scholar] [CrossRef]
- Montecucco, A.; Zanetta, F.; Biamonti, G. Molecular mechanisms of etoposide. EXCLI J. 2015, 14, 95–108. [Google Scholar] [CrossRef]
- Xiao, J.; Gao, M.; Sun, Z.; Diao, Q.; Wang, P.; Gao, F. Recent advances of podophyllotoxin/epipodophyllotoxin hybrids in anticancer activity, mode of action, and structure-activity relationship: An update (2010–2020). Eur. J. Med. Chem. 2020, 208, 112830. [Google Scholar] [CrossRef]
- Castro, M.A.; Miguel del Corral, J.M.; Gordaliza, M.; Garcia, P.A.; Gomez-Zurita, M.A.; Garcia-Grdvalos, M.D.; de la Iglesia-Vicente, J.; Gajate, C.; An, F.Y.; Mollinedo, F.; et al. Synthesis and biological evaluation of new selective cytotoxic cyclolignans derived from podophyllotoxin. J. Med. Chem. 2004, 47, 1214–1222. [Google Scholar] [CrossRef]
- Castro, M.A.; Miguel del Corral, J.M.; Gordaliza, M.; Garcia, P.A.; Gomez-Zurita, M.A.; San Feliciano, A. Synthesis and cytotoxic evaluation of C-9 oxidized podophyllotoxin derivatives. Bioorganic Med. Chem. 2007, 15, 1670–1678. [Google Scholar] [CrossRef]
- Castro, M.Á.; Miguel del Corral, J.M.; Gordaliza, M.; Grande, C.; Gómez-Zurita, A.; García-Grávalos, D.; San Feliciano, A. Synthesis and cytotoxicity of podophyllotoxin analogues modified in the A ring. Eur. J. Med. Chem. 2003, 38, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Miguel del Corral, J.M.; Gordaliza, M.; Gomez-Zurita, M.A.; de la Puente, M.L.; Betancur-Galvis, L.A.; Sierra, J.; San Feliciano, A. Synthesis, cytotoxicity and antiviral activity of podophyllotoxin analogues modified in the E-ring. Eur. J. Med. Chem. 2003, 38, 899–911. [Google Scholar] [CrossRef]
- Castro, M.A.; Miguel del Corral, J.M.; García, P.A.; Rojo, M.V.; de la Iglesia-Vicente, J.; Mollinedo, F.; Cuevas, C.; San Feliciano, A. Synthesis and biological evaluation of new podophyllic aldehyde derivatives with cytotoxic and apoptosis-inducing activities. J. Med. Chem. 2010, 53, 983–993. [Google Scholar] [CrossRef]
- Hernandez, A.P.; Diez, P.; García, P.A.; Miguel del Corral, J.M.; Pérez-Andrés, M.; Diez, D.; San Feliciano, A.; Fuentes, M.; Castro, M.Á. New Hybrids Derived from Podophyllic Aldehyde and Diterpenylhydroquinones with Selectivity toward Osteosarcoma Cells. ACS Med. Chem. Lett. 2018, 9, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.Á.; Miguel del Corral, J.M.; García, P.A.; Rojo, M.V.; Bento, A.C.; Mollinedo, F.; Francesch, A.M.; San Feliciano, A. Lignopurines: A new family of hybrids between cyclolignans and purines. Synthesis and biological evaluation. Eur. J. Med. Chem. 2012, 58, 377–389. [Google Scholar] [CrossRef]
- Hernandez, A.P.; Chamorro, P.; Rodriguez, M.L.; Miguel del Corral, J.M.; Garcia, P.A.; Francesch, A.; San Feliciano, A.; Castro, M.A. New Antineoplastic Naphthohydroquinones Attached to Labdane and Rearranged Diterpene Skeletons. Molecules 2021, 26, 474. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Gamito, A.M.; Tangarife-Castatno, V.; Zapata, B.; Miguel del Corral, J.M.; Mesa-Arango, A.C.; Betancur-Galvis, L.; San Feliciano, A. Synthesis and antifungal activity of terpenyl-1,4-naphthoquinone and 1,4-anthracenedione derivatives. Eur. J. Med. Chem. 2013, 67, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Gamito, A.M.; Tangarife-Castano, V.; Roa-Linares, V.; Miguel del Corral, J.M.; Mesa-Arango, A.C.; Betancur-Galvis, L.; Francesch, A.M.; Feliciano, A.S. New 1,4-anthracenedione derivatives with fused heterocyclic rings: Synthesis and biological evaluation. RSC Adv. 2015, 5, 1244–1261. [Google Scholar] [CrossRef] [Green Version]
- Miguel del Corral, J.M.; Gordaliza, M.; Castro, M.A.; Mahiques, M.M.; San Feliciano, A.; García-Grávalos, M.D. Further antineoplastic terpenylquinones and terpenylhydroquinones. Bioorganic Med. Chem. 1998, 6, 31–41. [Google Scholar] [CrossRef]
- Molinari, A.; Oliva, A.; Ojeda, C.; Miguel del Corral, J.M.; Castro, M.A.; Cuevas, C.; Feliciano, A.S. New cytotoxic-antineoplastic prenyl-1,2-naphthohydroquinone derivatives. Bioorganic Med. Chem. 2005, 13, 6645–6650. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.-P.; Micaelo, A.; Pinol, R.; Garcia-Vaquero, M.L.; Aramayona, J.J.; Criado, J.J.; Rodriguez, E.; Ignacio Sanchez-Gallego, J.; Landeira-Vinuela, A.; Juanes-Velasco, P.; et al. Comprehensive and systematic characterization of multi-functionalized cisplatin nano-conjugate: From the chemistry and proteomic biocompatibility to the animal model. J. Nanobiotechnol. 2022, 20, 341. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.P.; Diez, P.; García, P.A.; Pérez-Andrés, M.; Ortega, P.; Jambrina, P.G.; Diez, D.; Castro, M.A.; Fuentes, M. A Novel Cytotoxic Conjugate Derived from the Natural Product Podophyllotoxin as a Direct-Target Protein Dual Inhibitor. Molecules 2020, 25, 4258. [Google Scholar] [CrossRef]
- Ravelli, R.B.G.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Brooks, C.L., 3rd; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [Green Version]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [Green Version]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.M.; Mittal, J.; Feig, M.; MacKerell, A.D. Optimization of the Additive CHARMM All-Atom Protein Force Field Targeting Improved Sampling of the Backbone ϕ, ψ and Side-Chain χ1 and χ2 Dihedral Angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef] [Green Version]
- Hart, K.; Foloppe, N.; Baker, C.M.; Denning, E.J.; Nilsson, L.; MacKerell, A.D. Optimization of the CHARMM Additive Force Field for DNA: Improved Treatment of the BI/BII Conformational Equilibrium. J. Chem. Theory Comput. 2012, 8, 348–362. [Google Scholar] [CrossRef] [Green Version]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [Green Version]
- Gil-Guerrero, S.; Melle-Franco, M.; Peña-Gallego, Á.; Mandado, M. Clar Goblet and Aromaticity Driven Multiradical Nanographenes. Chem. Eur. J. 2020, 26, 16138–16143. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, I.C.; Huang, Y.-J.; Chiang, T.-I.; Yeh, C.-W.; Hsu, L.-S. Shikonin Induces Apoptosis through Reactive Oxygen Species/Extracellular Signal-Regulated Kinase Pathway in Osteosarcoma Cells. Biol. Pharm. Bull. 2010, 33, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Nerella, S.; Kankala, S.; Paidakula, S.; Gavaji, B. Synthesis of D-ring modified acid hydrazide derivatives of podophyllotoxin and their anticancer studies as Tubulin inhibiting agents. Bioorganic Chem. 2020, 94, 103384. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, B.A.; Banday, J.A.; Bhat, B.A.; Ara, T. Synthesis and In vitro Anticancer Activity of Triazolyl Analogs of Podophyllotoxin, a Naturally Occurring Lignin. Russ. J. Org. Chem. 2021, 57, 2039–2047. [Google Scholar] [CrossRef]
- Li, J.; Hua, H.M.; Tang, Y.B.; Zhang, S.P.; Ohkoshi, E.; Lee, K.H.; Xiao, Z.Y. Synthesis and evaluation of novel podophyllotoxin analogs. Bioorganic Med. Chem. Lett. 2012, 22, 4293–4295. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, C.; Ma, X.; Gao, Y.; Liu, J.; Wu, L. Synthesis and biological evaluation of NQO1-activated prodrugs of podophyllotoxin as antitumor agents. Bioorganic Med. Chem. 2020, 28, 115821. [Google Scholar] [CrossRef]
- Cai, D.-S.; Lou, S.-Y.; Huo, S.; Gao, F.; Pi, W.M.; Chen, K.-D.; Wang, C.; Yang, X.-Y.; Jiao, J.-Y.; Xu, B.; et al. Synthesis and biological activity evaluation of podophyllotoxin-linked bile acid derivatives as potential anti-liver cancer agents. Bioorganic Chem. 2022, 128, 106066. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Zhao, D.; Ran, X.; Zhang, L.; Zhao, D. Novel Hybrids of Podophyllotoxin and Coumarin Inhibit the Growth and Migration of Human Oral Squamous Carcinoma Cells. Front. Chem. 2021, 8, 626075. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xie, Q.; Zeng, X.; Tao, N.; Xu, Y.; Chen, Y.; Wang, J.; Zhang, L. Novel hybrids of podophyllotoxin and formononetin inhibit the growth, migration and invasion of lung cancer cells. Bioorganic Chem. 2019, 85, 445–454. [Google Scholar] [CrossRef] [PubMed]









| IC50 (µM) | ||||||
|---|---|---|---|---|---|---|
| Compound | MG-63 | MCF-7 | HT-29 | |||
| 24 h | 72 h | 24 h | 72 h | 24 h | 72 h | |
| 1 | 18.3 | 0.00707 | >100 | 0.0284 | >100 | 0.0664 |
| 2 | 1.23 | 0.0663 | >100 | 0.477 | >100 | 0.0283 |
| 3 | 1.11 | 4.23 | 8.23 | 4.93 | 4.33 | 8.51 |
| 16 | 2.80 | 2.26 | 1.38 | 2.33 | 4.77 | 0.834 |
| 17 | 2.70 | 0.260 | 8.81 | 2.34 | 4.16 | 0.652 |
| 18 | 0.752 | 0.292 | 13.1 | 1.78 | 4.12 | 0.0405 |
| Compounds | −ΔG (kcal/mol) | −log IC50 | |||
|---|---|---|---|---|---|
| MG-63 | MCF-7 | HT-29 | |||
| Cyclolignans | 1 | 9.50 | 8.15 | 7.55 | 7.18 |
| 2 | 8.50 | 7.22 | 6.32 | 7.54 | |
| Terpenylhydroquinones | 3 | 9.00 | 5.08 | 5.95 | 5.36 |
| 4 | 9.40 | 6.85 | 7.82 | 7.74 | |
| L-MHQs | 16 | 10.40 | 5.65 | 5.63 | 6.08 |
| 17 | 9.90 | 6.59 | 5.63 | 6.19 | |
| 18 | 10.50 | 6.54 | 5.75 | 7.39 | |
| L-DHQs | 19 | 9.00 | 5.65 | 5.66 | 5.82 |
| 20 | 10.10 | 5.69 | 6.00 | 5.72 | |
| 21 | 10.10 | 6.82 | 5.99 | 5.84 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, Á.-P.; Díez, P.; García, P.A.; Pérez-Andrés, M.; Veselinova, A.; Jambrina, P.G.; San Feliciano, A.; Díez, D.; Fuentes, M.; Castro, M.Á. Improving Properties of Podophyllic Aldehyde-Derived Cyclolignans: Design, Synthesis and Evaluation of Novel Lignohydroquinones, Dual-Selective Hybrids against Colorectal Cancer Cells. Pharmaceutics 2023, 15, 886. https://doi.org/10.3390/pharmaceutics15030886
Hernández Á-P, Díez P, García PA, Pérez-Andrés M, Veselinova A, Jambrina PG, San Feliciano A, Díez D, Fuentes M, Castro MÁ. Improving Properties of Podophyllic Aldehyde-Derived Cyclolignans: Design, Synthesis and Evaluation of Novel Lignohydroquinones, Dual-Selective Hybrids against Colorectal Cancer Cells. Pharmaceutics. 2023; 15(3):886. https://doi.org/10.3390/pharmaceutics15030886
Chicago/Turabian StyleHernández, Ángela-Patricia, Paula Díez, Pablo A. García, Martín Pérez-Andrés, Anzhela Veselinova, Pablo G. Jambrina, Arturo San Feliciano, David Díez, Manuel Fuentes, and Mᵃ Ángeles Castro. 2023. "Improving Properties of Podophyllic Aldehyde-Derived Cyclolignans: Design, Synthesis and Evaluation of Novel Lignohydroquinones, Dual-Selective Hybrids against Colorectal Cancer Cells" Pharmaceutics 15, no. 3: 886. https://doi.org/10.3390/pharmaceutics15030886